Controlled-release of Chlorine Dioxide in a Perforated Packaging System to Extend the Storage Life and Improve the Safety of Grape Tomatoes
Summary
Here, we describe a protocol for the application of a novel, slow-release ClO2 product that reduces spoilage and extends the shelf life of fresh fruit. The slow-release ClO2 product was added to standard commercial grape tomato packaging and tested against Escherichia coli and Alternaria alternata.
Abstract
A controlled-release chlorine dioxide (ClO2) pouch was developed by sealing a slurry form of ClO2 into semipermeable polymer film; the release properties of the pouch were monitored in containers with or without fruit. The pouch was affixed to the inside of a perforated clamshell containing grape tomatoes, and the effect on microbial population, firmness, and weight loss was evaluated during a 14 day storage period at 20 °C. Within 3 days, the ClO2 concentration in the clamshells reached 3.5 ppm and remained constant until day 10. Thereafter, it decreased to 2 ppm by day 14. The ClO2 pouch exhibited strong antimicrobial activity, reducing Escherichia coli populations by 3.08 log CFU/g and Alternaria alternata populations by 2.85 log CFU/g after 14 days of storage. The ClO2 treatment also reduced softening and weight loss and extended the overall shelf life of the tomatoes. Our results suggest that ClO2 treatment is useful for extending the shelf life and improving the microbial safety of tomatoes during storage without impairing their quality.
Introduction
A diet rich in fresh fruits and vegetables may help to reduce the risk of many diseases, including coronary heart disease and specific types of cancers1. However, there are a number of foodborne microbial pathogens, such as Escherichia coli, Salmonella enterica, and Listeria monocytogenes, associated with the consumption of fresh fruits and vegetables that can cause illness or even death among consumers who eat contaminated produce2. For example, E. coli O157:H7 outbreaks have been associated with grapes, tomatoes, and strawberries3,4, and hepatitis A outbreaks have been associated with fresh blueberries5. In addition, microbial contamination can cause substantial product loss through postharvest decay6. Alternaria alternata is an important plant pathogenic fungus that is known to cause leaf spots and other diseases in over 380 host species of plants7. It has been shown to be the cause of an Alternaria black spot8, a stem canker disease and a leaf blight of tomatoes9. Therefore, a safe and effective postharvest decontamination treatment is needed to both control foodborne pathogens and to prevent postharvest decay in fresh produce.
Low- and non-residue technologies are new trends for alternative sanitizers. A variety of postharvest fungicides have been used to reduce spoilage organisms and to prevent foodborne illness. Ozone, a strong antimicrobial agent, has been shown to preserve the quality and freshness of strawberries and blueberries10,11. However, ozone may cause oxidation of fruit surface tissue and can result in discoloration and the deterioration of flavor quality12. Chlorine has been used to sanitize fresh produce, such as blueberries and apples13. While effective, chlorine can react with nitrogen-containing compounds or ammonia, resulting in carcinogenic byproducts14, especially when used for the sanitization of fresh fruit15.
Chlorine dioxide (ClO2), an alternative sanitizer, was approved by both China and the US for the postharvest treatment of fruits and vegetables16. ClO2 is a water-soluble oxidizing agent with an oxidation capacity 2.5 times greater than that of free chlorine17. ClO2 is highly effective at low concentrations and with a short contact time18. ClO2 has low toxicity and minimal corrosiveness at the concentrations used for disinfection, and it is recognized as one of the most effective bactericidal and fungicidal agents for use in a variety of settings19,20,21.
Numerous research results have shown that ClO2 can control foodborne pathogens and postharvest decay16. For example, ClO2 gas has been used to inactivate L. monocytogenes, Salmonella, and E. coli O157:H7 and to prevent blueberry and strawberry spoilage22,23. ClO2 gas reduces the risk of microbial contamination while maintaining the attributes of fresh fruit, and it was effective at controlling the postharvest decay of strawberries24. However, it is unstable at high concentrations and non-transportable, historically requiring costly generators on site or inefficient two-part powder mixing.
However, a new ClO2 product with a ready-made, controlled-release formulation (i.e., it does not require a generator or the premixing of ingredients) has been shown to be highly effective at controlling food spoilage organisms and pathogens in preliminary experiments25. It is a safe, cost-effective, non-corrosive, easily transportable, and controlled-release form of ClO2, with no adverse effects on the environment. Previous experiments have demonstrated that this slow-release ClO2 powder wrapped in filtration material and placed in clamshell packaging significantly reduced the decay of fresh blueberries and strawberries, decreased berry water loss, and maintained fruit firmness during postharvest storage25,26. Recently, a controlled-release ClO2 packet was developed by sealing a slurry form of ClO2 in a semipermeable polymer film. The objectives of this work were to: 1) monitor ClO2 gas release properties in both a closed container and in perforated clamshells, 2) investigate the effect of a controlled-release ClO2 pouch enclosed in a container on foodborne pathogens and the decay of grape tomatoes, and 3) evaluate the effects of the controlled-release ClO2 on the storage quality of grape tomatoes.
Protocol
1. Measurement of Gaseous ClO2 in the Headspace of a Closed Chamber
- Obtain the materials: ClO2 pouch (0.5 g of ClO2 slurry (9.5% a.i.) in a polymer film selected for its release rate (total surface area of 6 cm2); the exact components are proprietary), a glass chamber (19.14 L), and a lid with switchable gas inlet and outlet.
- Attach the ClO2 pouch to the lid using double-sided tape.
- Close the chamber by sealing the lid with petroleum jelly.
- Connect the inlet and outlet of a ClO2 gas detector to the chamber.
NOTE: This is a gas circulation system, and no gas loss occurred when taking measurements. - Switch on the inlet and outlet gas flow and measure the ClO2 concentration in the chamber after incubating for 0, 1, 2, 3, 4, 24, 26, 28, and 48 h.
- Monitor the temperature and relative humidity (RH) in the chamber with temperature and RH data loggers.
2. Fruit Preparation and Storage
- Obtain 15 kg of fresh grape tomatoes (Solanum lycopersicum var. cerasiforme) from a local retailer. Ensure that the fruits are healthy and have no visual flaws.
- Preparation of inoculum
- Use strains of E. coli (wild type) and A. alternata from citrus fruit surfaces27 for inoculation.
- Culture E. coli on E. coli agar (ECA) at 35 °C for 1 day27 and then re-culture the organisms on a new plate for 1 day. Confirm the organisms by sampling the ECA plates with a bac-loop, streaking the bacteria on Levine eosin methylene blue (EMB) agar, and incubating for 24 h at 35 °C; cultures that turn reflective, metallic green are positive for E. coli.
- Culture A. alternata on potato dextrose agar (PDA) at 25 °C until spores appear.
- Scrape the E. coli cells from the agar plate into 50 mL of sterile distilled water until the estimated concentration reaches 9 log CFU/mL using a comparison with McFarland equivalence turbidity standards. Add 1,950 mL of sterile water containing 0.1% Tween-20 to make 2 L total of the final inoculum.
- Verify the cell concentration by dilution plating on EC agar plates. Scrape the A. alternata spores from the culture medium and suspend them into 2 L of sterile distilled water containing 0.1% Tween-20.
NOTE: The final E. coli population was 7.5 log CFU/g, and the A. alternata population was 5.5 log CFU/g.
- Place 7 kg of the tomatoes into a 10 L stainless steel pan that is completely covered by an autoclavable bag. Place the bag and pan in a safety hood. Apply the inoculum solution (2 L) to the fruits using a trigger sprayer applied from the top while gently stirring the fruits with a gloved hand.
- After 5 min, place the tomatoes in a single layer on sterilized sheets and allow them to air dry for 2 h. Put about 200 g of fruit each into twent-four 1 lb (~1.14 L) perforated clamshells.
- Carefully fold the contaminated foils and place them into the steel pan. Remove the gloves and put them into the pan. Wrap the autoclavable bag and autoclave all contaminated supplies at 121 °C for 25 min.
- Attach ClO2 pouches to the lids of 12 clamshells. Use the other 12 clamshells as controls. Weigh each whole clamshell. Store the fruit at 20 °C for 14 days.
- Take samples on days 3, 7, 10, and 14. Sample three clamshells, representing 3 replicates, per treatment per day.
3. Monitoring of ClO2 Concentration in the Clamshells
- Insert the inlet and outlet tubing of the ClO2 gas detector into the center of the clamshells, with a 2 cm distance between the two ends, and take the ClO2 measurement on days 3, 7, 10, and 14.
4. Determination of Microbial Population and Fruit Quality Attributes
- Agitate 5 fruits (about 60 g) from each replicate at 100 rpm for 1 h in a sterilized sampling bag along with 99 mL of sterile potassium phosphate buffer (0.01 M, pH 7.2) on an orbital shaker.
- Plate serial dilutions (1-, 10-, and 100-fold) of the buffer wash, 50 µL each, on ECA (for E. coli) and PDA (for A. alternata) using a spiral plater.
- Incubate the ECA plates at 35 °C for 24 h and the PDA plates at 25 °C for 3 days. Read the microbial colony count using an optical plate reader. Sanitize all equipment which contacted the contaminated fruit after use.
- Measure fruit firmness with a fruit firmness tester using the manufacturer's protocol. Calibrate the tester before each use. Measure 20 fruit for each replicate and express the results as the pressure force, Newton (N), required to compress the fruit by 1 mm (converted to N·m−1).
- Weigh the whole clamshell with the fruit at the beginning of and during storage and calculate the weight loss in comparison to the initial weight.
5. Statistical Analysis
- Replicate all experiments in triplicate. Analyze the data using analysis of variance (ANOVA). Determine the mean separation by Duncan's multiple range test; the significance is defined at p <0.05.
Representative Results
The release of ClO2 exhibited a linear pattern over the first few hours. The concentration increased about 2.38 ppm/h over the first 4 h. The release speed slowed after 24 h of incubation, and the ClO2 concentration reached 25.4 ppm. However, the concentration tended to be stable after 24 h of incubation (Figure 1).
The headspace ClO2 concentration in the clamshell with grape tomatoes was about 4 ppm between day 3 and day 10, it decreased after 10 days of storage, and it was about 2 ppm on day 14 (Figure 2). The initial populations of E. coli and A. alternata in the fruit after inoculation were 4.3 and 3.4 log CFU/g, respectively (Figure 3). Treatment with ClO2 pouches reduced the populations of E. coli and A. alternata by 3.08 and 2.85 log CFU/g, respectively, after 14 days of storage (Figure 3).
The effects of ClO2 treatment on fruit firmness and weight loss are presented in Figures 4 and 5. ClO2 prevented a loss of firmness and weight in the fruit, and these effects grew with extended storage time (Figures 4 and 5).
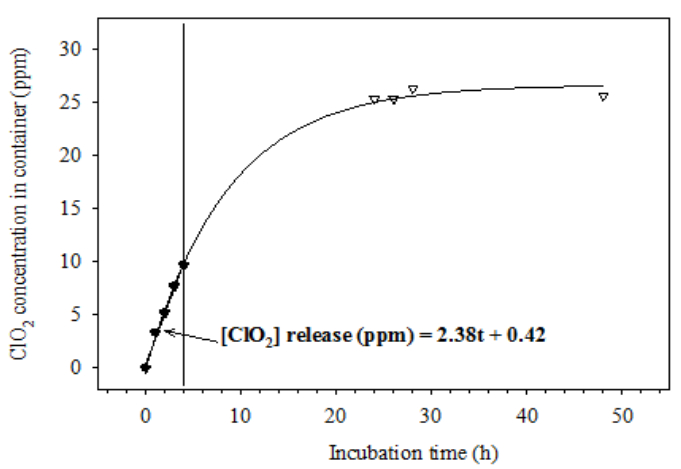
Figure 1: ClO2 release profile of a 0.5-g ClO2 pouch in a sealed, empty 19.14-L glass container at 20 °C and relative humidity 91%.
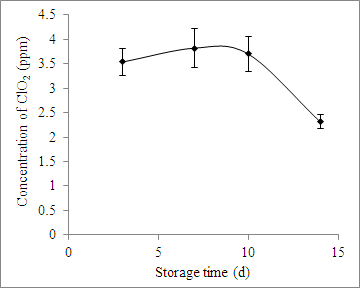
Figure 2: Concentration of ClO2 in 1 lb perforated clamshell packaging with 200 g of grape tomatoes at 20 °C. The values are the mean ± SD.
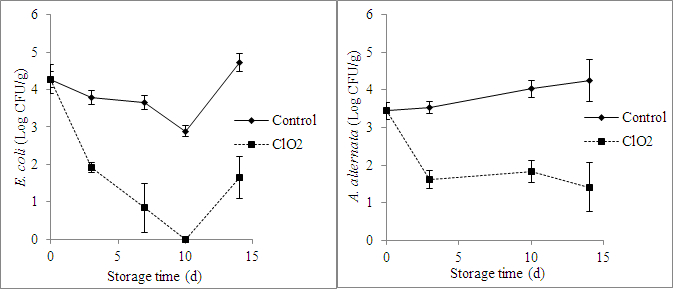
Figure 3: Effect of ClO2 treatment on E. coli and A. alternata populations on the surfaces of inoculated grape tomatoes stored for 14 days at 20 °C. The values are the mean ± SD.
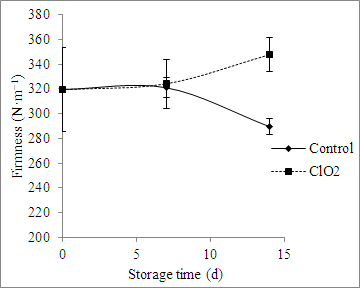
Figure 4: Effect of ClO2 treatment on the firmness of grape tomatoes stored for 14 days at 20 °C. The values are the mean ± SD.
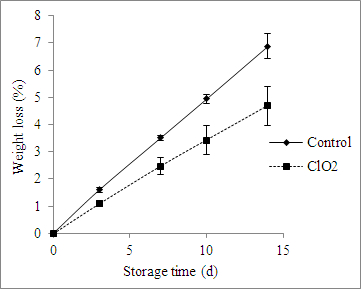
Figure 5: Effect of ClO2 treatment on the weight loss of grape tomatoes stored for 14 days at 20 °C. The values are the mean ± SD.
Discussion
Chlorine dioxide is an ideal biocide to prevent food decay. However, it is unstable at high concentrations and non-transportable, requiring costly generators or inefficient two-part powder mixing. This study examined the application of a stable, ready-to-use form of chlorine dioxide to reduce food spoilage and the incidence of foodborne illness. In contrast to other chlorine dioxide application technologies currently in use, the commercial ClO2 used here is cost effective, has a long shelf life, and does not require large generators or premixing. However, due to the strong oxidative properties of ClO2, the gas release properties of ClO2 are difficult to measure and therefore are rarely reported. In a previous study, a titration method was used to measure the release rate28. However, this method is less accurate and more complicated. Some research evaluated the concentration of ClO2 by absorbing it in water and then measured it using gas chromatography with mass spectrometric (GC-MS) detection29. However, this GC-MS instrument is complicated and expensive30. In our research, a ClO2 gas detector was used to measure the concentration of ClO2. This detector has multiple sensors that provide more accurate results in a shorter time.
In our protocol, for the preparation of the inoculum, the use of a deep-sided, 10 L steel pan as a basin for the application of inoculum, itself placed within an autoclavable bag, as well as sterile foil on which to dry the fruit, allows for quick clean-up and helps to avoid human exposure to possibly pathogenic organisms through incidental contact. Spraying the fruit within the confines of the autoclavable bag reduced the dispersion of microbial aerosols. Drying the fruit on foil allowed for complete removal and the subsequent sterilization of all surfaces with which the contaminated fruit had come into contact.
Chlorine dioxide exhibited strong antimicrobial activity against E. coli and A. alternata in grape tomatoes (Figure 3). ClO2 solution has been used to wash fruits and vegetables. Treatment with ClO2 gas at 4.1 mg/L (1,484 ppm) for 20 min at 23 °C significantly reduced the population of Salmonella, E. coli O157:H7, and L. monocytogenes on fresh-cut lettuce, cabbage, and carrots, without causing adverse effects on sensory properties31. Higher than 3-log reductions of E. coli O157:H7 were achieved after 4 mg/L (1,448 ppm) ClO2 gas treatments for 10 min at 21 °C and 90% RH on apple surfaces32. The effects of ClO2 treatment on fruit firmness and weight loss are presented in Figures 4 and 5. The firmness of ClO2-treated tomatoes increased compared to the control fruit (Figure 4). ClO2-treated fruit demonstrated inhibited enzyme activity, including in peroxidase and polyphenol oxidase, which was attributed to an important role in the softening process33, or inhibited respiration rates and ethylene production34,35. A linear relationship between softening and weight loss was demonstrated in blueberries36. It was suggested that ClO2 could reduce fruit metabolism in addition to preventing weight loss and retaining firmness37. It was concluded that the ClO2 pouch was a promising, non-thermal, pathogen-reduction technique for fresh fruits and vegetables. It maintained firmness and reduced the weight loss of grape tomatoes.
One limiting characteristic of this method of sanitation is that although this ClO2 technology may reduce the tomato surface inoculum of A. alternata, reducing the risk of new postharvest infections by this fungus, it will not be able to control established, latent infections of A. alternate38. Established infections are typically produced in the field before harvest and are the main cause of postharvest tomato black spots, which cause significant economic losses to the industry. Another limiting characteristic is the rapid reaction of ClO2, which prevents the product from effectively combating microorganisms that are deeply embedded within a water-rich environment or dense organic material39. Typically, the sanitizing potential of the product at low concentrations quickly loses effectiveness before being able to sufficiently penetrate to the interior of large fruits. The solution to this problem, a higher concentration of the product, carries with it problems of its own, including phytopathic effects and plant tissue bleaching. Therefore, for each unique application of commodity versus pathogen, it is necessary to find a sanitizer concentration that balances anti-microbial effectiveness with acceptable commodity damage.
In summary, ClO2 can be used as a sanitizer to control foodborne pathogens, yeasts, and molds on fruit. The findings in this study suggest that ClO2 at low concentrations for longer time durations in active packaging is useful for improving the microbial safety and reducing decay during storage without impairing the physical properties of the fruit. Future applications of this protocol include testing the effectiveness of slow-release ClO2 pouches as an addition to existing commercial packaging against the foodborne pathogens and spoilage organisms of any number of fresh food products, including fruits, vegetables, meats, and breads.
Divulgaciones
The authors have nothing to disclose.
Acknowledgements
We would like to thank the financial support provided by Worrell Water Technologies, LLC. Mention of a trademark or proprietary product is for identification only and does not imply a guarantee or warranty of the product by the U.S. Department of Agriculture.
Materials
| Curoxin chlorine dioxide pouch | Worrell Water Technologies | Slurry, a.i. 9.5% in sealed permeable polymer film | |
| Grape tomato | Santa Sweets, Inc | Santa Sweets Authentic | |
| ClO2 gas detector | Analytical Technology, Inc., Collegeville, PA | PortaSens II | |
| Perforated clamshell | Packaging Plus LLC, Yakima, WA | OSU #1, 1 lb | |
| Escherichia coli | Wild Type (WT) from fruit surface | ||
| Alternaria alternata | from fruit surface | ||
| E. coli agar | EC Broth, Oxoid, UK | EC Broth with 1.5% agar | |
| Potato dextrose agar | BD Difco, Sparks, MD | ||
| Levine eosin methylene blue agar | BD Difco, Sparks, MD | ||
| Trigger spray bottle | Impact Products, LLC., Toledo, OH | ||
| Sterilized sampling bag | Fisherbrand, Fisher Scientific, Pittsburgh, PA | ||
| Orbit shaker | New Brunswick Scientific, New Brunswick, NJ | Innova 2100 | |
| IUL Instruments Neutec Eddy jet spiral plater inoculation plating system | Neutec Group Inc., Farmingdale, NY | ||
| EZ micro optical plate reader | Synoptics, Ltd., Cambridge, UK | ProtoCOL | |
| Fruit firmness tester | Bioworks Inc, Wamego, KS | FirmTech 2 | |
| Tinytag temperature and RH data logger | Gemini Data Loggers, West Sussex, UK | ||
| McFarland equivalence turbidity standard | Fisherbrand, Fisher Scientific, Pittsburgh, PA |
Referencias
- Van Duyn, M. S., Pivonka, E. Overview of the health benefits of fruit and vegetable consumption for the dietetics professional: Selected literature. J Am Diet Assoc. 100 (12), 1511-1521 (2000).
- Beuchat, L. R. Ecological factors influencing survival and growth of human pathogens on raw fruits and vegetables. Microbes Infect. 4 (4), 413-423 (2002).
- Mahmoud, B. S. M., Bhagat, A. R., Linton, R. H. Inactivation kinetics of inoculated Escherichia coli O157 : H7, Listeria monocytogenes and Salmonella enterica on strawberries by chlorine dioxide gas. Food Microbiol. 24 (7-8), 736-744 (2007).
- Bean, N. H., Griffin, P. M. Foodborne disease outbreaks in the United-States, 1973-1987 – pathogens, vehicles, and trends. J Food Protect. 53 (9), 804-817 (1990).
- Calder, L., et al. An outbreak of hepatitis A associated with consumption of raw blueberries. Epidemiol Infect. 131 (1), 745-751 (2003).
- Chen, Z., Zhu, C. H. Combined effects of aqueous chlorine dioxide and ultrasonic treatments on postharvest storage quality of plum fruit (Prunus salicina L.). Postharvest Biol Technol. 61 (2-3), 117-123 (2011).
- Mmbaga, M. T., Shi, A. N., Kim, M. S. Identification of Alternaria alternata as a causal agent for leaf blight in syringa species. Plant Pathology J. 27 (2), 120-127 (2011).
- Fagundes, C., Palou, L., Monteiro, A. R., Perez-Gago, M. B. Hydroxypropyl methylcellulose-beeswax edible coatings formulated with antifungal food additives to reduce alternaria black spot and maintain postharvest quality of cold-stored cherry tomatoes. Sci Hortic-Amsterdam. 193, 249-257 (2015).
- Akhtar, K. P., Saleem, M. Y., Asghar, M., Haq, M. A. New report of Alternaria alternata causing leaf blight of tomato in Pakistan. Plant Pathol. 53 (6), 816 (2004).
- Spalding, D. H. Effect of ozone on appearance and decay of strawberries peaches and lettuce. Phytopathology. 56, 586 (1966).
- Bialka, K. L., Demirci, A. Decontamination of Escherichia coli O157 : H7 and Salmonella enterica on blueberries using ozone and pulsed UV-Light. J Food Sci. 72 (9), M391-M396 (2007).
- Kim, J. G., Yousef, A. E., Dave, S. Application of ozone for enhancing the microbiological safety and quality of foods: A review. J Food Protect. 62 (9), 1071-1087 (1999).
- Crowe, K. M., Bushway, A., Davis-Dentici, K. Impact of postharvest treatments, chlorine and ozone, coupled with low-temperature frozen storage on the antimicrobial quality of lowbush blueberries (Vaccinium angustifolium). LWT-Food Sci Technol. 47 (1), 213-215 (2012).
- Richardson, S. D., Plewa, M. J., Wagner, E. D., Schoeny, R., DeMarini, D. M. Occurrence, genotoxicity, and carcinogenicity of regulated and emerging disinfection by-products in drinking water: A review and roadmap for research. Mutat Res-Rev Mutat. 636 (1-3), 178-242 (2007).
- Soliva-Fortuny, R. C., Martin-Belloso, O. New advances in extending the shelf-life of fresh-cut fruits: a review. Trends Food Sci Tech. 14 (9), 341-353 (2003).
- Zhu, C. H., Chen, Z., Yu, G. Y. Fungicidal mechanism of chlorine dioxide on Saccharomyces cerevisiae. Ann Microbiol. 63 (2), 495-502 (2013).
- Han, Y., Sherman, D. M., Linton, R. H., Nielsen, S. S., Nelson, P. E. The effects of washing and chlorine dioxide gas on survival and attachment of Escherichia coli O157 : H7 to green pepper surfaces. Food Microbiol. 17 (5), 521-533 (2000).
- Chen, Z., Zhu, C. H., Han, Z. Q. Effects of aqueous chlorine dioxide treatment on nutritional components and shelf-life of mulberry fruit (Morus alba L). J Biosci Bioeng. 111 (6), 675-681 (2011).
- Gordon, G., Rosenblatt, A. A. Chlorine dioxide: The current state of the art. Ozone-Sci Eng. 27 (3), 203-207 (2005).
- Park, S. H., Kang, D. H. Antimicrobial effect of chlorine dioxide gas against foodborne pathogens under differing conditions of relative humidity. LWT-Food Sci Technol. 60 (1), 186-191 (2015).
- Wu, V. C. H., Kim, B. Effect of a simple chlorine dioxide method for controlling five foodborne pathogens, yeasts and molds on blueberries. Food Microbiol. 24 (7-8), 794-800 (2007).
- Mahmoud, B. S., Bhagat, A. R., Linton, R. H. Inactivation kinetics of inoculated Escherichia coli O157:H7, Listeria monocytogenes and Salmonella enterica on strawberries by chlorine dioxide gas. Food Microbiol. 24 (7-8), 736-744 (2007).
- Popa, I., Hanson, E. J., Todd, E. C., Schilder, A. C., Ryser, E. T. Efficacy of chlorine dioxide gas sachets for enhancing the microbiological quality and safety of blueberries. J Food Protect. 70 (9), 2084-2088 (2007).
- Jin, Y. Y., Kim, Y. J., Chung, K. S., Won, M., Bin Song, ., K, Effect of aqueous chlorine dioxide treatment on the microbial growth and qualities of strawberries during storage. Food Sci Biotechnol. 16 (6), 1018-1022 (2007).
- Sun, X. X., et al. Antimicrobial activity of controlled-release chlorine dioxide gas on fresh blueberries. J Food Protect. 77 (7), 1127-1132 (2014).
- Wang, Z., et al. Improving storability of fresh strawberries with controlled release chlorine dioxide in perforated clamshell packaging. Food Bioprocess Technol. 7 (12), 3516-3524 (2014).
- Narciso, J. A., Ference, C. M., Ritenour, M. A., Widmer, W. W. Effect of copper hydroxide sprays for citrus canker control on wild-type Escherichia coli. Lett Appl Microbiol. 54 (2), 108-111 (2012).
- Lee, S. Y., Costello, M., Kang, D. H. Efficacy of chlorine dioxide gas as a sanitizer of lettuce leaves. J Food Protect. 67 (7), 1371-1376 (2004).
- Shinb, H. S., Jung, D. G. Determination of chlorine dioxide in water by gas chromatography-mass spectrometry. J Chromatogr A. 1123, 92-97 (2006).
- Tzanavaras, P. D., Themelis, D. G., Kika, F. S. Review of analytical methods for the determination of chlorine dioxide. Cent Eur J Chem. 5 (1), 1-12 (2007).
- Sy, K. V., Murray, M. B., Harrison, M. D., Beuchat, L. R. Evaluation of gaseous chlorine dioxide as a sanitizer for killing Salmonella, Escherichia coli O157 : H7, Listeria monocytogenes, and Yeasts and molds on fresh and fresh-cut produce. J Food Protect. 68 (6), 1176-1187 (2005).
- Du, J., Han, Y., Linton, R. H. Efficacy of chlorine dioxide gas in reducing Escherichia coli O157 : H7 on apple surfaces. Food Microbiol. 20 (5), 583-591 (2003).
- Wang, Y. Z., Wu, J., Ma, D. W., Ding, J. D. Preparation of a cross-linked gelatin/bacteriorhodopsin film and its photochromic properties. Sci China Chem. 54 (2), 405-409 (2011).
- Guo, Q., et al. Chlorine dioxide treatment decreases respiration and ethylene synthesis in fresh-cut ‘Hami’ melon fruit. Int J Food Sci Tech. 48 (9), 1775-1782 (2013).
- Aday, M. S., Caner, C. The applications of ‘active packaging and chlorine dioxide’ for extended shelf life of fresh strawberries. Packag Technol Sci. 24 (3), 123-136 (2011).
- Paniagua, A. C., East, A. R., Hindmarsh, J. P., Heyes, J. A. Moisture loss is the major cause of firmness change during postharvest storage of blueberry. Postharvest Biol Technol. 79, 13-19 (2013).
- Gomez-Lopez, V. M., Ragaert, P., Jeyachchandran, V., Debevere, J., Devlieghere, F. Shelf-life of minimally processed lettuce and cabbage treated with gaseous chlorine dioxide and cysteine. Int J Food Microbiol. 121 (1), 74-83 (2008).
- Mahovic, M. J., Tenney, J. D., Bartz, J. A. Applications of chlorine dioxide gas for control of bacterial soft rot in tomatoes. Plant Dis. 91 (10), 1316-1320 (2007).
- Tan, H. K., Wheeler, W. B., Wei, C. I. Reaction of chlorine dioxide with amino-acids and peptides – kinetics and mutagenicity studies. Mutat Res. 188 (4), 259-266 (1987).

