The Calibration and Use of Capacitance Sensors to Monitor Stem Water Content in Trees
Summary
The hydraulic capacitance of biomass is a key component of the vegetation water budget, which serves as a buffer against short and long-term drought stresses. Here, we present a protocol for the calibration and use of soil moisture capacitance sensors to monitor water content in the stems of large trees.
Abstract
Water transport and storage through the soil-plant-atmosphere continuum is critical to the terrestrial water cycle, and has become a major research focus area. Biomass capacitance plays an integral role in the avoidance of hydraulic impairment to transpiration. However, high temporal resolution measurements of dynamic changes in the hydraulic capacitance of large trees are rare. Here, we present procedures for the calibration and use of capacitance sensors, typically used to monitor soil water content, to measure the volumetric water content in trees in the field. Frequency domain reflectometry-style observations are sensitive to the density of the media being studied. Therefore, it is necessary to perform species-specific calibrations to convert from the sensor-reported values of dielectric permittivity to volumetric water content. Calibration is performed on a harvested branch or stem cut into segments that are dried or re-hydrated to produce a full range of water contents used to generate a best-fit regression with sensor observations. Sensors are inserted into calibration segments or installed in trees after pre-drilling holes to a tolerance fit using a fabricated template to ensure proper drill alignment. Special care is taken to ensure that sensor tines make good contact with the surrounding media, while allowing them to be inserted without excessive force. Volumetric water content dynamics observed via the presented methodology align with sap flow measurements recorded using thermal dissipation techniques and environmental forcing data. Biomass water content data can be used to observe the onset of water stress, drought response and recovery, and has the potential to be applied to the calibration and evaluation of new plant-level hydrodynamics models, as well as to the partitioning of remotely sensed moisture products into above- and belowground components.
Introduction
Water stored in plant material plays an integral role in plants' ability to cope with short- and long-term water stress1,2. Plants store water in roots, stems, and leaves in both intracellular and extracellular (e.g., xylem vessels) spaces 2,3,4. This water has been shown to contribute between 10 and 50% of diurnally transpired water2,5,6,7,8. As such, plant hydraulic capacitance is a key component of the terrestrial water balance, can be used as an indicator of water stress, drought response, and recovery1, and is a critical factor necessary to correct for observed time lags between transpiration and sap flow9,10,11. Real-time monitoring of vegetation water content can also be used in agricultural applications to help constrain orchard and crop irrigation in order to increase watering efficiency12,13. However, measurements of continuous, in-situ stem-water content of woody species7,14,15,16,17,18,19 are rare relative to sap flux measurements20. Here, we outline a procedure for the calibration of capacitance sensors to monitor the volumetric water content within the stems of trees5,21.
Hydrodynamic behaviors and water-use regulation by vegetation are an integral component of the soil-plant-atmosphere continuum22,23 and are therefore important controls for the water and carbon fluxes between the biosphere and atmosphere24,25. The dynamics of stem water content are influenced by both biotic and abiotic factors. Depletion and recharge of stem-stored water are affected by short- and long-term trends in environmental conditions, in particular, vapor pressure deficit and soil water content1,26. The physical properties of the wood27 (e.g., density, vessel structure) and the emergent hydraulic strategy25 (e.g., iso- or anisohydric stomatal regulation) determine a plant's ability to store and use water19,26,28, and can vary widely by species29,30. Previous studies have demonstrated different roles of capacitance in tropical16,27,31,32,33 and temperate5,7,21 species, and in both angiosperms1,2,34 and gymnosperms6,11,17,19.
Improved knowledge of biomass water content will improve understanding of vegetation strategies for water acquisition and use1,2, along with species' vulnerability to predicted changes in precipitation regimes35,36. Further understanding of plant water use strategies will help predict shifting demographic patterns under future climate scenarios37,38. Through model-data fusion techniques39, stem water content data obtained using this methodology can be used to inform and test scalable, plant-level hydrodynamics models40,41,42,43,44 in order to improve calculations of stomatal conductance and, thereby, simulations of both transpiration and photosynthetic carbon uptake. These advanced hydrodynamic models may provide a significant reduction in uncertainty and error when incorporated into larger land-surface and Earth systems models25,45,46,47,48.
Methods used to monitor or calculate stem water content include tree coring33,49, electronic dendrometers2,15,50, electrical resistance51, gamma radiation attenuation52, deuterium tracers19, networks of sap flux sensors32,33,53, stem psychrometers49, and amplitude11 and time4,12,13 domain reflectometry (TDR). Recent efforts have tested the viability of capacitance sensors that have traditionally been used to measure soil volumetric water content5,18,21,27. Frequency domain reflectometry (FRD)-style capacitance sensors are low cost and use relatively small amounts of energy for continuous measurements, making them an attractive tool for high temporal resolution measurements in field scenarios. The ease of automation of FDR over TDR-style sensors facilitates the collection of continuous sun-hourly data sets, and eliminates many of the challenges inherent in TDR measurements requiring substantial cable lengths13. The use of in-situ capacitance sensors eliminates the need for repetitive coring or branch harvesting, and may provide enhanced accuracy for hardwood species. Woody species that withdraw water principally from extracellular spaces, such as xylem vessels, or have high wood or bark moduli of elasticity, are generally not good candidates for popular dendrometer measurement techniques due to low elastic stem expansion2. Capacitance sensors estimate dielectric permittivity, which can be directly converted to volumetric water content. However, capacitance measurements are sensitive to the density of the media surrounding the sensor. Therefore, we advocate for species-specific calibrations that convert the output of the sensors to volumetric wood-water content5,21.
We present a protocol for a species-specific calibration to convert capacitance sensor output to volumetric water content of wood. Also provided are instructions for field installation of capacitance sensors in mature trees and a discussion of the method's strengths, weaknesses, and assumptions. These techniques are designed to monitor volumetric water content in the trunk, the largest tree water storage reservoir8, but can be easily expanded to the whole tree with installation of additional sensors along the branches. Measurements of dynamic plant water content will advance the fields of vegetation hydrodynamics, biometeorology, and land-surface modeling.
Protocol
1. Select a Tree for Instrumentation
- Select trees for measurement. Ideally, select trees that are healthy with a generally round stem cross-section, and a diameter between 1-2 times the tine length, or a sapwood depth greater than the length of the sensor tines (~5 cm for the specific capacitance sensors demonstrated here). Measure the depth of the sapwood using tree cores, or for many species, calculate sapwood depth through allometric equations relating sapwood area to stem diameter 29,54, as measured by a standard diameter tape.
NOTE: However, some types of capacitance sensors may be able to be trimmed to an appropriate length following Step 1.2 without adversely affecting measurement accuracy. Only capacitance sensors with rigid measurement tines that do not contain wiring can be trimmed or cut. Separate calibration is necessary for trimmed sensors. Therefore, select the tree and determine the appropriate tine length prior to the calibration procedure. - Determine the appropriate length of the sensor tines based on the tree's diameter and sapwood depth.
NOTE: Capacitance sensors integrate moisture information over the length of the sensor tines, L. It is therefore assumed that the integrated observation they provide is representative of the entire stem when the diameter is between L and 2L. The bark and phloem will not affect the measurement as they are removed from the measurement area prior to installation (see section 4.2).- Trim the sensors for trees where L is greater than the stem diameter, (see Step 1.3) such that the tines do not penetrate through the opposite side of the stem.
- Measure the combined signal from the sapwood and heartwood with the sensor for trees where the diameter is larger than 2L, but sapwood depth is less than L.
NOTE: As water content differs in these two tissue types, this may cause a bias if it is assumed that the observation is representative of the whole stem. In such cases, or when the user is only interested in the sapwood water fluctuations, the sensor tines must be trimmed to the sapwood depth such that the observations will represent the water content fluctuations of the sapwood (active xylem) only. When the sapwood depth is larger than L, observations represent only the sapwood, but the sensor does not need to be trimmed.
- If necessary (as determined in Steps 1.2.1 and 1.2.2), cut the sensor to fit for the specified application. To cut the sensor tines, clamp the sensor securely to a workbench and, while wearing proper protective equipment, use a power rotary tool equipped with a steel cutting disk to cut each tine to precisely the same length.
2. Harvest and Prepare Wood Samples of All Species of Interest to Generate a Species-Specific Calibration
- Collect a trunk, juvenile stem, or large branch at least 6 cm in diameter from the species of interest. Larger diameters are preferred in order to maximize the amount of wood encasing the sensor and most closely approximate the trunk density for field measurements. Remove all attached branches or leaves, and any lichen or loose material.
NOTE: Refer to the Discussion section for further discussion of uncertainty due to differences in branch and stem density. - Segment the stem into 25 or more cylindrical sections of ~15 cm length.
- Label each segment and record the average diameter and length of each. Approximate the segments' volume as the volume of a cylinder.
- Separate the segments into two groups for differential rehydration and drying. Place roughly 1/3 of the segments in a water bath to rehydrate, and the other 2/3 of the segments in a drying oven at 60 °C to dehydrate. Keep two segments separate for immediate measurement: preferably one from the middle of the length of the stem, and one from an end.
NOTE: Typically, segments drying at 60 °C will be fully desiccated after ~2 weeks, and fully rehydrated after ~3 days. Remove individual segments from the oven/water bath and measure daily or twice-daily intervals (see Step 3.7). In order to produce a gradient of measurements spanning the largest range of possible volumetric water contents.
3. Create a Calibration Relationship between Sensor Output and Volumetric Water Content
- Connect a capacitance sensor to a data logging device, following the instructions provided by the manufacturer, and to a computer for real-time sensor readout visualization. Set the time interval for data collection to 30 s.
- Using a premade drilling template held securely in place to maintain alignment, and a drill bit slightly smaller than the diameter of the tines of the capacitance sensor (3.57 mm for the sensor used in this experiment), drill two vertical sets of three holes with the second set located approximately 150 ° away around the wood segment with a vertical separation of a few centimeters to ensure that there is no potential overlap between holes. Use the wood segments set aside in Step 2.4 for the first set of measurements.
- Weigh the segment and record the weight to the nearest 0.01 g at the time of measurement. Cap the ends of the segments with plastic wrap to prevent additional drying.
- Immediately after weighing, clean the tines of the capacitance sensor with an alcohol swab and insert it into the stem segment completely, such that no part of the steel tines is visible. Wait for the measurement reading on the output screen to stabilize (2 or 3 min, generally). Record the sensor outputs of temperature, electrical conductivity, and dielectric permittivity (εb) every 30 s for 5 min and compute the average of the 10 measurements.
- Gently remove the sensor from the segment, clean the tines with an alcohol swab, and wait for the output readings to return to zero. Repeat the measurement procedure from step 3.3 onward in the second set of pre-drilled holes.
- Remove plastic from the ends and place the stem segment in the drying oven. Allow it to desiccate completely (generally 2 weeks, or until weight has stabilized for several days).
- Repeat steps 3.3 through 3.7 for all segments. Remove segments from the drying oven and measure more frequently (twice daily) within the first several days of drying, due to the higher rate of initial moisture loss than during the last several days (daily). Dry excess water from the surface of segments removed from the water bath using a paper towel before weighing and measurement. Measure one rehydrated stem segment per day, until all rehydrated segments have been measured.
- After complete desiccation, record the final dry weight of all stem segments.
- Calculate the volumetric water content (VWC, cm3water /cm3wood) of each stem segment at the time of measurement, using the following formula:
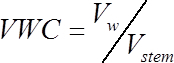 (1)
(1)
Where Vw is the volume of water (cm3), and Vstem is the volume of the stem segment (cm3) calculated in step 1.2.- Calculate the volume of water in each segment at the time of measurement as:
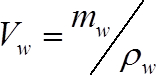 (2)
(2)
Where mw is the mass water in the stem segment (g) at the time of measurement, and ρw is the density of water (1 g cm-3) - Calculate the mass of water in each segment at the time of measurement as:
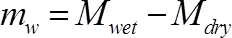 (3)
(3)
Where Mwet is the mass of the stem segment at the time of measurement (g), and Mdry is the final weight of the segment (g).
- Calculate the volume of water in each segment at the time of measurement as:
- Using a statistical analysis software package, create a best-fit linear regression between sensor-observed dielectric permittivity and VWC (Figure 1, Table 1).
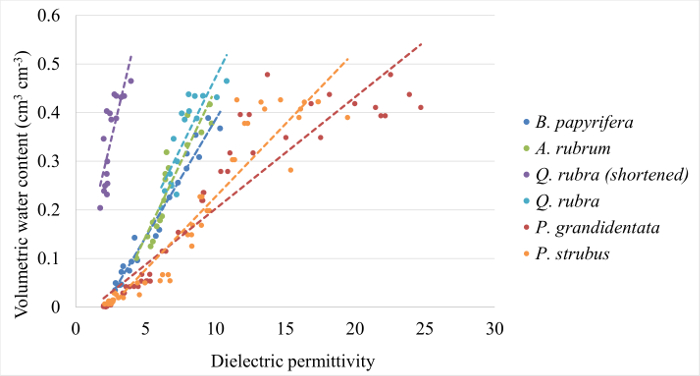
Figure 1: Example calibration curves. Calibration curves generated for Quercus rubra, Acer rubrum, Betula papyrifera, Populus grandidentata, and Pinus strobus following Parts 1 and 2 of this protocol. Equations and coefficients of determination are provided for each in Table 1. Please click here to view a larger version of this figure.
| m | b | R2 | |
| B. paprifera | 0.048 | -0.098 | 0.967 |
| A. rubrum | 0.067 | -0.158 | 0.853 |
| Q. rubra (shortened) | 0.120 | 0.041 | 0.636 |
| Q. rubra | 0.058 | -0.109 | 0.718 |
| P. grandidentata | 0.023 | -0.028 | 0.887 |
| P. strobus | 0.030 | -0.072 | 0.900 |
Table 1: Calibration equations for the conversion of εb a VWC for five temperate tree species. Coefficients 'm' and 'b' are presented for a linear equation in the standard form: VWC = m*εb+b.
4. Installing Capacitance Sensors in Trees for Field Measurements
- Prior to sensor installation, record the stem diameter and the height above the ground surface for each sensor location. Typically, to monitor VWC in the trunk, place one sensor ~0.5 m above the ground surface, and place a second one just below the first major branching split (~7.5 m above the ground, Figure 2).
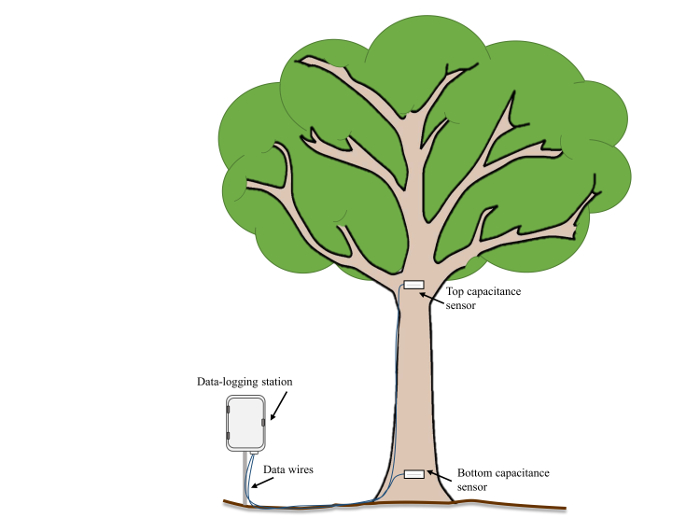
Figure 2: Example field experiment setup. A schematic diagram of sensor locations and orientation in a mature tree adjacent to a data logging station. Please click here to view a larger version of this figure.
- Remove the bark to the cambium and create a flat surface where the sensor will be installed using a draw blade. Ensure that this surface is wide and flat enough that the sensor sits flush against the surface of the tree when it is installed such that no part of the tines is exposed. Remove the bark and cambium to ensure that measurements include only water content in the xylem, while excluding water content in the bark or phloem.
- Drill the holes for the tines. Use a drill bit slightly smaller than the diameter of the tines for softer wood, while using a bit closer to the true size of the tines for harder wood (3.57 mm as used in this protocol). For high quality measurements, ensure that the sensor tines make good contact with the wood. Since there will not be a need to remove the sensor regularly, as was the case during the calibration procedure, a slightly smaller drill bit may be used here than the one used for calibration.
- Clean the sensor tines with an alcohol swab to remove any dirt or skin oils, and insert the sensor into the pre-drilled holes. If the sensor meets too much resistance upon insertion, gently back it out and re-drill the holes to widen them slightly. Ensure that all three tines are fully inserted and the body of the sensor sits flush against the tree trunk.
- Use a silicon-based sealant to seal the sensor against the tree trunk to help keep stem flow from entering the holes and to prevent pest infestation.
- Cover the sensor with reflective insulation to avoid external heating.
- Following the manufacturers' specifications, connect all sensors to a 12 V power source and a compatible data logger. Use data collection intervals of 5 min for sensors deployed in the field, but use longer intervals to conserve power in sites where power is limiting, e.g., solar/battery operated sites.
5. Process the Raw Data to Stem-Water Storage Using the Calibration Curve
- Using the equation of the calibration curve generated in step 2.9, convert the sensor output to VWC for all observations. VWC is generally expressed as cm3 /cm3 or as a percent. The percent VWC will therefore range between 0 and 100%.
- Integrate the total volume of interest (cm3) between the two measurement heights (e.g., de Figure 2: 0.5 m and 7.5 m) by assuming stem diameter changes linearly with height. For trees where the observation represents the whole stem (i.e., trees of diameters between L and 2L, using unmodified sensors, or thinner trees where the sensor was trimmed to the stem diameter, see section 2.2), use equation 4:
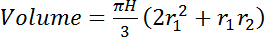 (4)
(4)
where H is the height difference (vertical distance) between the sensors (e.g., in Figure 2: 700 cm) and r1 and r2 are the radii of the stem (cm) at the bottom and top sensors' locations, respectively.
For trees where sensors provide an estimate only of the sapwood water content (see sensor length determination, section 1.2) use eq. 5:
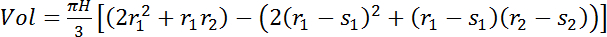 (5)
(5)
where s1 and s2 are the sapwood depths (cm) at the bottom and top sensors' locations, respectively. - Calculate the volume of stem- or sapwood- stored water by multiplying the tree's average VWC by the total volume of interest.
Representative Results
In this section, we present calibration data for five common eastern forest tree species, followed by a detailed analysis of field measurements of stem-water storage in three Acer rubrum individuals during the 2016 growing season. Calibration curves were generated for Acer rubrum, Betula papyrifera, Pinus strobus, Populus grandidentata, and Quercus rubra (Figure 1). Slopes of the curves differed by as much as 97.7% for P. grandidentata and A. rubrum (Table 1) demonstrating the need to perform species-specific calibration to obtain accurate VWC measurements. Volumetric water content was monitored at the base of the tree stem 0.5 m above the ground surface, and at the base of the live crown, 7.5 m above the ground surface (Figure 2). Diurnal depletion and replenishment of VWC were observed in both locations, with VWC at the top measurement point showing larger diurnal variability than the lower location (Figure 3). VWC at the top measurement location ranged from 0.55 to 0.88 cm3/cm3, while VWC at the lower measurement location ranged from 0.72 to 0.95 cm3/cm3. During transpiration, water is lost from leaves and depleted most quickly from distal branches. Therefore, it follows that VWC measured at the base of the live crown should be lower than at the base of the trunk when storage is being actively withdrawn. Measurements at both locations showed the same general trends during the growing season (Figure 3).
To evaluate the observed diurnal dynamics of changing stem water content, we calculated the time rate of change in stem-average total water content, ΔStorage (g/s), (Figure 4). ΔStorage was compared to sap flux data collected simultaneously in the same tree using Granier-style thermal dissipation sensors55. Negative values in ΔStorage indicate the rate of reduction in trunk water storage. Changes in storage began to occur shortly after dawn (6:00 am), while sap flux lagged behind between an hour and a half and two hours (~8:00 am). In general, lag-times between sap flux and transpiration are estimated at slightly more than an hour9. The slowing depletion of storage between noon and 4:00 pm can be attributed to mid-day stomatal closure, which is also responsible for the asymmetric shape of the sap flux curve throughout the afternoon56,57. The congruence in results obtained using well-established sap flux techniques and our storage measurement procedure demonstrate the ability of capacitance sensors to capture fast dynamics of changes in stem water storage in mature trees.
Three different mature Acer rubrum (diameters at breast height, DBH: 29.1 cm, 28.3 cm, 22.7 cm) in a forest research site in northern Michigan were monitored during the 2016 growing season. While total stem water storage is dependent on the volume of the trunk (Figure 5A), all three individuals exhibited patterns of recharge and replenishment consistent with seasonal trends in sap flux (Figure 5B) and available soil moisture within the top 1 m depth (Figure 5C). Stem-stored water, sap flux, and soil moisture were all at their lowest during the end-of-summer extended interstorm period, day-of-year (DOY) 200-225. At this time, sap flux and stem water content were reduced significantly in all monitored individuals. We estimated the drought recovery time in response to the observed decline in soil water content from 10% to 5.6% to be approximately 10 days for A. rubrum, based on the time required for stem-water storage to rebound to pre-drought levels.
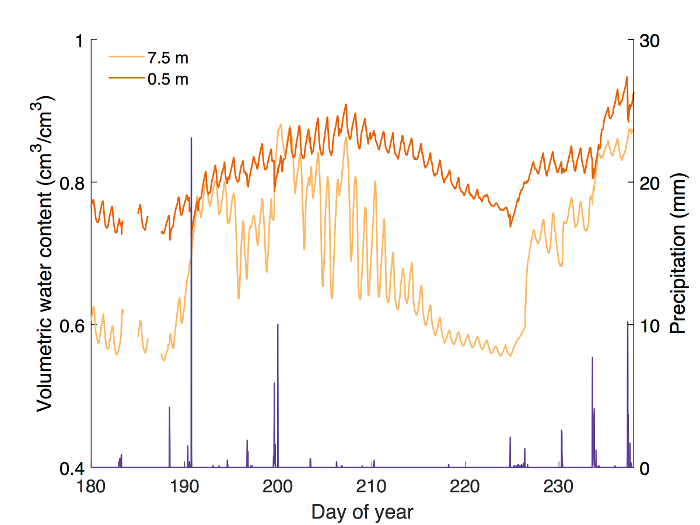
Figure 3: Example results from a field experiment monitoring stem water content at 0.5 and 7.5 m heights in Acer rubrum (DBH = 29.1 cm). Water content at 7.5 m above the ground generally remained lower than water content at 0.5 m. Diurnal fluctuations in water content at 7.5m were larger than those recorded closer to ground level. Increases in stem water content at both heights corresponded to days following precipitation (shown in purple), with a large decrease in stem water content between DOY 210 and 225 of 2016 when little to no precipitation occurred. Missing data are the result of temporary power interruption. Please click here to view a larger version of this figure.
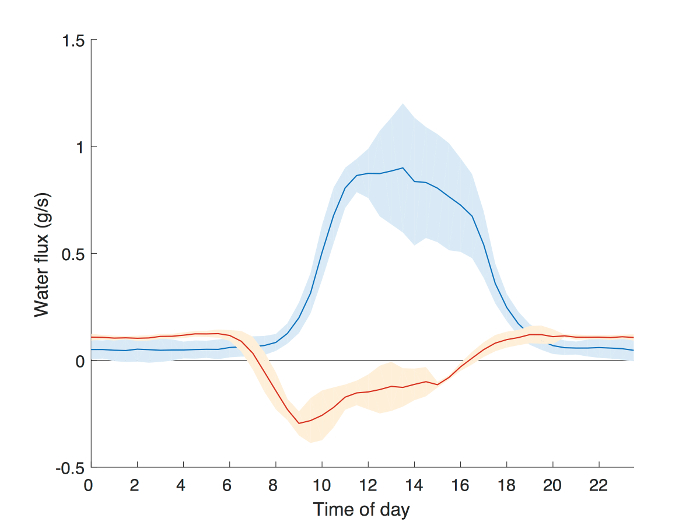
Figure 4: Example results from a field experiment comparing the time lag in water flux as measured by sap flow and the rate of change of stem water content. Sap flow was monitored simultaneously with stem water content in an Acer rubrum following the methodology outlined in Matheny, et al.29 The average diurnal sap flow rate (blue) and the time derivative of stem water storage (ΔStorage, orange) as calculated following Matheny, et al.5 are shown for 5 days when soil moisture was non-limiting (DOY 245-250, 2016). Shaded areas represent the standard deviation across the 5-day window. ΔStorage begins to decline shortly after dawn (6:00), while sap flux begins to increase roughly 2 h later (8:00). Please click here to view a larger version of this figure.
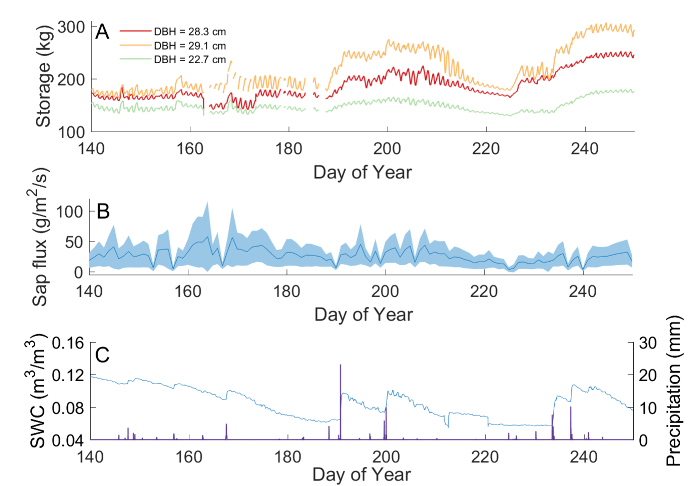
Figure 5: Example results from a field experiment monitoring stem water storage in three Acer rubrum over the course of 110 days. Stem water storage was monitored in the trunks of three Acer rubrum of different trunk sizes throughout the course of the 2016 growing season (A). Seasonal trends in stem water storage corresponded to trends seen in sap flux monitored in 15 Acer rubrum in the same forest following the procedures of Matheny, et al.29 (B). Shaded area corresponds to the standard deviation between individual trees, while the solid line represents the mean daily integrated sap flux. Both stem water storage and sap flux from Acer rubrum followed the patterns of soil water content (SWC), as integrated over the top 1 m of soil depth, and precipitation (C). Missing data are the result of temporary power interruption. Please click here to view a larger version of this figure.
Discussion
Seasonal and diurnal patterns in stem water content observed via capacitance sensors align with trends in concurrent sap flux and environmental forcing measurements (Figure 3, Figure 4, Figure 5). Reservoirs of stem water storage are depleted diurnally when the pace of transpiration surpasses the rate of recharge through woody tissues, and seasonally when soil moisture limits root-water availability5. This internal capacitance provides a valuable buffer against hydraulic limitations to stomatal conductance and is responsible for the time lag between the onset of transpiration and sap flux9. In this study, we observed a maximum daily water withdrawal from storage ranging between 23.3 and 49.9 L (14.6 and 22.3%) of the total available stem water content. The relatively fast increase in stem water content after rainfall events observed in this study was consistent across all monitored maples and has been shown previously5. This type of behavior may suggest cavitation refill or rapid increases in water content in other surrounding tissues21. There is a need for further research to connect these measurements with observations of cavitation refilling21,58. The use of capacitance sensors facilitates monitoring this critical aspect of the vegetation water balance continuously, and in situ in field environments. This methodology was developed using the GS-3 ruggedized soil moisture sensor. These sensors feature three rigid stainless-steel tines (3.26 mm diameter, 5.5 cm length). While the method can be adapted for use with other capacitance sensors, it is dependent on the presence of rigid measurement tines.
Species-specific calibration permits the conversion of measured dielectric permittivity to volumetric water content for tree species with differing wood densities (Figure 1). However, there are several inherent assumptions in the presented calibration procedure. Most notable is the assumption that the wood density of the branch or stem segments used for calibration is roughly equivalent to the density of the wood at the installation location in the field. While branch and stem densities have been shown to be in good agreement for some species59, this is not always the case60, due to differences in xylem vessel taper and arrangement. Another important consideration during calibration is the amount of wood surrounding the sensor tines. Some FDR-style sensors require a substantial depth of media embedment (≥5 cm) on all sides to avoid inclusion of other material (i.e., air) in readings. The use of larger diameter segments for calibration may significantly improve the agreement between lab calculated VWC and εb by helping to minimize the impact of both density differences and embedment depths.
Radial and circumferential differences in sap flow are well-documented61,62,63,64,65,66. It follows that differences in wood water content would likewise vary with location and depth. In a study using x-ray computed tomography, Fromm, Sautter, et al.67 demonstrated that this is indeed the case. The work of Fromm, et al.67 also evidenced significant density and VWC differences between early- and latewood in the ring porous species, Q. robur. Challenges due to circumferential differences in flow and conductivity can be overcome by the use of additional sensors; however, radial and temporal density variations present more difficulty. The present methodology assumes that density is constant in both time and space. For longer-term measurement campaigns, changes in density and flow patterns due to wounding effects68 may also be of concern. Some differences in water content with radial depth may be addressed by modification to the length of the sensor tines. The style of capacitance sensor used in this work employs 5.5 cm long solid stainless-steel tines that can be cut without damaging the sensor function. It is important that all tines be cut to precisely the same length and a new calibration be performed using the modified sensor (see Figure 1 and Table 1, Q. rubra shortened). Using sensors with tines shortened to 2 cm, Matheny, et al.5 demonstrated different dynamics between shallow actively conducting sapwood and bulk sapwood in Q.rubra. While this method provides a means to examine water content in shallow depths (0 < cut tine length < 5.5 cm), it lacks the ability to distinguish between specific depths (i.e.,VWC between 2 and 4 cm) or at depths greater than the initial sensor length.
The most common difficulties with this methodology arise during installation, and are frequently the result of under-drilled or misaligned holes. It is necessary to ensure good contact between the sensor and the wood, but the use of excessive force during installation can cause the sensor to break. In the event that the pre-drilled holes or the sensor tines themselves are out of alignment, flexure to the sensor body can also result in instrument failure. The close fit required to maintain sufficient contact with the wood also makes removal from the tree or sample segment difficult, and extra care is necessary during the calibration procedure when sensors must be removed and reused after each measurement. Given the ability of skin oils to influence readings, it is reasonable to assume that the use of grease or another lubricant to facilitate installation and removal would likewise affect sensor output. During field operation, the most likely cause of instrument failure is power or communication loss due to cable damage. Large spikes in VWC have been observed, likely resulting from stem flow penetrating the sensor if the silicon seal around the sensor is damaged or incomplete. These spikes should be filtered from the data during post processing.
Frequency domain reflectometry-style capacitance sensors enable volumetric water content to be measured in the stems of large trees at higher temporal resolution than most previous methodologies. This technique can also provide significant insights into the availability and use of stem-water capacitance particularly for hardwood trees with low elastic moduli that are the least likely to generate the elastic expansions and contractions monitored by electronic microdentrometers2. VWC measurements may also be used to improve the accuracy of sap flux measurements as collected by widely-used methods, such as the thermal dissipation technique69, which rely on a reference maximum temperature that has been demonstrated to be sensitive to changes in stem VWC. The low cost, low power nature of the sensors facilitates the expansion of the presented methodology to branches and roots, to more easily assess the whole-tree water balance. The combination of water content and water flow data, as monitored by sap flux, provides advanced insight into vegetation hydraulic functions.
The approach we present here is generalizable to any tree species, and therefore could be used to measure any tree's water storage, within a range of diameters thicker than half a tine length, but not so thick that the tines are too short to represent the dynamics within sapwood. The low cost and operation requirements of the presented approach make it possible to instrument many trees in order to obtain a large and meaningful sample size within a whole forest plot, even in cases of high species diversity. Potential applications include forest hydrology and ecohydrology, inclusive of studies requiring the water budget of a forest stand to be known and monitored, studies of plant transpiration and response to water availability, and studies of plant response to drought and potential mortality due to water stress. Data obtained via this methodology can be used for calibration of plant-level hydrodynamic models. Observations of volumetric stem water content also provide a unique resource for validation of radar measurements of soil moisture from remote sensing, for which above-ground vegetation water storage is a noise term that may now be eliminated. This technique can also be used in agricultural plantations to monitor the effective water status of trees and facilitate the dynamic optimization of irrigation schemes.
Divulgaciones
The authors have nothing to disclose.
Acknowledgements
Funding for this study was provided by U.S. Department of Energy's Office of Science, Office of Biological and Environmental Research, Terrestrial Ecosystem Sciences Program Award No. DE-SC0007041, Ameriflux Management program under Flux Core Site Agreement No. 7096915 through Lawrence Berkeley National Laboratory, and the National Science Foundation Hydrological Science grant 1521238. Any opinions, findings, and conclusions or recommendations expressed in this material are those of the authors and do not necessarily reflect the views of the funding agencies.
Materials
| Ruggedized Soil Moisture Sensor | METER Group Inc. | GS-3 | Capacitance sensors |
| 1/8" drill bit | Any | N/A | |
| 9/64" drill bit | Any | N/A | |
| Drying oven | Any | N/A | |
| Chainsaw | Any | N/A | |
| Electric drill | Any | N/A | |
| Bucket for water bath | Any | N/A | |
| Alcohol swabs | Any | N/A | |
| Draw knife | Any | N/A | |
| Data logger | Any | N/A | |
| Silicon sealant | Any | N/A |
Referencias
- Matheny, A. M., et al. Contrasting strategies of hydraulic control in two codominant temperate tree species. Ecohydrol. 10 (3), e1815 (2017).
- Kocher, P., Horna, V., Leuschner, C. Stem water storage in five coexisting temperate broad-leaved tree species: significance, temporal dynamics and dependence on tree functional traits. Tree Physiol. 33 (8), 817-832 (2013).
- Holbrook, N. M., Gartner, B. L. Chapter 7. Plant stems: physiology and functional morphology. , 151-174 (1995).
- Wullschleger, S. D., Hanson, P. J., Todd, D. E. Measuring stem water content in four deciduous hardwoods with a time-domain reflectometer. Tree Physiol. 16 (10), 809-815 (1996).
- Matheny, A. M., et al. Observations of stem water storage in trees of opposing hydraulic strategies. Ecosphere. 6 (9), 165 (2015).
- Waring, R. H., Running, S. W. Sapwood water storage: its contribution to transpiration and effect upon water conductance through the stems of old-growth Douglas-fir. Plant Cell Environ. 1 (2), 131-140 (1978).
- Cermak, J., Kucera, J., Bauerle, W. L., Phillips, N., Hinckley, T. M. Tree water storage and its diurnal dynamics related to sap flow and changes in stem volume in old-growth Douglas-fir trees. Tree Physiol. 27 (2), 181-198 (2007).
- Betsch, P., et al. Drought effects on water relations in beech: The contribution of exchangeable water reservoirs. Agric. For. Meteorol. 151 (5), 531-543 (2011).
- Schäfer, K. V. R., Oren, R., Tenhunen, J. D. The effect of tree height on crown level stomatal conductance. Plant Cell Environ. 23 (4), 365-375 (2000).
- Burgess, S. S. O., Dawson, T. E. Using branch and basal trunk sap flow measurements to estimate whole-plant water capacitance: a caution. Plant Soil. 305 (1-2), 5-13 (2008).
- Kumagai, T., Aoki, S., Otsuki, K., Utsumi, Y. Impact of stem water storage on diurnal estimates of whole-tree transpiration and canopy conductance from sap flow measurements in Japanese cedar and Japanese cypress trees. Hydrol. Process. 23 (16), 2335-2344 (2009).
- Nadler, A., Raveh, E., Yermiyahu, U., Green, S. Stress induced water content variations in mango stem by time domain reflectometry. Soil Sci. Soc. Am. J. 70 (2), 510-520 (2006).
- Nadler, A., Raveh, E., Yermiyahu, U., Green, S. R. Evaluation of TDR use to monitor water content in stem of lemon trees and soil and their response to water stress. Soil Sci. Soc. Am. J. 67 (2), 437-448 (2003).
- Hernandez-Santana, V., Martinez-Fernandez, J. TDR measurement of stem and soil water content in two Mediterranean oak species. Hydrolog Sci J. 53 (4), 921-931 (2008).
- Cocozza, C., et al. Simultaneous measurements of stem radius variation and sap flux density reveal synchronisation of water storage and transpiration dynamics in olive trees. Ecohydrol. 8 (1), 33-45 (2015).
- Andrade, J. L., et al. Regulation of water flux through trunks, branches, and leaves in trees of a lowland tropical forest. Oecologia. 115 (4), 463-471 (1998).
- Domec, J. C., Gartner, B. L. Cavitation and water storage capacity in bole xylem segments of mature and young Douglas-fir trees. Trees-Struct. Funct. 15 (4), 204-214 (2001).
- Holbrook, N. M., Burns, M. J., Sinclair, T. R. Frequency and time-domain dielectric measurements of stem water-content in the arborescent palm, Sabal palmetto. J. Exp. Bot. 43 (246), 111-119 (1992).
- Meinzer, F. C., et al. Dynamics of water transport and storage in conifers studied with deuterium and heat tracing techniques. Plant Cell Environ. 29 (1), 105-114 (2006).
- Poyatos, R., et al. SAPFLUXNET: towards a global database of sap flow measurements. Tree Physiol. 36 (12), 1449-1455 (2016).
- Hao, G. Y., Wheeler, J. K., Holbrook, N. M., Goldstein, G. Investigating xylem embolism formation, refilling and water storage in tree trunks using frequency domain reflectometry. J. Exp. Bot. 64 (8), 2321-2332 (2013).
- Bonan, G. B., Williams, M., Fisher, R. A., Oleson, K. W. Modeling stomatal conductance in the earth system: linking leaf water-use efficiency and water transport along the soil-plant-atmosphere continuum. Geosci. Model Dev. 7 (5), 2193-2222 (2014).
- Brantley, S. L., et al. Reviews and syntheses: on the roles trees play in building and plumbing the critical zone. Biogeosciences Discuss. 2017, 1-41 (2017).
- Bonan, G. B. Forests and climate change: Forcings, feedbacks, and the climate benefits of forests. Science. 320 (5882), 1444-1449 (2008).
- Matheny, A. M., Mirfenderesgi, G., Bohrer, G. Trait-based representation of hydrological functional properties of plants in weather and ecosystem models. Plant Diversity. 39 (1), 1-12 (2017).
- Chapotin, S. M., Razanameharizaka, J. H., Holbrook, N. M. Water relations of baobab trees (Adansonia spp.L.) during the rainy season: does stem water buffer daily water deficits. Plant Cell Environ. 29 (6), 1021-1032 (2006).
- Oliva Carrasco, L., et al. Water storage dynamics in the main stem of subtropical tree species differing in wood density, growth rate and life history traits. Tree Physiol. 35 (4), 354-365 (2015).
- Wullschleger, S. D., Meinzer, F. C., Vertessy, R. A. A review of whole-plant water use studies in trees. Tree Physiol. 18 (8-9), 499-512 (1998).
- Matheny, A. M., et al. Species-specific transpiration responses to intermediate disturbance in a northern hardwood forest. J. Geophys. Res. 119 (12), 2292-2311 (2014).
- Ford, C. R., Hubbard, R. M., Vose, J. M. Quantifying structural and physiological controls on variation in canopy transpiration among planted pine and hardwood species in the southern Appalachians. Ecohydrol. 4 (2), 183-195 (2011).
- Holbrook, N. M., Sinclair, T. R. Water-Balance in the arborescent palm, Sabal palmetto. II. Transpiration and stem water storage. Plant Cell Environ. 15 (4), 401-409 (1992).
- Goldstein, G., et al. Stem water storage and diurnal patterns of water use in tropical forest canopy trees. Plant Cell Environ. 21 (4), 397-406 (1998).
- Borchert, R. Soil and stem water storage determine phenology and distribution of tropical dry forest trees. Ecology. 75 (5), 1437-1449 (1994).
- Hernandez-Santana, V., Martinez-Fernandez, J., Moran, C. Estimation of tree water stress from stem and soil water monitoring with time-domain reflectometry in two small forested basins in Spain. Hydrol. Process. 22 (14), 2493-2501 (2008).
- . . Climate change 2013: the physical science basis contribution of working group I to the fifth assessment report of the intergovernmental panel on climate change. , 1535 (2013).
- Konings, A. G., Williams, A. P., Gentine, P. Sensitivity of grassland productivity to aridity controlled by stomatal and xylem regulation. Nat. Geosci. , (2017).
- Fei, S., et al. Divergence of species responses to climate change. Science Advances. 3 (5), (2017).
- Fisher, R. A., et al. Vegetation demographics in Earthsystem models: a review of progress and priorities. Glob. Change Biol. , (2017).
- Dietze, M. C., Lebauer, D. S., Kooper, R. O. B. On improving the communication between models and data. Plant Cell Environ. 36 (9), 1575-1585 (2013).
- Bohrer, G., et al. Finite element tree crown hydrodynamics model (FETCH) using porous media flow within branching elements: A new representation of tree hydrodynamics. Water Resour. Res. 41 (11), (2005).
- Mirfenderesgi, G., et al. Tree level hydrodynamic approach for resolving aboveground water storage and stomatal conductance and modeling the effects of tree hydraulic strategy. J. Geophys. Res. 121 (7), 1792-1813 (2016).
- Gentine, P., Guérin, M., Uriarte, M., McDowell, N. G., Pockman, W. T. An allometry-based model of the survival strategies of hydraulic failure and carbon starvation. Ecohydrol. 9 (3), 529-546 (2015).
- Huang, C. -. W., et al. The effect of plant water storage on water fluxes within the coupled soil-plant system. New Phytol. 213 (3), 1093-1106 (2017).
- Bittner, S., et al. Functional-structural water flow model reveals differences between diffuse- and ring-porous tree species. Agric. For. Meteorol. 158, 80-89 (2012).
- Matheny, A. M., et al. Characterizing the diurnal patterns of errors in the prediction of evapotranspiration by several land-surface models: an NACP analysis. J. Geophys. Res. 119 (7), 1458-1473 (2014).
- Matthes, J. H., Goring, S., Williams, J. W., Dietze, M. C. Benchmarking historical CMIP5 plant functional types across the Upper Midwest and Northeastern United States. J. Geophys. Res. 121 (2), 523-535 (2016).
- Musavi, T., et al. The imprint of plants on ecosystem functioning: A data-driven approach. Int. J. Appl. Earth Obs. Geoinf. 43, 119-131 (2015).
- Wullschleger, S. D., et al. Plant functional types in Earth system models: past experiences and future directions for application of dynamic vegetation models in high-latitude ecosystems. Ann. Bot. 114 (1), 1-16 (2014).
- Scholz, F. G., et al. Biophysical properties and functional significance of stem water storage tissues in Neotropical savanna trees. Plant Cell Environ. 30 (2), 236-248 (2007).
- Scholz, F. G., et al. Temporal dynamics of stem expansion and contraction in savanna trees: withdrawal and recharge of stored water. Tree Physiol. 28 (3), 469-480 (2008).
- Borchert, R. Electric resistance as a measure of tree water status during seasonal drought in a tropical dry forest in Costa Rica. Tree Physiol. 14 (3), 299-312 (1994).
- Edwards, W. R. N., Jarvis, P. G. A method for measuring radial differences in water content of intact tree stems by attenuation of gamma radiation. Plant Cell Environ. 6 (3), 255-260 (1983).
- Phillips, N. G., Scholz, F. G., Bucci, S. J., Goldstein, G., Meinzer, F. C. Using branch and basal trunk sap flow measurements to estimate whole-plant water capacitance: comment on Burgess and Dawson (2008). Plant Soil. 315 (1-2), 315-324 (2009).
- Bovard, B. D., Curtis, P. S., Vogel, C. S., Su, H. -. B., Schmid, H. P. Environmental controls on sap flow in a northern hardwood forest. Tree Physiol. 25, 31-38 (2005).
- Granier, A. Evaluation of transiration in a Douglas-Fir stand by means of sap flow measurements. Tree Physiol. 3 (4), 309-319 (1987).
- Brodribb, T. J., Holbrook, N. M. Stomatal closure during leaf dehydration, correlation with other leaf physiological traits. Plant Physiol. 132 (4), 2166-2173 (2003).
- Brodribb, T. J., Holbrook, N. M. Stomatal protection against hydraulic failure: a comparison of coexisting ferns and angiosperms. New Phytol. 162 (3), 663-670 (2004).
- Taneda, H., Sperry, J. S. A case-study of water transport in co-occurring ring- versus diffuse-porous trees: contrasts in water-status, conducting capacity, cavitation and vessel refilling. Tree Physiol. 28 (11), 1641-1651 (2008).
- Schuldt, B., Leuschner, C., Brock, N., Horna, V. Changes in wood density, wood anatomy and hydraulic properties of the xylem along the root-to-shoot flow path in tropical rainforest trees. Tree Physiol. 33 (2), 161-174 (2013).
- Sarmiento, C., et al. Within-individual variation of trunk and branch xylem density in tropical trees. Am. J. Bot. 98 (1), 140-149 (2011).
- Barij, N., Cermak, J., Stokes, A. Azimuthal variations in xylem structure and water relations in cork oak (Quercus suber). Iawa J. 32 (1), 25-40 (2011).
- Domec, J. C., Pruyn, M. L., Gartner, B. L. Axial and radial profiles in conductivities, water storage and native embolism in trunks of young and old-growth ponderosa pine trees. Plant Cell Environ. 28 (9), 1103-1113 (2005).
- Ewers, B. E., Oren, R. Analyses of assumptions and errors in the calculation of stomatal conductance from sap flux measurements. Tree Physiol. 20 (9), 579-589 (2000).
- Fan, Z. X., Cao, K. F., Becker, P. Axial and radial variations in xylem anatomy of angiosperm and conifer trees in Yunnan, China. Iawa J. 30 (1), 1-13 (2009).
- James, S. A., Clearwater, M. J., Meinzer, F. C., Goldstein, G. Heat dissipation sensors of variable length for the measurement of sap flow in trees with deep sapwood. Tree Physiol. 22 (4), 277-283 (2002).
- James, S. A., et al. Axial and radial water transport and internal water storage in tropical forest canopy trees. Oecologia. 134 (1), 37-45 (2003).
- Fromm, J. H., et al. Xylem water content and wood density in spruce and oak trees detected by high-resolution computed tomography. Plant Physiol. 127 (2), 416-425 (2001).
- Steppe, K., De Pauw, D. J. W., Doody, T. M., Teskey, R. O. A comparison of sap flux density using thermal dissipation, heat pulse velocity and heat field deformation methods. Agric. For. Meteorol. 150 (7-8), 1046-1056 (2010).
- Vergeynst, L. L., Vandegehuchte, M. W., McGuire, M. A., Teskey, R. O., Steppe, K. Changes in stem water content influence sap flux density measurements with thermal dissipation probes. Trees. 28 (3), 949-955 (2014).

