Three-dimensional Organotypic Cultures of Vestibular and Auditory Sensory Organs
Summary
Three-dimensional organotypic cultures of the murine utricle and cochlea in optically clear collagen I gels preserve innate tissue morphology, allow for mechanical stimulation through adjustment of matrix stiffness, and permit virus-mediated gene delivery.
Abstract
The sensory organs of the inner ear are challenging to study in mammals due to their inaccessibility to experimental manipulation and optical observation. Moreover, although existing culture techniques allow biochemical perturbations, these methods do not provide a means to study the effects of mechanical force and tissue stiffness during development of the inner ear sensory organs. Here we describe a method for three-dimensional organotypic culture of the intact murine utricle and cochlea that overcomes these limitations. The technique for adjustment of a three-dimensional matrix stiffness described here permits manipulation of the elastic force opposing tissue growth. This method can therefore be used to study the role of mechanical forces during inner ear development. Additionally, the cultures permit virus-mediated gene delivery, which can be used for gain- and loss-of-function experiments. This culture method preserves innate hair cells and supporting cells and serves as a potentially superior alternative to the traditional two-dimensional culture of vestibular and auditory sensory organs.
Introduction
The study of most aspects of mammalian organ development has been facilitated by in vitro systems. Two principal methods are now used for the culture of vestibular sensory organs: free-floating1 and adherent2 preparations. Both methods permit the investigation of hair cell vulnerabilities3 and regeneration1,4 in vitro. In addition, the developmental roles of the Notch5,6, Wnt7,8, and epidermal growth factor receptor (EGFR)9,10 signaling cascades in the inner ear have been established, in part, through the use of in vitro cultures of sensory epithelia. However, cell growth and differentiation are controlled, not only through signaling by morphogens, but also through physical and mechanical cues such as intercellular contacts, the stiffness of extracellular matrix, and mechanical stretching or constriction. The role of such mechanical stimuli is challenging to investigate in the developing inner ear in vivo. Moreover, existing free-floating and adherent culture methods are not suitable for such studies in vitro. Here we describe a method for three-dimensional organotypic culture in collagen I gels of varying stiffness. This method largely preserves the in vivo architecture of the vestibular and cochlear sensory organs and allows investigation of the effects of mechanical force on growth and differentiation11.
Because mechanical stimuli are known to activate downstream molecular events, such as the Hippo signaling pathway12,13,14,15, it is important to be able to combine mechanical stimulation with biochemical and genetic manipulations. The culture method described here permits virus-mediated gene delivery and can therefore be used to study both mechanical and molecular signaling during inner ear development11.
Protocol
All methods described here have been approved by the Animal Care and Use Committees of Rockefeller University and of the University of Southern California.
1. (Optional) Preparation of Collagen I Solution from Mouse-tail Tendons
Note: Collagen I solutions are available commercially. Follow the manufacturer's instructions for gel preparation.
- Euthanize 5 – 10 young adult (3 – 5 weeks old) mice of any wild type strain with carbon dioxide in accordance with the protocol approved by the relevant Institutional Animal Care and Use Committee16. Collect the tails and disinfect them by submerging in 70% ethanol for a minimum of 4 h at room temperature.
Note: Incubation for over 48 h should be avoided, as it results in excessive tissue dehydration and impedes the tendon extraction process. - Remove the skin from each tail by introducing a longitudinal cut with a scalpel blade and retracting the whole skin with forceps. Transfer the skinned tails to a 100-mm Petri dish filled with clean 70% ethanol and cut them into 10-mm segments.
Note: Discard the thinnest parts of the tails, which are difficult to manipulate. - Using forceps, secure each segment of the tail to the bottom of the Petri dish and use a second pair of fine forceps to extract the tendons from the tail one at a time. Individual tendon fibers should emerge with minimal resistance.
Note: Old, dull #5 forceps work well for this step. - Prepare 100 mL of a 0.1% solution (by volume) of acetic acid in sterile, molecular-grade water and add 10 mL of that solution to a sterile 100-mm Petri dish. Transfer the tendons to the Petri dish and leave for 1 h at room temperature to denature the collagen I.
- Use a sterile scalpel or iridectomy scissors with blunt, curved tips to mince the tendon into 1 – 2 mm fragments. Transfer the minced tendons to a sterile 50-mL tube and add 0.1% acetic acid to bring the volume to 50 mL.
Note: Use four or five tails for each 50 mL of acid to achieve a collagen I concentration of 2.0 – 2.5 mg/mL. - Refrigerate the collagen I solution at 4 °C for a minimum of 48 h to facilitate complete protein denaturation. Vortex with a tabletop vortex set to the maximal speed for 1 min twice a day.
- Measure the protein concentration of the solution using the bicinchoninic acid assay, adjust the collagen I concentration to 2.0 – 2.5 mg/mL by adding 0.1% solution of acetic acid, and store at 4 °C.
Note: Collagen I solution can be stored for one to two years. - (Optional) Centrifuge the collagen I solution at 2,000 x g for 1 h at 4 °C and use the translucent top fraction (approximately half of the volume), to achieve optically clear collagen gels in Section 4.
2. Dissection of Vestibular and Auditory Organs
- Euthanize pregnant mice of any strain with carbon dioxide in accordance with the protocol approved by the relevant Institutional Animal Care and Use Committee16. Extract and decapitate the embryos.
Note: Lfng-CreERT2/tdTomato mice can be used to allow permanent labeling of supporting cells upon exposure to 4-hydroxytamoxifen17. - Sterilize all the working surfaces and dissection instruments, including two pairs of #5 forceps and a hair knife18, by cleaning them with 70% ethanol.
- Split each head in two halves by introducing a longitudinal cut with a sterile scalpel blade or by using two pairs of #5 forceps. Extract the temporal bones, which contain the inner ears19, and place them into 15 mL of ice-cold Hank's balanced salt solution (HBSS) in a 60-mm Petri dish. Keep the dish on ice.
Note: Embryos at a range of stages from E13.5 to E18.5 have been used successfully. - (Optional) Wash the inner ears by gently shaking them in a 50 mL conical tube containing 30 mL ice-cold HBSS and replacing the HBSS three or four times. Keep the tube on ice.
Note: This step limits possible contamination of the tissue culture and quickly brings the temperature to 4 °C, which prevents tissue degradation and cell death. - Using two pairs of fine #5 forceps, separate the inner ears from the temporal bone and transfer the ears, three or four at a time, into a 60-mm Petri dish filled with ice-cold HBSS.
- Dissect the vestibular sensory organs.
- Orient the ears medial side up and locate the utricle. With two pairs of #5 forceps, remove the cartilage surrounding the vestibular organs. Gently sever the vestibular nerve, the connection between the utricle and saccule, and the semicircular canals, as shown in Figure 1. Gently pull the utricle and the attached ampullae of the superior and horizontal semicircular canals from the ear.
Note: This method can be used on inner ears of stages E16.5 – E18.5. Preserve the cartilage fragments for Section 3 below.
- Orient the ears medial side up and locate the utricle. With two pairs of #5 forceps, remove the cartilage surrounding the vestibular organs. Gently sever the vestibular nerve, the connection between the utricle and saccule, and the semicircular canals, as shown in Figure 1. Gently pull the utricle and the attached ampullae of the superior and horizontal semicircular canals from the ear.
- Dissect the cochlea.
- Remove the cartilaginous tissue surrounding the hearing organ with two pairs of #5 forceps. Gently sever the connection between the cochlear base and the saccule as shown in Figure 1.
Note: For stages E13.5 – E14.5, soften the cartilage prior to dissection by treating the inner ears with 0.25% collagenase I in phosphate-buffered saline (PBS) solution for 5 min at room temperature. Preserve the cartilage fragments for Section 3 below.
- Remove the cartilaginous tissue surrounding the hearing organ with two pairs of #5 forceps. Gently sever the connection between the cochlear base and the saccule as shown in Figure 1.
- Using a 200-µL pipet fitted with a wide nonstick tip, transfer the utricles and cochleae to a 30-mm Petri dish filled with growth medium comprising DMEM/F12 supplemented with 33 mM Dglucose, 19 mM sodium bicarbonate, 15 mM 4(2hydroxyethyl)-1piperazineethanesulfonic acid (HEPES), 1 mM glutamine, 1 mM nicotinamide, 20 mg/L epidermal growth factor, 20 mg/L fibroblast growth factor, 10 mg/L insulin, 5.5 mg/L transferrin, and 5 µg/L sodium selenite.
- Maintain the utricle preparations for up to 3 h at 37 °C in a tissue-culture incubator gassed with 5% carbon dioxide to allow healing of the cuts in the epithelium introduced during the dissection. Cochlea preparations should be transferred to the collagen gel 10 min after the dissection.
Note: Because the dissection is performed in ice-cold HBSS, there is no need to pre-warm the growth medium to 37 °C.
3. (Optional) Adjust Collagen I Gel Stiffness by Adding Varying Concentrations of Chondrocytes
Note: The method for chondrocyte isolation was modified from Gosset et al.20
- Prepare a 1% solution of collagenase I in sterile 1x PBS and store 100 – 200 µL aliquots at -80 °C. Defrost on ice when needed, avoiding multiple freeze-thaw cycles.
- Use fine forceps to collect the pieces of cartilage left over from the dissection of the vestibular and auditory organs from 10 – 12 ears. Separate connective and inner ear tissues from the cartilage. Transfer the cartilage to a 30-mm Petri dish and add 300 µL of sterile growth medium.
- Use a sterile scalpel blade or iridectomy scissors with blunt curved tips to mince the tissue to achieve cartilage pieces approximately 0.5 mm in length.
- Add collagenase I to the medium with cartilage to achieve a final enzyme concentration of 0.25%. Transfer the dish to a tissue-culture incubator at 37 °C gassed with 5% carbon dioxide. Pipet vigorously using a 1,000 µL pipet every 20 min until pieces of cartilage dissociate and are no longer distinguishable in the solution, then incubate for an additional 20 min.
Note: In case of the utricle preparations, chondrocyte isolation can be performed during step 2.9; it takes approximately 2 h (3 – 4 pipetting rounds). - Collect the cell suspension in a 15 mL conical tube and adjust the volume to 10 mL with sterile 1x PBS. Centrifuge at 800 x g for 5 min at 4 °C. Remove the supernatant and resuspend the cells in 10 mL of sterile 1x PBS. Centrifuge at 800 x g for 5 min at 4 °C and remove the supernatant.
Note: It is critical to wash the cells twice; any remaining collagenase I will digest the collagen I gel. - Using a 200-µL pipet, add 100 µL of culture medium to the cell pellet and pipet gently to resuspend. Count the cells using a hemocytometer and maintain them on ice prior to use.
- To increase gel stiffness, add chondrocytes to the neutralized collagen I gel solution (Section 1) and mix rapidly to distribute the cells throughout the gel prior to solidification.
Note: The elastic modulus of the collagen I gel (a measure of stiffness), increases linearly with the number of chondrocytes added11. The relationship is described by the experimentally determined linear function E = 206·Nc + 15, in which E is the elastic modulus in Pascals and Nc is the number of chondrocytes in millions. For a detailed protocol for gel stiffness measurements please refer to the original article11.
4. Place the Vestibular or Auditory Sensory Organ in a Collagen I Gel
- In a sterile 1.5-mL tube, prepare collagen I polymerization solution by mixing 160 µL of 10x PBS with phenol red pH indicator, 133 µL of 0.34 M sodium hydroxide, 70 µL of 0.9 M sodium bicarbonate, and 40 µL of 1 M HEPES. Keep the tube on ice.
Note: This recipe provides the polymerization solution needed to prepare 2 mL of collagen I gel, but can be scaled as needed. - Mix 100 µL of polymerization solution and 400 µL of collagen I solution on ice by gently pipetting up and down in a chilled 1.5-mL tube. To increase gel stiffness, add 50 µL of growth medium containing chondrocytes and mix gently as described in Section 3.
- Transfer 500 µL of the neutralized collagen I solution to a chilled 30-mm Petri dish with a 10-mm glass-bottom insert or to a well of a four-well plate.
Note: It is critical to keep all the reagents on ice to prevent rapid and uneven polymerization of the collagen I. - Quickly transfer the cochleae or utricles to the neutralized collagen I solution and adjust the organs to their desired positions with a sterile hair knife18 or pair of fine forceps.
Note: Collagen I polymerization becomes noticeable after 1 – 2 min as the solution becomes turbid. - After the solution around the tissue has polymerized, place the Petri dish or four-well plate for 20 min at 37 °C in a tissue-culture incubator gassed with 5% carbon dioxide to ensure complete polymerization.
- Add 3 mL of growth medium supplemented with 0.5% fetal bovine serum (FBS) per Petri dish or 500 µL of the same medium per well of a four-well plate. Maintain the culture at 37 °C in a tissue-culture incubator gassed with 5% carbon dioxide. If desired, supplement the growth medium with 10 µM 5-ethynyl-2´-deoxyuridine (EdU) to label proliferating cells.
Note: Higher FBS concentrations can be used if desired.
5. Viral Injections in Three-dimensional Cultures of Vestibular and Auditory Sensory Organs
- Defrost the desired virus on ice and mix with trypan blue solution in a 0.5 mL conical tube to achieve a final dye concentration of 0.05%. Use 10 – 20x dye to avoid substantial dilution of the virus. Keep on ice.
Note: Adenovirus serotype 5 works best for infecting supporting cells in the utricle19, whereas adeno-associated virus Anc80 can infect both hair cells and supporting cells in the utricle and the cochlea21. - Break the tip of a glass needle prepared on a micropipette puller with clean fine forceps while observing it at the highest magnification of a binocular dissecting microscope.
Note: It is important to optimize the settings on the needle puller. Needles with 9 – 12-mm shanks and 20 – 30-µm openings work best. - Remove a sensory-organ culture from the incubator. Attach the needle to the microinjector and fill it with 2 – 3 µL of dye and virus mixture. Advance the needle into the sensory organ while observing it under the binocular dissecting microscope.
- Gently drive the needle tip through mesenchymal and epithelial layers of the roof of a three-dimensional utricular culture. Inject the viral mixture until the cavities of the utricle and ampullae fill with the blue dye.
Note: Because it allows easy access to sensory organs, a 10-mm glass-bottom Petri dish is optimal for the injections. - Incubate at 37 °C in a tissue-culture incubator gassed with 5% carbon dioxide.
Note: When green or red fluorescent protein is used in the viral construct, the fluorescence is apparent 24 h after viral transduction.
Representative Results
Vestibular and auditory sensory organs from embryonic ears, cultured in 40-Pa collagen I gels mimicking low stiffness embryonic conditions11, retain relatively normal three-dimensional structures (Figure 1) and maintain hair cells and supporting cells (Figure 2 and Figure 3). Although supporting cell density decreases by over 30% (Student's t-test: n = 4, p <0.004) and hair cell density decreases by 60% (Student's t-test: n = 5 , p <0.0001) after 3 days in utricular cultures (Figure 2), the area of the macula more than doubles over the same period of time11 (n = 3, p = 0.0002). This demonstrates that the method for three-dimensional culture described here allows for a significant increase in the number of supporting cells11, while maintaining 80% of existing hair cells in the utricle over a 3 day period. In the three-dimensional cultures established from E14.5 cochlea, Sox2-positive progenitor cells differentiate as morphologically distinguishable rows of inner and outer hair cells after 3 days in culture (Figure 3).
Gene expression can be manipulated in three-dimensional cultures of vestibular and auditory sensory organs by means of viral infection. 4hydroxytamoxifen is added to the culture medium to label supporting cells in the cochlear explants established from E15.5 embryos of Lfng-CreERT2/tdTomato mice17. Injection of adenovirus type 5 into the lumen of the culture results in infection of supporting cells at the organ's base (Figure 4A). Injection of the same virus into the lumen of utricular culture established from E17.5 embryo, results primarily in infection of supporting cells throughout the sensory epithelium (Figure 4B).
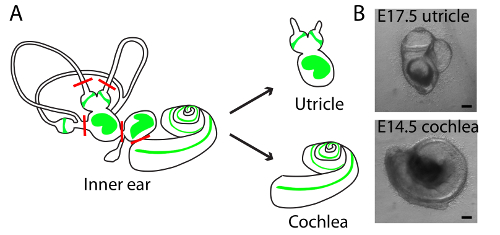
Figure 1. Schematic diagrams of dissections and light-microscopic images of representative cultures of utricle and cochlea in three-dimensional collagen I gels. (A) A schematic drawing portrays the sensory epithelia (green) of the six receptor organs of the murine inner ear. The red lines delineate the cuts introduced during the dissection of an utricle and cochlea. (B) Light microscopy images portray the E17.5 utricle (upper panel) and E14.5 cochlea (lower panel) embedded in collagen I gel and cultured for 48 h. The scale bars represent 100 µm. This figure has been modified from Gnedeva et al.11 Please click here to view a larger version of this figure.
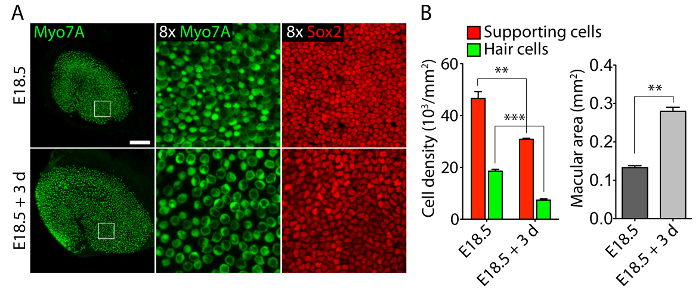
Figure 2. Hair cells and supporting cells in three-dimensional utricular cultures. (A) Confocal-microscopic images portray an E18.5 utricle prior to explantation (top panels) and after 3 days in a three-dimensional culture in 40-Pa collagen I gel (bottom panels). Hair cells are labeled for Myo7A (green) and supporting cells for Sox2 (red). The scale bar represents 50 µm. (B) Quantifications of supporting cell densities (red bars), hair cell densities (green bars), and macular areas (grey bars) in E18.5 utricles prior to explantation and after 3 days in three-dimensional organ culture are represented as means ± SEMs (p <0.001 is represented as ** and p <0.0001 as ***). This figure has been modified from Gnedeva et al.11 Please click here to view a larger version of this figure.
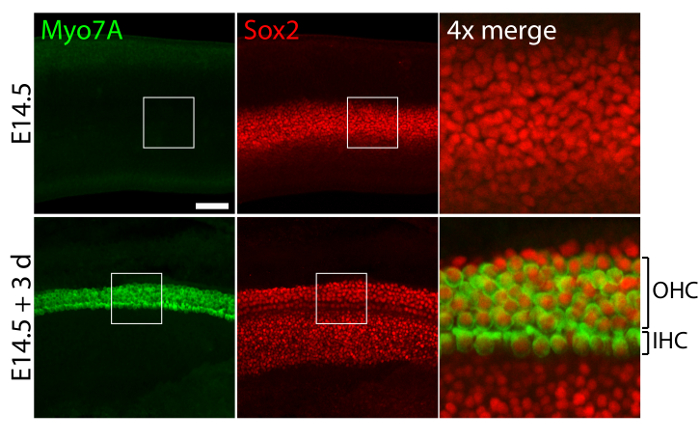
Figure 3. Hair cells and supporting cells in three-dimensional cochlear cultures. Confocal-microscopic images portray an E14.5 cochlea prior to explantation (top panels) and after 3 days in a three-dimensional culture in 40-Pa collagen I gel (bottom panels). Myo7A- and Sox2-positive inner hair cells (IHC) and outer hair cells (OHC) appear in 4 – 5 rows after 3 days in culture. Supporting cells are also labeled for Sox2 (red). The scale bar represents 25 µm. Please click here to view a larger version of this figure.
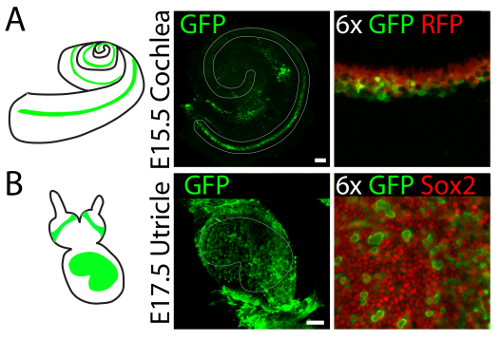
Figure 4. Representative results of viral infections in three-dimensional organ cultures of the cochlea and utricle. (A) Injection of adenovirus serotype 5 into three-dimensional cochlear cultures established from Lfng-CreERT2/tdTomato26 E15.5 embryos results in infection (GFP, green) of supporting cells (Tomato, red) at the base of the organ. The sensory epithelium is delineated in gray. The scale bar represents 100 µm. (B) An identical injection into a three-dimensional utricular culture established from an E17.5 embryo results in infection (GFP, green) of supporting cells (Sox2, red) throughout the organ. The sensory epithelium is delineated in gray. The scale bar represents 100 µm. This figure has been modified from Gnedeva et al.11 Please click here to view a larger version of this figure.
Discussion
The molecular signals that mediate growth and differentiation in the inner ear during development have been studied extensively5,6,7,8,9,10. However, evidence obtained from the utricular model system suggests that mechanical cues, sensed through cell junctions and the activation of Hippo signaling, also play an important role in these processes2,11,22. Moreover, both the extrusion of dying hair cells from the sensory epithelium and the subsequent formation of new sensory receptors through transdifferentiation can affect the mechanical force sensed by the residual supporting cells, causing them to re-enter the cell cycle during regeneration. The three-dimensional culture system described here provides an experimental means of investigating both the role of mechanical force in growth control during inner ear development11 and, potentially, the role of same force during hair cell regeneration. Moreover, the approach facilitates viral infection that provides a method of altering gene expression in the sensory epithelium, thus permitting a combination of mechanical and molecular manipulations for the investigation of growth and regeneration in the ear's sensory organs11.
The limitations of the method relate to the minimal information available on the endogenous forces and mechanical stimuli during inner ear embryogenesis. Measurements of tissue stiffness in the developing inner ear do not exist to our knowledge; hence it is hard to estimate what stiffness of collagen I gel corresponds to in vivo conditions. Our observations and the model suggest that the force opposing growth of the utricle is low initially and increases as the organ approaches its final size11. We therefore hypothesize that a collagen I gel without chondrocytes is a physiologically relevant substrate in which to culture embryonic vestibular and auditory sensory organs.
The three-dimensional culture method described here induces formation of new supporting cells in the utricular macula11, while also maintaining over 80% of hair cells after 3 days in culture (Figure 2). The method, therefore, represents a superior alternative to two-dimensional cultures of the utricle, in which only 40 – 50% of hair cells survive after the first 24 h in culture5,8, and can be used to study hair cell vulnerabilities and regeneration in vitro.
Although we demonstrate the formation of anatomically distinguishable organized rows of inner and outer hair cells in the cultured organ of Corti, more work is required to determine whether the three-dimensional culture method described here supports normal cochlear duct elongation during the process of convergent extension (CE)23,24,25. CE is a highly dynamic process involving cell migration, rearrangement, and cell-cell contact changes26 that is likely to be affected by the external force produced by the tissues surrounding the developing cochlear duct. This method does preserve relatively normal three-dimensional tissue architecture, and could potentially be beneficial for the study of CE in vitro.
Divulgaciones
The authors have nothing to disclose.
Acknowledgements
We thank Dr. A. Jacobo, Dr. J. Salvi, and A. Petelski for their contributions to the original research on which this protocol is based. We also thank J. Llamas and W. Makmura for technical assistance and animal husbandry. We acknowledge NIDCD Training grant T32 DC009975, NIDCD grant R01DC015530, Robertson Therapeutic Development Fund, and the Caruso Family Foundation for funding. Finally, we acknowledge support from Howard Hughes Medical Institute, of which Dr. Hudspeth is an Investigator.
Materials
| #10 Surgical Blades | Miltex | 4-110 | |
| #5 Forceps | Dumont | 11252-20 | |
| 100 mm Petri dish | Sigma | P5856-500EA | |
| 250 uL large orifice pipette tips | USA Scientific | 1011-8406 | |
| 30 mm glass-bottom Petri dish | Matsunami Glass USA Corporation | D35-14-1.5-U | |
| 4 well plate | Thermo Fisher Scientific | 176740 | |
| 4-Hydroxytamoxifen | Sigma | H7904 | |
| 60 mm Petri dish | Thermo Fisher Scientific | 123TS1 | |
| Acetic acid | Sigma | 537020 | |
| Ad-GFP | Vector Biolabs | 1060 | |
| Anti-GFP, chicken IgY fraction | Invitrogen | A10262 | |
| Anti-Myo7A | Proteus Biosciences | 25-6790 | |
| Anti-Sox2 Antibody (Y-17) | Santa Cruz | sc-17320 | |
| Bicinchoninic acid assay | Thermo Fisher Scientific | 23225 | |
| Click-iT EdU Alexa Fluor 647 Imaging Kit | Thermo Fisher Scientific | C10340 | |
| Collagenase I | Gibco | 17100017 | |
| D-glucose | Sigma | G8270 | |
| DMEM/F12 | Gibco | 11320033 | |
| Epidermal growth factor | Sigma | E9644 | |
| Fetal Bovine Serum (FBS) | Thermo Fisher Scientific | 16140063 | |
| Fibroblast growth factor | Sigma | F5392 | |
| Flaming/Brown Micropipette Puller | Sutter Instrument | P-97 | |
| Glutamine | Sigma | G8540 | |
| HBSS | Gibco | 14025092 | |
| Hemocytometer | Daigger | EF16034F | |
| HEPES | Sigma | H4034 | |
| Insulin | Sigma | I3536 | |
| Iridectomy scissors | Zepf Medical Instruments | 08-1201-10 | |
| Microinjector | Narishige | IM-6 | |
| Nicotinamide | Sigma | N0636 | |
| PBS (10X), pH 7.4 | Gibco | 70011044 | |
| PBS (1X), pH 7.4 | Gibco | 10010023 | |
| Phenol Red pH indicator | Sigma | P4633 | |
| Pure Ethanol, 200 Proof | Decon Labs | 2716 | |
| RFP antibody | ChromoTek | 5F8 | |
| Sodium bicarbonate | Sigma | S5761 | |
| Sodium hydroxide | Sigma | S8045 | |
| Sodium selenite | Sigma | S5261 | |
| Tabletop vortex | VWR | 97043-562 | |
| Transferrin | Sigma | T8158 | |
| Trypan blue | Sigma | T6146 |
Referencias
- Oesterle, E. C., Tsue, T. T., Reh, T. A., Rubel, E. W. Hair-cell regeneration in organ cultures of the postnatal chicken inner ear. Hear Res. 70 (1), 85-108 (1993).
- Meyers, J. R., Corwin, J. T. Shape change controls supporting cell proliferation in lesioned mammalian balance epithelium. J Neurosci Off J Soc Neurosci. 27 (16), 4313-4325 (2007).
- Cunningham, L. L. The adult mouse utricle as an in vitro preparation for studies of ototoxic-drug-induced sensory hair cell death. Brain Res. 1091 (1), 277-281 (2006).
- Warchol, M. E., Lambert, P. R., Goldstein, B. J., Forge, A., Corwin, J. T. Regenerative proliferation in inner ear sensory epithelia from adult guinea pigs and humans. Science. 259 (5101), 1619-1622 (1993).
- Lin, V., Golub, J. S., Nguyen, T. B., Hume, C. R., Oesterle, E. C., Stone, J. S. Inhibition of Notch activity promotes nonmitotic regeneration of hair cells in the adult mouse utricles. J Neurosci Off J Soc Neurosci. 31 (43), 15329-15339 (2011).
- Wu, J., et al. Co-regulation of the Notch and Wnt signaling pathways promotes supporting cell proliferation and hair cell regeneration in mouse utricles. Sci Rep. 6, 29418 (2016).
- Chai, R., et al. Wnt signaling induces proliferation of sensory precursors in the postnatal mouse cochlea. Proc Natl Acad Sci U S A. 109 (21), 8167-8172 (2012).
- Wang, T., et al. Lgr5+ cells regenerate hair cells via proliferation and direct transdifferentiation in damaged neonatal mouse utricle. Nat Commun. 6, 6613 (2015).
- Doetzlhofer, A., White, P. M., Johnson, J. E., Segil, N., Groves, A. K. In vitro growth and differentiation of mammalian sensory hair cell progenitors: a requirement for EGF and periotic mesenchyme. Dev Biol. 272 (2), 432-447 (2004).
- White, P. M., Stone, J. S., Groves, A. K., Segil, N. EGFR signaling is required for regenerative proliferation in the cochlea: conservation in birds and mammals. Dev Biol. 363 (1), 191-200 (2012).
- Gnedeva, K., Jacobo, A., Salvi, J. D., Petelski, A. A., Hudspeth, A. J. Elastic force restricts growth of the murine utricle. eLife. 6, (2017).
- Aragona, M., et al. A mechanical checkpoint controls multicellular growth through YAP/TAZ regulation by actin-processing factors. Cell. 154 (5), 1047-1059 (2013).
- Dong, J., et al. Elucidation of a universal size-control mechanism in Drosophila and mammals. Cell. 130 (6), 1120-1133 (2007).
- Low, B. C., Pan, C. Q., Shivashankar, G. V., Bershadsky, A., Sudol, M., Sheetz, M. YAP/TAZ as mechanosensors and mechanotransducers in regulating organ size and tumor growth. FEBS Lett. 588 (16), 2663-2670 (2014).
- Zhao, B., et al. Inactivation of YAP oncoprotein by the Hippo pathway is involved in cell contact inhibition and tissue growth control. Genes Dev. 21 (21), 2747-2761 (2007).
- . . AVMA Guidelines for the Euthanasia of Animals: 2013 Edition. , (2013).
- Semerci, F., et al. Lunatic fringe-mediated Notch signaling regulates adult hippocampal neural stem cell maintenance. eLife. 6, (2017).
- Tuan, R. S., Lo, C. W. Developmental biology protocols. Methods in molecular biology. , 137 (2000).
- Brandon, C. S., Voelkel-Johnson, C., May, L. A., Cunningham, L. L. Dissection of adult mouse utricle and adenovirus-mediated supporting-cell infection. J Vis Exp JoVE. (61), (2012).
- Gosset, M., Berenbaum, F., Thirion, S., Jacques, C. Primary culture and phenotyping of murine chondrocytes. Nat Protoc. 3 (8), 1253-1260 (2008).
- Landegger, L. D., et al. A synthetic AAV vector enables safe and efficient gene transfer to the mammalian inner ear. Nat Biotechnol. 35 (3), 280-284 (2017).
- Burns, J. C., et al. Reinforcement of cell junctions correlates with the absence of hair cell regeneration in mammals and its occurrence in birds. J Comp Neurol. 511 (3), 396-414 (2008).
- Wang, J., et al. Regulation of polarized extension and planar cell polarity in the cochlea by the vertebrate PCP pathway. Nat Genet. 37 (9), 980-985 (2005).
- Chacon-Heszele, M. F., Ren, D., Reynolds, A. B., Chi, F., Chen, P. Regulation of cochlear convergent extension by the vertebrate planar cell polarity pathway is dependent on p120-catenin. Dev Camb Engl. 139 (5), 968-978 (2012).
- Yamamoto, N., Okano, T., Ma, X., Adelstein, R. S., Kelley, M. W. Myosin II regulates extension, growth and patterning in the mammalian cochlear duct. Dev Camb Engl. 136 (12), 1977-1986 (2009).
- Tada, M., Heisenberg, C. -. P. Convergent extension: using collective cell migration and cell intercalation to shape embryos. Dev Camb Engl. 139 (21), 3897-3904 (2012).

