Continuous Noninvasive Measuring of Crayfish Cardiac and Behavioral Activities
Summary
This article presents a noninvasive biomonitoring system for the continuous recording and analyses of crayfish cardiac and locomotor activities. This system consists of a near-infrared optical sensor, a video-tracking module, and software for evaluating crayfish heartbeats that reflects its physiological condition and characterizes crayfish behavior during heartbeat fluctuations.
Abstract
A crayfish is a pivotal aquatic organism that serves both as a practical biological model for behavioral and physiological studies of invertebrates and as a useful biological indicator of water quality. Even though crayfish cannot directly specify the substances that cause water quality deterioration, they can immediately (within a few seconds) warn humans of water quality deterioration via acute changes in their cardiac and behavioral activities.
In this study, we present a noninvasive method that is simple enough to be implemented under various conditions due to a combination of simplicity and reliability in one model.
This approach, in which the biological organisms are implemented into environmental evaluation processes, provides a reliable and timely alarm for warning of and preventing acute water deterioration in an ambient environment. Therefore, this noninvasive system based on crayfish physiological and ethological parameter recordings was investigated for the detection of changes in an aquatic environment. This system is now applied at a local brewery for controlling quality of the water used for beverage production, but it can be used at any water treatment and supply facility for continuous, real-time water quality evaluation and for regular laboratory investigations of crayfish cardiac physiology and behavior.
Introduction
The subject of aquatic organisms' applications, both as model organisms for various laboratory investigations1,2 and as tools for monitoring industrial and natural/environmental water quality3,4, appears to be well studied. Nevertheless, this topic is still of noteworthy interest for humans, irrespective of whether they belong to the scientific community or to other occupations. In spite of the existence of a number of advanced methods for monitoring certain parameters (so-called "biomarkers")5,6,7,8, the most important requirements for selecting an indicator consist of three simple factors: (i) simplicity, (ii) reliability, and (iii) general availability.
Crayfish, as an essential representative of freshwater fauna, distinguishes itself because it is found worldwide, is widespread, and, in most cases9, has a sufficiently large and hard carapace suitable for manipulation. This crustacean belongs to the group of higher invertebrates that provide sufficient development of vital physiological systems and respective organs while, at the same time, maintaining a relatively simple organization10.
Methods based on the assessment of the range of crayfishes' biological and/or behavioral parameters, as described in the scientific literature, have significantly contributed to the development of biomonitoring and crayfish studies in general. Most of the currently available invasive methods for crayfish heart rate measurements are based on electrocardiogram recordings that require a complex and precise surgical procedure11,12,13; such manipulations can cause significant stress to and may require prolonged adaptation by the crayfish. Also, it is not known how long a crayfish can carry such electrodes and whether it will successfully molt while carrying such an attachment. The described noninvasive methods are based on plethysmographic recordings, which are complicated by hardware complexity and require a conditioning circuit for signal filtering14 and an amplification or precise and expensive optic components15,16.
In this study, we described an approach that contributes to existing results and offers new alternatives for improving current crayfish heart rate measurement procedures. Among the advantages, there are (i) a fast and noninvasive attachment that does not require a prolonged physiological adaptation; (ii) crayfishes' capability to carry the sensor within a period of a few months from molting to molting; (iii) the software capable of monitoring real-time cardiac and behavioral activities and the evaluation of data obtained concurrently from multiple crayfish; (iv) a low manufacturing price and simplicity. The biomonitoring system that we describe permits the noninvasive and continuous monitoring of crayfish cardiac and locomotor activities based on changes in crayfishes' etho-physiological characteristics. This system can easily be applied in laboratory examinations of the crayfish cardiac physiology and/or ethology, in addition to industrial implementations for controlling water quality at water treatment and supply facilities.
Protocol
1. Crayfish Selection
- In order to successfully apply the current approach to crayfish, select the respective adult specimens with sufficient carapace sizes (which is a carapace length of at least 30 mm) for sensor attachment, visually examine it for the absence of diseases, and check whether it lifts both chelae when it is touched. The above-mentioned parameters indicate an eligible state of crayfish health.
NOTE: If several crayfish are expected to be used in the trial and are exposed to the same conditions, the experimental group should be formed based on several parameters: (i) similar weight and length; (ii) comparable heart rate; (iii) pronounced nocturnal activity; (iv) regular food consumption; (v) inter-molting period17. Sometimes, it is hard to define whether a crayfish is near to molting by the heart rate measurements or visual or tactile examinations only; therefore, the analyses of the crayfish's hemolymph total protein content can be helpful. Protein content is expected to be higher when the crayfish is closer to molting than in the inter-molting state18.
2. Recording of Crayfish Cardiac Activity and Behavior
- In order to noninvasively measure crayfish heart rates, preliminarily prepare the sensor for this procedure. Before this, put a crayfish into the tank with water and let it acclimate there for a few days as the preparation of the sensor19 will also take a few days.
- Axially couple an IR light-emitting diode (LED) with a phototransistor. Attach the optical sensor circuit onto a board; it will require a power supply of 5 V. For the LED connection, place a 200 Ω resistor on the IR sensor board; in order to connect the phototransistor, place a 220 Ω resistor on the board.
- When attached to the crayfish, the sensor output is modulated by the amount of hemolymph filling the crayfish cardiac muscle and scatters an incident light from the LED. In order to avoid reciprocal interference of the illuminated IR light by the LED and the reflected IR light from the crayfish heart, which is received by the phototransistor, place a small wall (0.5 x 1.5 x 4 mm, thickness x height x width) made of black antistatic plastic between the LED and the phototransistor.
- Place the LED in a waterproof package, and cover the surface of the sensor with the waterproof dielectric gel from the side adjacent to the carapace for the protection of the electronic components from potential damage (Figure 1). Let the gel dry for 3 days in order to gain its best protective properties.
- For an analog signal, attach thin flexible cables (about 3 m long) to the sensor and connect to the analogue-to-digital converter (ADC); from this, a digitized signal will be transferred to a personal computer over a USB interface, at which point the information about the crayfish cardiac activity is saved, analyzed in real-time with special software (see Table of Materials), and stored for further detailed analyses.
- As soon as the sensor is prepared, attach it to the crayfish. In order to do this, switch the computer on and run the software. Determine the number of crayfish to be fixed to the sensors and recorded heart rate to be saved to the date file.
- Remove the crayfish from the water and wipe its dorsal carapace side with a paper towel. Wrap the chelae and abdomen of the crayfish in the paper towel in order to avoid any damages by human hand and to eliminate additional stress on the crayfish caused by warm human hands.
NOTE: Do not use a previous cooling of the crayfish on ice or in the freezer for its immobilization before manipulations with the sensor attachment. The difference in temperatures leads to crayfish dorsal surface weeping which, in turn, leads to unreliable sensor fastening and quick adhesive detachment from the crayfish's carapace. - Prepare a surface (i.e., take a small flat piece of plastic or tear a piece of sticky tape and fix it to a table) and a stick for mixing the glue. Press out two small drops (of a diameter of about 0.5 cm) from tubes A and B containing epoxy glue and quickly mix them.
- Attach the sensor to the crayfish dorsal carapace and try to find the place in which the cardiac signal amplitude would be maximal. Hold the crayfish with the sensor in one hand and, using the other free hand, put a drop of mixed glue on each of the four auxiliary wires located on the sensor (fix them in between steps 2.1.1 and 2.1.4.). Do not move the sensor at least 5 min until the glue hardens (the glue hardening depends on the ambient temperature and humidity).
NOTE: When fixing the sensor to the crayfish carapace, examine thoroughly the whole cardiac area from the carapace side in order to define the area with the best (maximal) cardiac signal amplitude. That will help the software to provide more precise heart rate calculations. - Touch the glue using a free hand, and if it is not sticky, put the unwrapped crayfish with the attached sensor (Figure 2) to the box without water for few more minutes until the glue is completely dry.
NOTE: An optimal temperature for crayfish and glue manipulation varies from 18 to 22 °C. At these temperatures, the glue hardens within 5 to 7 min and is completely dry within 8 to 10 min. At lower temperatures, the stress in the crayfish is less pronounced; however, the glue needs more time to harden, about 15 and 20 min under 15 °C and 10 °C, respectively. At higher temperatures, particularly above 25 °C, the glue hardens within 3 min, but the crayfish undergoes much more stress; therefore, try to minimize the exposure of the crustacean to extreme conditions without water. - Before moving the crayfish back into the tank, dip its cephalothorax into the water several times with short intervals of a few seconds in order to allow a discharge of the air that has accumulated in the gills, and leave the crayfish in the water for approximately 1 h to remove any excess chemicals. After this process is complete, release the crayfish into the water and allow it to acclimate for one to two weeks under experimental conditions, depending on observed physiological indices. Optimal water exchange during the acclimation periods is every other day.
NOTE: Characteristics of crayfish that have acclimated and are in a healthy state include pronounced circadian cardiac and locomotor activities, regular food consumption, and spending most daylight in a specialized shelter (if provided).
3. Camera and Software Setup
- Start the software; the video camera will automatically switch on.
- Select an option of movement detection, thoroughly detect area of each tank on the screen and the software will start tracking the behavior and linking it with the cardiac activity recordings.
NOTE: A crayfish motion detection module consists of a video camera that tracks crayfish behavior from below the tank and the software that combines the behavior with cardiac activity. The data from the module are used to facilitate more precise cardiac activity data processing by eliminating periods in which the crayfish demonstrates high locomotive activity. Sudden crayfish movements (i.e., an escape reaction or feeding initiation) can result in fluctuations or short-time spikes in cardiac signals that may reduce the precision of cardiac interval calculations.
Representative Results
As a result, we obtained a combination of crayfish cardiac and behavioral activities, recorded and saved in a txt-format file (Figure 3). Besides the number of experimental crayfish, the date, and the sampling rate, the file consists of three columns: (1) the continual time in hh:mm:ss format; (2) the heart rate automatically calculated in beats per minute; (3) the locomotion registered as absence (0) or presence (1) of any movement. When the crayfish was inactive, zero was assigned to the cell responsible for movement, and when it moved, then number one appeared in the respective cell. When continuously recording, the data file was automatically created every day at 00:00 hours (12:00 AM). It was crucial to include locomotion since it could have caused changes in the heart rate (Figure 4). After 10 s, a food odor (milled, filtered, and diluted Chironomidae larvae) was delivered into the tank containing the crayfish, using a peristaltic pump. At 14 s, the crayfish recognized the stimulus, and its heart rate slightly decreased due to the so-called orienting response. After 20 s, the heart rate increased, thus resulting in a decrease in cardiac intervals. At 26 s, the crayfish moved toward the stimulus source, and both the physiological excitation caused by the food odor and the locomotion initiation resulted in a substantial heart rate increase. At 37 s, there was also evidence of abrupt crayfish motion. Additionally, locomotion could have substantially contributed to the heart rate growth during the crayfish's reactions to certain stimuli (Figure 5). A disturbed crayfish typically has an increase in heart rate, as seen during the 30- to 40-min interval with occasional locomotion. However, during the 45 to 50 min interval, the locomotion is much more pronounced. This locomotion contributed to a heart rate that is significantly higher than that seen during the period with decreased locomotion. If the data from the file is transferred to another application or the above programming algorithm is used, the data containing just the cardiac activity of the crayfish could be obtained and subsequently processed if necessary (Figure 6). The heart rate of undisturbed crayfish is characterized by a monotonic amplitude of the heartbeat curve and by approximately equal cardiac intervals between each cardiac peak.
In order to analyze crayfish behavioral patterns (such as passed distance, preference of a certain area in the tank or arena, and locomotion velocity), it would be possible to exchange the current camera with a standard video camera with a flat wide-angle lens, as the currently used camera does not make a recording but just tracks locomotion. Alternatively, a recording with any of the online applications for catching a video from the screen could be used.
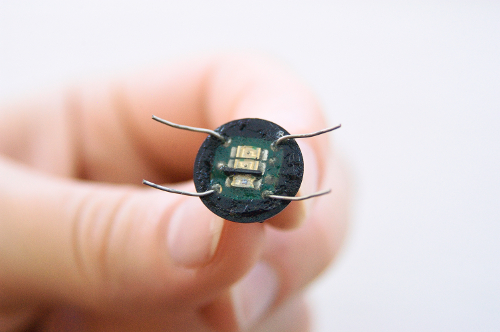
Figure 1: Noninvasive infrared optoelectronic sensor. Please click here to view a larger version of this figure.

Figure 2: Signal crayfish, Pacifastacus leniusculus, holding the sensor on its carapace. Please click here to view a larger version of this figure.
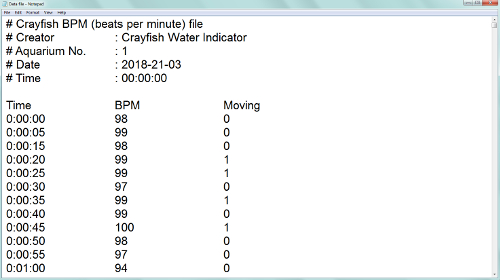
Figure 3: An example of the data file. Please click here to view a larger version of this figure.
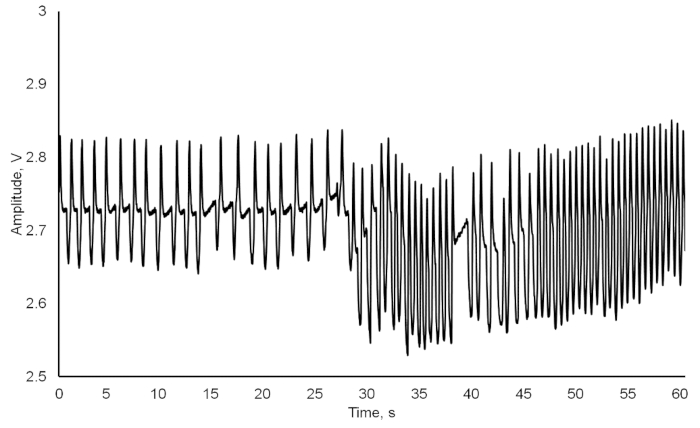
Figure 4: Crayfish heartbeat during the change from normal to disturbed conditions when exposed to food odors. Please click here to view a larger version of this figure.
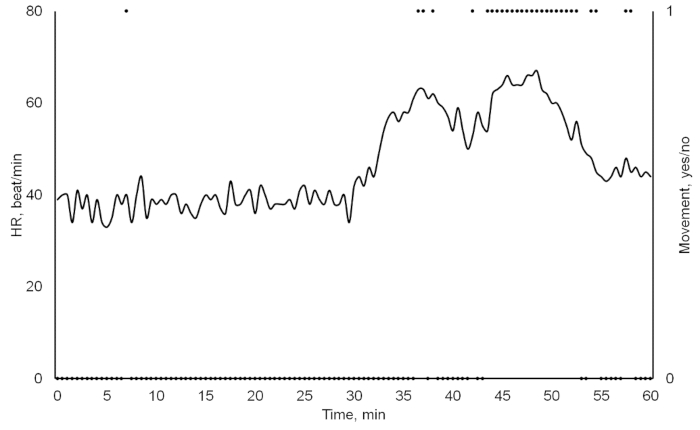
Figure 5: Heart rate and locomotion activities of a crayfish in undisturbed (0–30 min) and disturbed (30–60 min) conditions. Please click here to view a larger version of this figure.
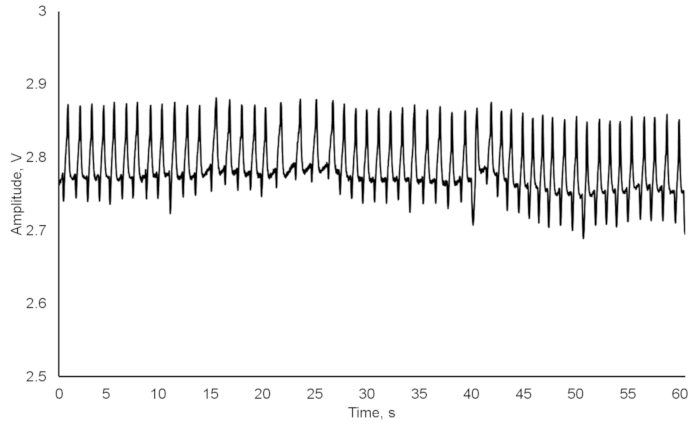
Figure 6: Undisturbed crayfish heart rate. Please click here to view a larger version of this figure.
Discussion
It has been widely suggested that the measurement of certain physiological parameters (such as heart or ventilation rate or both) is a more reliable method for recording crayfish reactions than the evaluation of behavioral responses that do not always occur immediately11. However, it is evident that the most efficient approach for assessing real crayfish reactions to environmental changes is the combination of cardiac activity and behavior recordings since that makes it possible to see the reason(s) for the crayfish heartbeat changes and whether or not they occur as a result of chemical alterations in the ambient environment or because of locomotion initiation. During water quality monitoring, it is crucial to eliminate all outside influences on the changes in crayfish physiological markers, including abrupt movements that have increasing effects on the heart rate but do not present an alarm for the biomonitoring system.
Another possibility for facilitating a more precise and informative heartbeat evaluation are the chronotropic and inotropic parameter analyses of crayfish cardiac activities mainly related to specific shapes in crayfish cardiac signals19. Such analyses confirmed that even when the heartbeat changed only a few beats per minute, some of the secondary parameters can indicate significant changes in crayfish cardiac activities19.
Despite the number of benefits in using the described approach, research around monitoring crayfish has moved toward an absolute minimization of tactile crayfish manipulations. In the recently developed contactless system20, the elimination of sensors and their respective wires means that crayfish of any size can be used for the monitoring procedure. It is also possible to keep multiple crayfish in one experimental area since the absence of any wires prevents wire tangling and crayfish movement restrictions. The crayfish will carry just two tiny pieces of a highly reflective tape that indicates its cardiac area. These pieces of tape can be attached to the crayfish even after a few post-molting days. Crayfish cardiac activities and behaviors are recorded by the video camera and analyzed in real-time by the coordinating software. Along with other technical advances, the modified approach will cause a significant decrease in the price of the monitoring system due to limited hardware.
Divulgaciones
The authors have nothing to disclose.
Acknowledgements
This study was supported by the Ministry of Education, Youth and Sports of the Czech Republic-projects “CENAKVA” No. CZ.1.05/2.1.00/01.0024 and “CENAKVA II” No. LO1205 under the National Sustainability Program I, by the Grant Agency of the University of South Bohemia in České Budějovice (012/2016/Z), and by the Grant Agency of the Czech Republic (No. 16-06498S)
Materials
| IR LED diode | KINGBRIGHT ELECTRONIC | KP-3216F3C | |
| Phototransistor | EVERLIGHT | ELPT15-21C | |
| Resistor | ROYAL OHM | 0805S8J0201T5E | |
| Resistor | ROYAL OHM | 0805S8F2200T5E | |
| Capacitor | KEMET | C0805C334K5RACTU | |
| Cable | TECHNOKABEL | FTP KAT.5E 4X2X0,14C | |
| Connector | HARTING | 21348100380005 | |
| Connector | HARTING | 21348000380005 | |
| Dielectric gel | KRAYDEN | Sylgard 535 | |
| Analogue-to-digital convertor | TEDIA | UDAQ-1416CA | |
| Glue | KUPSITO.SK | 7338723044 | |
| Kinect video camera | ABCSTORE.CZ | GT3-00002 | |
| Analysis software | University of South Bohemia in Ceske Budejovice, Faculty of Fisheries and Protection of Waters, Institute of Complex Systems | Link to the software: www.frov.jcu.cz/crayfishmonitoring User name: frov Password: CF2018 |
Referencias
- Bownik, A., Sokołowska, N., Ślaska, B. Effects of apomorphine, a dopamine agonist, on Daphnia magna: Imaging of swimming track density as a novel tool in the assessment of swimming activity. Science of the Total Environment. 635, 249-258 (2018).
- Jeong, T. Y., Yoon, D., Kim, S., Kim, H. Y., Kim, S. D. Mode of action characterization for adverse effect of propranolol in Daphnia magna. based on behavior and physiology monitoring and metabolite profiling. Environmental Pollution. 233, 99-108 (2018).
- do Nascimento, M. T. L., et al. Determination of water quality, toxicity and estrogenic activity in a nearshore marine environment in Rio de Janeiro, Southeastern Brazil. Ecotoxicology and Environmental Safety. 149, 197-202 (2018).
- Xiao, G., et al. Water quality monitoring using abnormal tail-beat frequency of crucian carp. Ecotoxicology and Environmental Safety. 111, 185-191 (2015).
- Aagaard, A., Andersen, B. B., Depledge, M. H. Simultaneous monitoring of physiological and behavioral activity in marine organisms using non-invasive, computer aided techniques. Marine Ecology Progress Series. 73 (2), 277-282 (1991).
- Bloxham, M. J., Worsfold, P. J., Depledge, M. H. Integrated biological and chemical monitoring: in situ. physiological responses of freshwater crayfish to fluctuations in environmental ammonia concentrations. Ecotoxicology. 8 (3), 225-237 (1999).
- Depledge, M. H., Andersen, B. B. A computer-aided physiological monitoring system for continuous, long-term recording of cardiac activity in selected invertebrates. Comparative Biochemistry and Physiology. A, Comparative Physiology. 96 (4), 473-477 (1990).
- Depledge, M. H., Galloway, T. S. Healthy animals, healthy ecosystems. Frontiers in Ecology and the Environment. 3 (5), 251-258 (2005).
- Holdich, D. M., Reynolds, J. D., Souty-Grosset, C., Sibley, P. J. A review of the ever increasing threat to European crayfish from non-indigenous crayfish species. Knowledge and Management of Aquatic Ecosystems. 11, 394-395 (2009).
- Vogt, G., Holdich, D. M. Functional anatomy. Biology of freshwater crayfish. , 53-151 (2002).
- Bierbower, S. M., Cooper, R. L. Measures of heart and ventilatory rates in freely moving crayfish. Journal of Visualized Experiments. (32), e1594 (2009).
- Li, H., Listerman, L. R., Doshi, D., Cooper, R. L. Heart rate in blind cave crayfish during environmental disturbances and social interactions. Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology. 127 (1), 55-70 (2000).
- Listerman, L. R., Deskins, J., Bradacs, H., Cooper, R. L. Heart rate within male crayfish: social interactions and effects of 5-HT. Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology. 125 (2), 251-263 (2000).
- Burnett, N. P., et al. An improved noninvasive method for measuring heartbeat of intertidal animals. Limnology and Oceanography: Methods. 11 (2), 91-100 (2013).
- Fedotov, V. P., Kholodkevich, S. V., Strochilo, A. G. Study of contractile activity of the crayfish heart with the aid of a new non-invasive technique. Journal of Evolutionary Biochemistry and Physiology. 36 (3), 288-293 (2000).
- Kholodkevich, S. V., Ivanov, A. V., Kurakin, A. S., Kornienko, E. L., Fedotov, V. P. Real time biomonitoring of surface water toxicity level at water supply stations. Environmental Bioindicators. 3 (1), 23-34 (2008).
- Kuznetsova, T. V., Sladkova, S. V., Kholodkevich, S. V. Evaluation of functional state of crayfish Pontastacus leptodactylus in normal and toxic environment by characteristics of their cardiac activity and hemolymph biochemical parameters. Journal of Evolutionary Biochemistry and Physiology. 46 (3), 241-250 (2010).
- Sladkova, S. V., Kholodkevich, S. V. Total protein in hemolymph of crawfish Pontastacus leptodactylus as a parameter of the functional state of animals and a biomarker of quality of habitat. Journal of Evolutionary Biochemistry and Physiology. 47 (2), 160-167 (2011).
- Pautsina, A., Kuklina, I., Štys, D., Císař, P., Kozák, P. Noninvasive crayfish cardiac activity monitoring system. Limnology and Oceanography: Methods. 12 (10), 670-679 (2014).
- Císař, P., Saberioon, M., Kozák, P., Pautsina, A. Fully contactless system for crayfish heartbeat monitoring: Undisturbed crayfish as bio-indicator. Sensors and Actuators B: Chemical. 255, 29-34 (2018).

