Low-cost Protocol of Footprint Analysis and Hanging Box Test for Mice Applied the Chronic Restraint Stress
Summary
The low-cost protocol consisting of footprint analysis and hanging box test after restraint stress is useful for evaluating the movement disorders of mouse model.
Abstract
Gait disturbance is frequently observed in patients with movement disorders. In mouse models used for movement disorders, gait analysis is important behavioral test to determine whether the mice mimic the symptoms of patients. Motor deficits are often induced by stress when no spontaneous motor phenotype is observed in the mouse models. Therefore, gait analysis followed by stress loading would be a sensitive method for evaluating the motor phenotype in mouse models. However, researchers face the requirement of an expensive apparatus to obtain quantitative results automatically from gait analysis. For stress, stress loading by simple methods without expensive apparatuses required for electric shock and forced running is desirable. Therefore, we introduce a simple and low-cost protocol consisting of footprint analysis with paper and ink, hanging box test to evaluate motor function, and stress loading defined by restraint with a conical tube. The motor deficits of mice were successfully detected by this protocol.
Introduction
Movement disorders are defined as disturbances of the nervous system showing an excess or paucity of voluntary or automatic movements1. In particular, gait disturbance is frequently documented among patients with movement disorders2,3,4. Therefore, gait analysis is a suitable behavioral test for the validation of animal models of movement disorders. In mice, automated gait analyses have been performed for walking at natural speed5 and at adjustable speeds by treadmill6,7. These analyses provide quantitative results of gait automatically. An alternative method to detect gait disturbance is called footprint analysis. After labeling the bottoms of the feet with ink, mice walk on paper, and the footprints are analyzed. Initially, Vaseline and powdered charcoal were used to visualize the footprint8, and then were replaced by ink on polygraph paper9 and photographic developer on photographic paper10. A cheaper and less toxic method using ink and paper than the other methods remains to date11. Footprint analysis is less expensive compared with automated analysis5,6,7 and would be useful to evaluate the movement disorders in mouse models for the researchers without abundant research funds.
The hanging box test is a kind of four limb hanging tests using wire cage lid12 and wire mesh screen13. The box is an apparatus with rotatable mesh lid on the top of box along a center bar. In addition to gait analysis, the test can be inexpensively and easily performed. Therefore, we conducted the hanging box test to evaluate grip strength and balance, in addition to the footprint analysis in this protocol.
Stress induces the symptoms of movement disorders14,15. Motor deficits are often induced by several chronic stresses even when no spontaneous motor phenotype is observed in the mouse models of a movement disorder16,17,18. Restraint is one of the commonly used methods for stress loading in mice, because the animal is not physically harmed19 and cost is less compared with other methods such as electric shock with dedicated apparatus and forced running with use of a treadmill. Restraint by a tube, which is performed by confining a mouse in a holed 50 mL conical tube, is easier than other methods such as wire mesh strainer, taped limb, and wrapping of animal with gauze (reviewed20). In this paper, we summarize the protocols of footprint analysis and the hanging box test after restraint by a tube. This protocol would help us to use mouse models of movement disorders without spontaneous motor phenotype.
Protocol
All animal experiments were conducted in a humane manner. The Institutional Animal Experiment Committee of Jichi Medical University approved the study. The study was conducted in accordance with the Institutional Regulation for Animal Experiment and Fundamental Guideline for Proper Conduct of Animal Experiment and Related Activities in Academic Research Institutions under the jurisdiction of the MEXT of Japan. Mice used in this protocol have been described previously21.
1. Hanging Box Test
- Record the weight of each mouse. Mark the tail by marking pen for individual discrimination (e.g., a line, double lines, and triple lines).
NOTE: Growth curves are used for an index of general health22. - Place the mice in the experimental room at least 30 min before the behavioral test. Set the hanging box, which consists of a clear box (25 x 25 x 40 cm3) with a rotatable mesh lid on the top (Figure 1). The mesh lid can be rotated along a central bar so that the top is flipped 180 degrees.
- Put a mouse in the center of the mesh lid. Carefully turn the mesh lid up side down.
- Measure the fall latency (hanging time) of the mouse from the mesh lid.
NOTE: If the mouse does not fall within 5 min, record the latency as 5 min. - Return the mouse to the home cage. Clean the hanging box with 70% ethanol after every test.
2. Footprint Analysis
NOTE: Following the hanging box test, perform the footprint analysis.
- Set up the Runway (Figure 2A).
- Cut a piece of white paper (29.7 cm x 42 cm x 0.09 mm) longitudinally into three lengths of equal width. Set a piece of the white paper (9.9 cm x 42 cm) on the table.
- Put the dark goal box at the distal end of the paper. Put other boxes (approximately the same length as that of the paper) with the walls on both sides of the runway, preventing the escape of mice.
- Put black ink and red ink into separate Petri dishes (35 mm in diameter).
- Training session.
NOTE: Perform the training session only at 4 weeks of age.- Put a mouse on the proximal end of the paper (Face the head toward the goal box). Let the mouse walk from the proximal end to the goal box. Remove the mouse from the goal box. If the mouse stops on the paper, gently push the mouse to the goal box by finger.
- Hold the mouse by grasping the scruff between the thumb and forefinger to limit the movement of forelimbs. Then, grasp the back and the tail between the ball of the thumb and the other fingers to limit the movement of hindlimbs.
NOTE: Insufficient holding of a mouse results in blots of ink on clothing. - Immerse the bottoms of forelimbs in red ink and the bottoms of hindlimbs in black ink. Immediately put the mouse on the proximal end of the paper (Face the head toward the goal box). Let the mouse walk from the proximal end to the goal box. If the mouse stops on the paper, gently push the mouse to the goal box by finger.
- Remove the mouse from the goal box. Go to the test session.
- Test Session.
- Following the training session, set up the runway for footprints with a new cut piece of white paper.
- Hold the mouse by grasping the scruff between the thumb and forefinger to limit the movement of forelimbs. Then, grasp the back and the tail between the ball of the thumb and the other fingers to limit the movement of hindlimbs.
- Immerse the bottoms of forelimbs in red ink and the bottoms of hindlimbs in black ink. Immediately put the mouse on the proximal end of the paper. Let the mouse walk from the proximal end to the goal box.
NOTE: Because mice prefer the dark, walking becomes steadier as the mouse approaches the dark goal box. If the mouse stops on the paper, gently push the mouse to the goal box by finger. Then, if reliable footprints are not obtained for analysis (see step 2.4. Analysis of footprints for details) because the mouse stopped, retry the test session. - Return the mouse to the home cage from the goal box. Clean the goal box with 70% ethanol after each test session. Air-dry the foot-printed paper.
- Analysis of footprints
- Obtain three measurements of each parameter (stride lengths of forelimbs and hindlimbs, front and hind base widths, overlap between forelimb and hindlimb, Figure 2B) with a ruler from foot-printed paper.
NOTE: Because footprints of proximal and distal ends frequently show large variations because of stopping or running, choose the part with a steady gait pattern of footprints. The middle part of the foot-printed paper will usually be suitable for the analysis.- For the stride length, measure the distances between the same parts of the paw (e.g., paw pad or toe).
- For the front base width, draw a line between consecutive right (or left) front footprints. Then, measure the length of the vertical line from the pad of the left (or right) front footprint to the line drawn between the right (or left) footprints.
- For the hind base width, draw a line between consecutive right (or left) hind footprints. Then, measure the length of the vertical line from the pad of the left (or right) hind footprint to the line drawn between the right (or left) footprints.
- For overlap, measure the distance between pads of left (or right) front and hind footprints.
- Average the three measurements for each individual. Use the individual average of each parameter for the statistical analysis.
- For the stride length, use the average of the individual averages of the left and right strides.
- For asymmetry of stride length, use the absolute value of the difference between individual averages of left limb and right limb stride length.
- For the statistical analysis of the other parameters (front base width, hind base width, and overlap), use the individual average directly.
- Obtain three measurements of each parameter (stride lengths of forelimbs and hindlimbs, front and hind base widths, overlap between forelimb and hindlimb, Figure 2B) with a ruler from foot-printed paper.
3. Restraint Stress Loading
- Preparation of Restraint Tubes.
- Make 16 holes (approximately 2 mm in diameter) in a 50 ml conical tube (30 mm in diameter x 115 mm in length) along the scale marks (5, 10, 15, 20, 25, 30, 35, 40 mL) and the backside of each scale mark by square drill (Figure 3). Make a hole on the tip of the 50 mL conical tube (approximately 5 mm in diameter) for breathing by cutting off the tip. Make a hole (approximately 4 mm in diameter) in the tube cap to pass the tail of mice.
- Stress Loading
- Place the mice in the experimental room.
- Hold a mouse by grasping the scruff between the thumb and the forefinger. Enter the mouse into the restraint tube from the head. Pass the tail through the hole in the cap. Close the cap.
NOTE: Limit the forelimb movement, because mice reject entering the tube by the forelimbs. - Keep the mouse enclosed for 2 h on a desk at room temperature. Remove the mouse from the restraint tube and return to the home cage.
NOTE: The restraint tubes can be reused after a wash and dry.
4. Experimental Schedule (Figure 4):
- Perform the hanging box test and the footprint analysis on the same day at 4 weeks of age (see step 1. Hanging box test and step 2. Footprint analysis for details) as a baseline measurement on all mice prior to the grouping into ‘stress group’ and a ‘non-stress group’.
NOTE: About 8-10 mice in 2-3 litters may be suitable to use in an experiment. Footprint analysis at 4 weeks of age consists of training and test sessions. - Randomly divide the mice into a ‘stress group’ and a ‘non-stress group’.
NOTE: When the mice are used consisting of several litters, divide littermates evenly into both groups. Number in each group consists of about 4-5 mice. - Apply the restraint stress to the ‘stress group’ 6 times over the course of two weeks (see step 3. Restraint stress loading for details).
NOTE: 6 times of restraint are applied every two weeks, followed by a hanging box test and footprint analysis from 6-12 weeks of age. Do not apply the restraint stress on the test day of the hanging box test and the footprint analysis. - Perform the hanging box test and the test session of footprint analysis on the same day at 6, 8, 10, and 12 weeks of age.
Representative Results
The heterozygous male mice of Atp1a3 (Atp1a3+/−) that are the mouse model for rapid onset dystonia parkinsonism and wild-type littermates were used in this protocol. Atp1a3+/− showed significantly shorter stride lengths of forelimb and hindlimb than those of the wild type at 4 weeks of age (Figure 5A and Figure 5B, open circle and square). 'Stressed' Atp1a3+/− showed significantly shorter stride lengths of both limbs than those of 'non-stressed' Atp1a3+/− at 8 weeks of age (Figure 5A and Figure 5B, closed and open circle). Asymmetries of stride lengths of both limbs were not significantly different in all groups of mice at all ages (Figure 5C and Figure 5D). The front base and overlaps of both limbs were also similar in all groups at all ages (Figure 5E, G, H). The hind base was significantly wider in the 'stressed' Atp1a3+/− than that in the 'stressed' wild-type mice at 10 weeks of age (Figure 5F, closed circle and square). Thus, restraint stress caused motor deficits (short stride and wide base) of Atp1a3+/−.
The hanging box test was performed to evaluate the grip strength and balance on the test day of footprint analysis. No significant differences in hanging time were observed in 4 to 10 weeks old mice (Figure 6). At 12 weeks of age, the hanging time of 'stressed' wild-type mice was significantly longer than that of the other groups (Figure 6, closed square). Restraint stress prolonged hanging time in wild-type mice only, but not in Atp1a3+/−. Thus, the motor deficit of Atp1a3+/− was distinguishable from wild-type mice by restraint stress.
Body weight is an index of general health22. We measured the body weight of mice on the test day of the hanging box test and footprint analysis, and no significant differences were observed in all groups of mice at all ages (Figure 7). Thus, restraint stress did not affect the general health of mice.

Figure 1: Hanging box apparatus with a rotatable mesh lid. A mouse was placed in the center of the mesh lid, and then the mesh lid was turned up side down. The fall latency of the mouse from the mesh lid is to be measured. Please click here to view a larger version of this figure.
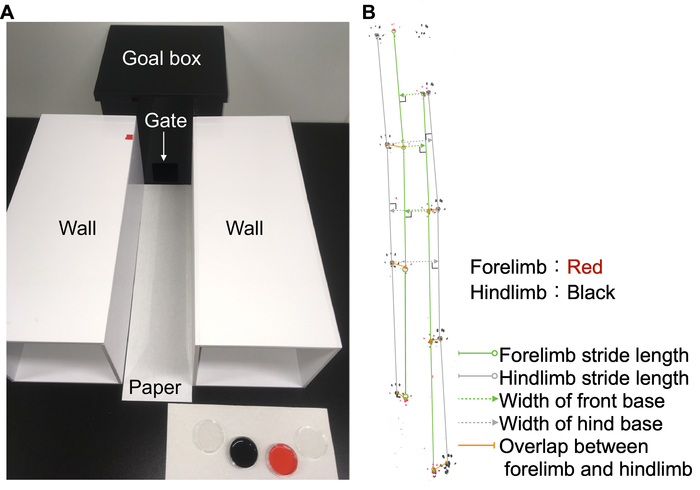
Figure 2: Footprint analysis. (A) Runway for footprint analysis. A foot-painted mouse (forelimbs: red ink; hindlimbs: black ink) was allowed to walk from the proximal end to the goal box. (B) Representative image of footprint and measurement of parameters. Three measurements of each parameter (forelimb and hindlimb stride lengths, widths of front and hind base, overlap between forelimb and hindlimb) were obtained from foot-printed paper. Please click here to view a larger version of this figure.
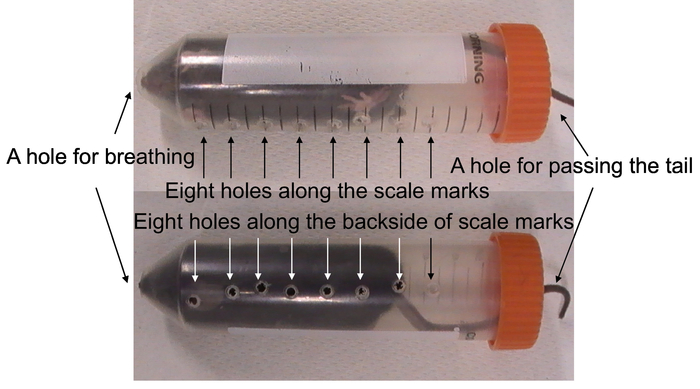
Figure 3: Restraint stress by holed 50 mL conical tube. The restraint tube has a hole for breathing, a hole for passing the tail, and 16 holes for air circulation. Mouse was kept in the tube for 2 h at room temperature. Please click here to view a larger version of this figure.
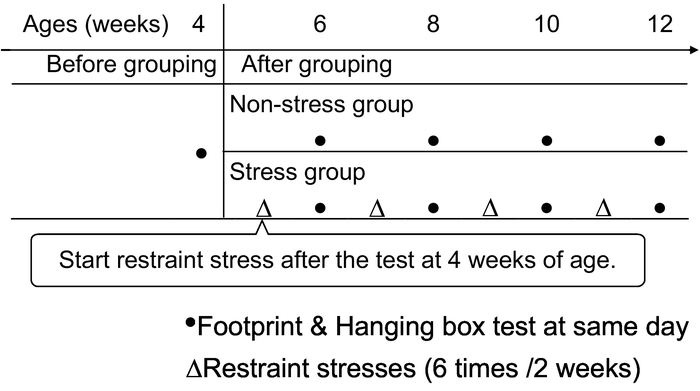
Figure 4: Experimental schedule of the hanging box test and the footprint analysis with the chronic restraint stress loading. Footprint analysis and the hanging box test were performed at 4 weeks of age. Then, the mice were split into 'stressed' and 'non-stressed' groups. For the 'stressed' group, the restraint stress was applied 6 times in 2 weeks until 12 weeks of age. For both groups, footprint analysis and the hanging box test were performed once per 2 weeks until 12 weeks of age. Please click here to view a larger version of this figure.
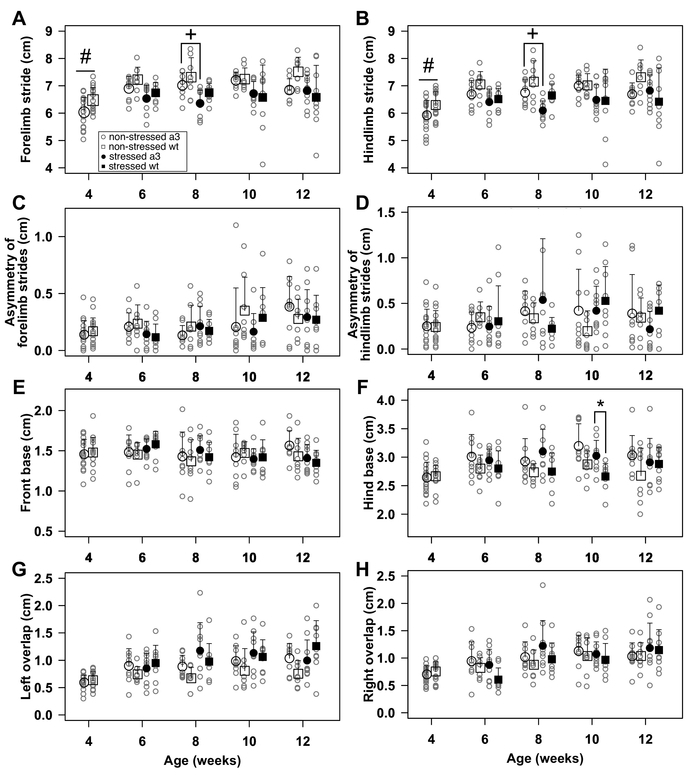
Figure 5: Representative results of footprint analyses. Footprint analysis was performed with 4-week-old Atp1a3+/− (a3) and wild-type (wt) mice (N = 19 and 17, respectively). Then, Atp1a3+/− and wild-type mice were split into 'stressed' and 'non-stressed' groups. Footprint analysis was conducted for 'non-stressed' Atp1a3+/−, 'non-stressed' wild-type, 'stressed' Atp1a3+/−, and 'stressed' wild-type mice from 6-12 weeks of age (open circles, N = 9; open squares, N = 8; solid circles, N = 10; solid squares, N = 9, respectively). (A) Stride length of forelimb. (B) Stride length of hindlimb. (C) Asymmetry of forelimb strides length. (D) Asymmetry of hindlimb strides length. (E) Width between forelimbs. (F) Width between hindlimbs. (G) Overlap between left forelimb and hindlimb. (H) Overlap between right forelimb and hindlimb. Data are the mean ± SD. Statistical analyses (t-test for 4-week-old mice and pairwise t-test with the Holm adjustment method for 6-12-week-old mice) were performed by R23. # p < .05, for 'non-stressed' Atp1a3+/− and 'non-stressed' wild-type mice. * p < .05, for 'stressed' Atp1a3+/− and 'stressed' wild-type mice. + p < .05, for 'non-stressed' and 'stressed' Atp1a3+/− mice. Figure 5A-F has been modified from reference18 with permission of Elsevier. Please click here to view a larger version of this figure.

Figure 6: Representative results of hanging box tests. The hanging box test was performed with Atp1a3+/− (a3) and wild-type (wt) mice at 4 weeks of age. Then, Atp1a3+/− and wild-type mice were split into 'stressed' and 'non-stressed' groups. Hanging time of 4-week-old Atp1a3+/− (open circles, N = 19) and wild-type (open squares, N = 17) mice and hanging time of 6-12-week-old 'non-stressed' Atp1a3+/− (open circles, N = 9), 'non-stressed' wild-type (open squares, N = 8), 'stressed' Atp1a3+/− (solid circles, N = 10), and 'stressed' wild-type (solid squares, N = 9) mice were plotted on a logarithmic scale. Data are the mean ± SD. Statistical analyses (t-test for 4-week-old mice and pairwise t-test with the Holm adjustment method for 6-12-week-old mice) were performed by R23. * p < .05, for 'stressed' Atp1a3+/− and 'stressed' wild-type mice. $ p < .05, for 'non-stressed' and 'stressed' wild-type mice. + p < .05, for 'non-stressed' Atp1a3+/− and 'stressed' wild-type mice. Data has been reprinted from reference18 with permission of Elsevier. Please click here to view a larger version of this figure.
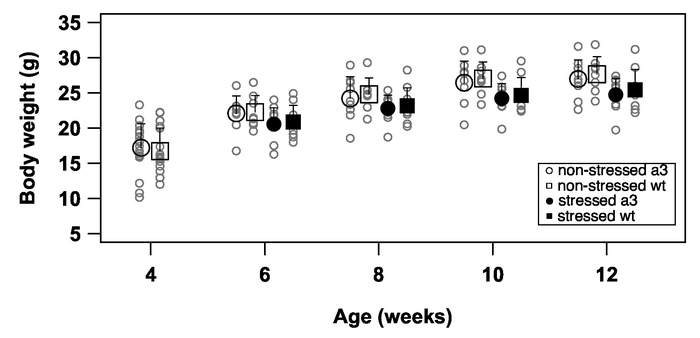
Figure 7: Representative results of growth curves. Body weights were measured of Atp1a3+/− (a3) and wild-type (wt) mice at 4 weeks of age (open circles, N = 19; open squares, N = 17, respectively). Then, Atp1a3+/− and wild-type mice were split into 'stressed' and 'non-stressed' groups. Body weights between 6 and 12 weeks of age of 'non-stressed' Atp1a3+/− (open circles, N = 9), 'non-stressed' wild-type (open squares, N = 8), 'stressed' Atp1a3+/− (solid circles, N = 10), and 'stressed' wild-type (solid squares, N = 9) mice were measured. Data are the mean ± SD. Statistical analyses (t-test for 4-week-old mice and pairwise t-test with the Holm adjustment method for 6-12-week-old mice) were performed by R23. Figure 7 has been modified from reference18 with permission of Elsevier. Please click here to view a larger version of this figure.
Discussion
The footprint analysis and the hanging box test are simple and inexpensive behavioral tests for the motor function of mice. The neurobehavioral phenotypes in several mouse models have been successfully detected by these tests. For example, shortened stride length in amyotrophic lateral sclerosis24, increased length of asymmetrical stride in ataxia-telangiectasia25, increased length of overlap in Huntington's disease26 and dystonia27, and widened base in ataxia28,29, Angelman syndrome30 and dystonia31 were demonstrated by the footprint analysis. Additionally, shorter hanging time than that of the control mice was observed in mouse model of Duchenne muscular dystrophy32. Thus, both tests described in this paper would be useful for evaluating motor dysfunction of mice.
There is a critical step of this protocol to obtain the sharp footprints. It is very important to put a mouse on paper immediately after immersing the bottoms of limbs in ink to avoid drying. It is necessary to finish the immersing steps (steps 2.2.3 and 2.3.3) in a short time. A disadvantage of this protocol of footprint analysis is that the parameters obtained are limited to simple ones that do not include temporal information (e.g., duration of gait cycle and step sequence of each foot) and physical information (e.g., pressure of footprint based on touch-sensitive LED panel). When temporal and physical information is required, an automated gait analysis apparatus must be used. Alternatively, temporal information of gait pattern can be obtained by high-speed camera33. Another disadvantage is that measuring the parameters on foot-printed paper is more laborious than automated gait analysis. For future application, the development of a program for semiautomated or automated data collection from foot-printed paper will be required to decrease the effort expended measuring parameters.
For the stress loading, total number and duration per session can be varied in this protocol. Restraint stress has been conducted with various durations and number of applications (e.g., single for 5 min34, single for 24 h35, 12 h x 5 sessions36, and 6 h x 31 sessions37, reviewed in ref. 20). To our knowledge, no systematic study concerns the effects of total number and duration per session on the motor function of mice. The activity of mice in the open field is affected by single stress loading of 1 h38 or 2 h but not by that of 15 min19. Prolonged duration of stress loading may lead to severe motor deficits. For the number of stress loadings, the stance of mice becomes wider than that of non-stressed mice after 36 times of stress loading (1 h twice weekly), but not after 30 times39. Chronic restraint stress (6 h per day for 31 consecutive days) aggravates rotarod performance in the mouse model of Parkinson's disease37. Our results showed the hanging time of 'stressed' Atp1a3+/− mice became shorter than that of the 'stressed' wild-type mice after 24 times of stress loading (at 12 weeks of age), but not after 18 times or less (at 4, 6, 8, and 10 weeks of age). Therefore, repeated stress loading is required for induction of certain motor deficits in mice, although the induction of motor deficits after a long period from the start of a single or several times of stress loading cannot be excluded. When mice do not show a neurobehavioral phenotype in this protocol, the recommendation is to increase the total number and duration of stress loading per session. By contrast, when the mice show gait abnormality from a single or several times of stress loading, duration and number can be decreased.
Finally, performance of walking and hanging can be affected by emotional behavioral characters (e.g., anxiety and activity) other than those of motor dysfunction. Mice with high anxiety and with hyperactivity often run (instead of walk) to the goal box. Mice showing depression-like behavior may walk with frequent stops. Therefore, an aberrant footprint pattern may not be due to motor deficits. Thus, to confirm the gait abnormality resulting only from motor deficits, performing additional tests that evaluate emotional characteristics (activity: open field test; anxiety: open field and elevated plus maze test; depression-like behavior: forced swim test) after this protocol as described previously18 is advisable.
Divulgaciones
The authors have nothing to disclose.
Acknowledgements
This work was supported by JSPS (Japan Society for the Promotion of Science) KAKENHI (Grant-in-Aid for Scientific Research C), Grant number 18K07373 (H.S.) and Subsidies for Private Universities.
Materials
| Hanging box | O’hara & Co. | http://ohara-time.co.jp/products/wire-hanging-test/ | |
| Marking pen | ZEBRA | MO-120-MC-BK | |
| Goal box | O’hara & Co. | http://ohara-time.co.jp/products/balanced-beam-test/ | Accessory for apparatus of balanced beam test |
| Boxes | O’hara & Co. | – | Side wall of runway |
| Black ink | Shin-asahi | – | |
| Red ink | Maruyamakogyo | BC-6 | |
| Disposable Petri Dish | Corning | 351008 | Petri dishe (35 mm in diameter) |
| Askul Multipaper Super White J Monochrome A3 | Askul | 701-712 | White paper (29.7 cm x 42 cm x 0.09mm) |
| 50 mL Conical tube | Corning | 430829 | |
| Square drill | KAKURI Corporation | DIY FACTORY (K32-0313) |
Referencias
- Warner, T. T. Movement disorders. Practical Guide to Neurogenetics. , (2008).
- Brashear, A., DeLeon, D., Bressman, S. B., Thyagarajan, D., Farlow, M. R., Dobyns, W. B. Rapid-onset dystonia-parkinsonism in a second family. Neurology. 48 (4), 1066-1069 (1997).
- Linazasoro, G., Indakoetxea, B., Ruiz, J., Van Blercom, N., Lasa, A. Possible sporadic rapid-onset dystonia-parkinsonism. Movement Disorders. 17 (3), 608-609 (2002).
- Svetel, M., Ozelius, L. J., et al. Rapid-onset dystonia-parkinsonism: case report. Journal of Neurology. 257 (3), 472-474 (2010).
- Vrinten, D. H., Hamers, F. F. T. “CatWalk” automated quantitative gait analysis as a novel method to assess mechanical allodynia in the rat; a comparison with von Frey testing. PAIN. 102 (1), 203-209 (2003).
- Berryman, E. R. DigigaitTM quantitation of gait dynamics in rat rheumatoid arthritis model. Journal of Musculoskeletal and Neuronal Interactions. 9 (2), 89-98 (2009).
- Beare, J. E., Morehouse, J. R., et al. Gait analysis in normal and spinal contused mice using the TreadScan system. Journal of Neurotrauma. 26 (11), 2045-2056 (2009).
- Rushton, R., Steinberg, H., Tinson, C. Effects of a single experience on subsequent reactions to drugs. British Journal of Pharmacology and Chemotherapy. 20, 99-105 (1963).
- Lee, C. C., Peters, P. J. Neurotoxicity and behavioral effects of thiram in rats. Environmental health perspectives. 17, 35-43 (1976).
- van der Zee, C. E., Schuurman, T., Traber, J., Gispen, W. H. Oral administration of nimodipine accelerates functional recovery following peripheral nerve damage in the rat. Neuroscience Letters. 83 (1-2), 143-148 (1987).
- Leroy, T., Stroobants, S., Aerts, J. -. M., D’Hooge, R., Berckmans, D. Automatic analysis of altered gait in arylsulphatase A-deficient mice in the open field. Behavior Research Methods. 41 (3), 787-794 (2009).
- Sango, K., McDonald, M. P., et al. Mice lacking both subunits of lysosomal beta-hexosaminidase display gangliosidosis and mucopolysaccharidosis. Nature Genetics. 14 (3), 348-352 (1996).
- Deacon, R. M. J. Measuring the Strength of Mice. Journal of Visualized Experiments. (76), e2610 (2013).
- Djamshidian, A., Lees, A. J. Can stress trigger Parkinson’s disease?. Journal of Neurology, Neurosurgey, and Psychiatry. 85 (8), 879-882 (2014).
- Brashear, A., Dobyns, W. B., et al. The phenotypic spectrum of rapid-onset dystonia-parkinsonism (RDP) and mutations in the ATP1A3. Brain. 130 (Pt 3), 828-835 (2007).
- Kirshenbaum, G. S., Saltzman, K., Rose, B., Petersen, J., Vilsen, B., Roder, J. C. Decreased neuronal Na+,K+-ATPase activity in Atp1a3 heterozygous mice increases susceptibility to depression-like endophenotypes by chronic variable stress. Genes, Brain and Behavior. 10 (5), 542-550 (2011).
- DeAndrade, M. P., Yokoi, F., van Groen, T., Lingrel, J. B., Li, Y. Characterization of Atp1a3 mutant mice as a model of rapid-onset dystonia with parkinsonism. Behavioral Brain Research. 216 (2), 659-665 (2011).
- Sugimoto, H., Ikeda, K., Kawakami, K. Heterozygous mice deficient in Atp1a3 exhibit motor deficits by chronic restraint stress. Behavioral Brain Research. 272, 100-110 (2014).
- Zimprich, A., Garrett, L., et al. A robust and reliable non-invasive test for stress responsivity in mice. Frontiers in Behavioral Neuroscience. 8, 125 (2014).
- Buynitsky, T., Mostofsky, D. I. Restraint stress in biobehavioral research: recent developments. Neuroscience and Biobehavioral Reviews. 33 (7), 1089-1098 (2009).
- Ikeda, K., Satake, S., et al. Enhanced inhibitory neurotransmission in the cerebellar cortex of Atp1a3-deficient heterozygous mice. The Journal of Physiology. 591 (13), 3433-3449 (2013).
- Crawley, J. N. Motor functions. What’s Wrong with My Mouse?. , (2007).
- . R: A language and environment for statistical computing Available from: https://www.R-project.org/ (2014)
- Wils, H., Kleinberger, G., et al. TDP-43 transgenic mice develop spastic paralysis and neuronal inclusions characteristic of ALS and frontotemporal lobar degeneration. Proceedings of the National Academy of Sciences of the United States of America. 107 (8), 3858-3863 (2010).
- Eilam, R., Peter, Y., et al. Selective loss of dopaminergic nigro-striatal neurons in brains of Atm-deficient mice. Proceedings of the National Academy of Sciences of the United States of America. 95 (21), 12653-12656 (1998).
- Lin, C. -. H., Tallaksen-Greene, S., et al. Neurological abnormalities in a knock-in mouse model of Huntington’s disease. Human Molecular Genetics. 10 (2), 137-144 (2001).
- Dang, M. T., Yokoi, F., et al. Generation and characterization of Dyt1 ΔGAG knock-in mouse as a model for early-onset dystonia. Experimental Neurology. 196 (2), 452-463 (2005).
- Glynn, D., Drew, C. J., Reim, K., Brose, N., Morton, A. J. Profound ataxia in complexin I knockout mice masks a complex phenotype that includes exploratory and habituation deficits. Human Molecular Genetics. 14 (16), 2369-2385 (2005).
- Becker, E. B. E., Oliver, P. L., et al. A point mutation in TRPC3 causes abnormal Purkinje cell development and cerebellar ataxia in moonwalker mice. Proceedings of the National Academy of Sciences of the United States of America. 106 (16), 6706-6711 (2009).
- Heck, D. H., Zhao, Y., Roy, S., LeDoux, M. S., Reiter, L. T. Analysis of cerebellar function in Ube3a-deficient mice reveals novel genotype-specific behaviors. Human Molecular Genetics. 17 (14), 2181-2189 (2008).
- Kirshenbaum, G. S., Dawson, N., et al. Alternating hemiplegia of childhood-related neural and behavioural phenotypes in Na+,K+-ATPase α3 missense mutant mice. PLoS ONE. 8 (3), e60141 (2013).
- Klein, A., Wessolleck, J., Papazoglou, A., Metz, G. A., Nikkhah, G. Walking pattern analysis after unilateral 6-OHDA lesion and transplantation of foetal dopaminergic progenitor cells in rats. Behavioral Brain Research. 199 (2), 317-325 (2009).
- Geldenhuys, W. J., Guseman, T. L., Pienaar, I. S., Dluzen, D. E., Young, J. W. A novel biomechanical analysis of gait changes in the MPTP mouse model of Parkinson’s disease. PeerJ. 3 (Pt 7), e1175 (2015).
- Cecchi, M., Khoshbouei, H., Morilak, D. A. Modulatory effects of norepinephrine, acting on alpha1 receptors in the central nucleus of the amygdala, on behavioral and neuroendocrine responses to acute immobilization stress. Neuropharmacology. 43 (7), 1139-1147 (2002).
- Chu, X., Zhou, Y., et al. 24-hour-restraint stress induces long-term depressive-likephenotypes in mice. Scientific Reports. 6, 32935 (2016).
- Freeman, M. L., Sheridan, B. S., Bonneau, R. H., Hendricks, R. L. Psychological Stress Compromises CD8+ T cell control of latent herpes simplex virus type 1 infections. The Journal of Immunology. 179 (1), 322-328 (2007).
- Lauretti, E., Di Meco, A., Merali, S., Praticò, D. Chronic behavioral stress exaggerates motor deficit and neuroinflammation in the MPTP mouse model of Parkinson’s disease. Translational Psychiatry. 6, e733 (2016).
- Quartermain, D., Stone, E. A., Charbonneau, G. Acute stress disrupts risk assessment behavior in mice. Physiology and Behavior. 59 (4-5), 937-940 (1996).
- Bannon, D. . The Behavioural effects of stress and aluminum toxicity on a mouse model of amyotrophic lateral sclerosis Parkinsonism-dementia complex. , 1-186 (2015).

