Expression, Solubilization, and Purification of Eukaryotic Borate Transporters
Summary
Here we present a protocol to express, solubilize, and purify several eukaryotic borate transporters with homology to the SLC4 transporter family using yeast. We also describe a chemical cross-linking assay to assess the purified homomeric proteins for multimeric assembly. These protocols can be adapted for other challenging membrane proteins.
Abstract
The Solute Carrier 4 (SLC4) family of proteins is called the bicarbonate transporters and includes the archetypal protein Anion Exchanger 1 (AE1, also known as Band 3), the most abundant membrane protein in the red blood cells. The SLC4 family is homologous with borate transporters, which have been characterized in plants and fungi. It remains a significant technical challenge to express and purify membrane transport proteins to homogeneity in quantities suitable for structural or functional studies. Here we describe detailed procedures for the overexpression of borate transporters in Saccharomyces cerevisiae, isolation of yeast membranes, solubilization of protein by detergent, and purification of borate transporter homologs from S. cerevisiae, Arabidopsis thaliana, and Oryza sativa. We also detail a glutaraldehyde cross-linking experiment to assay multimerization of homomeric transporters. Our generalized procedures can be applied to all three proteins and have been optimized for efficacy. Many of the strategies developed here can be utilized for the study of other challenging membrane proteins.
Introduction
The difficulty in obtaining sufficient quantities of purified membrane protein remains a critical bottleneck in the pursuit of structural and functional studies of receptors, ion channels, and transporters. Many protocols exist for moderately high throughput pipelines to screen and find candidate membrane proteins that express well enough to enable subsequent in vitro studies1,2,3,4. Typically, proteins are tagged with an N- or C-terminal green fluorescent protein (GFP), and expression levels are monitored either by in-gel fluorescence or by fluorescence-detection size exclusion chromatography (FSEC)5. Such approaches enable the triaging of membrane protein candidates into high expressing proteins, moderate or low expressing proteins, or proteins that express poorly or not at all. This approach works well when the experimental design is to investigate large numbers of candidate genes with the intention of selecting whichever protein expresses the best. However, in some cases, an experimental approach is predicated on studying a particular membrane protein, which can be challenging when that protein's expression levels are in the moderate or low range. Additionally, sometimes in these cases, expression levels can be only minimally increased by altering the construct via truncations, thermostabilizing mutations, or codon optimization. It is, therefore, sometimes necessary to optimize membrane protein expression and purification protocols for membrane proteins that express only moderately well.
The SLC4 family of transporters includes the bicarbonate transporter Anion Exchanger 1 (also known as Band 3), the most abundant membrane protein in red blood cells6, and a key driver of cellular respiration. The SLC4 family is homologous with borate transporters, which are critical in plants and have been shown to exhibit a similar structure to Anion Exchanger 1 as well as to sodium-coupled SLC4 transporters7,8,9,10. Here we report optimized protein expression and purification protocols using S. cerevisiae to purify three different borate transporters found in yeast and plants. We highlight in detail key steps we took to optimize the yield and efficiency of the purifications to homogeneity. Additionally, we report a chemical cross-linking assay using glutaraldehyde to monitor the multimeric assembly of these transporters in the context of a purified protein-detergent-lipid complex. The cross-linking experiment can help evaluate homolog and detergent suitability by assessing the multimeric state of oligomeric transporters after purification.
This protocol assumes that the desired transporter has been cloned into a 2 µ derived plasmid under inducible control of the GAL1 promoter for expression in S. cerevisiae. For these procedures, DNA was used encoding full-length wild-type borate transporters Saccharomyces cerevisiae Bor1 (ScBor1)11, Arabidopsis thaliana Bor1 (AtBor1)12, and Oryza sativa Bor3 (OsBor3)13. The C-terminus in each construct is appended with a 10-His-tag and a thrombin cleavage site inserted between the transporter and the 10-His-tag to enable its removal if desired. The protocol will also assume that the plasmid has already been transformed into the DSY-5 expression strain of S. cerevisiae on complete supplemental selective media (CSM) lacking histidine.
Protocol
1. Preparation of media and important buffers
- Prepare 500 mL of 40% galactose by adding 200 g of galactose and de-ionized water to a total volume of 500 mL. Use a hot plate or microwave to increase the rate of galactose getting into solution. Sterile filter with a vacuum filtration apparatus consisting of a 0.2 µm filter top attached to a sterile glass bottle.
NOTE: All media solutions are prepared using de-ionized water. - Prepare 500 mL of 40% glucose by adding 200 g of glucose and water to a total volume of 500 mL. Autoclave to sterilize.
- Into each of the four 2 L flasks, add 0.4 g of complete supplement mixture without histidine (CSM-His), 3.35 g yeast nitrogen base with ammonium sulfate (YNB + nitrogen), and 475 mL water. Autoclave. Add 25 mL of 40% glucose to achieve a final glucose concentration of 2%.
- Add, to a glass bottle, 50 g peptone and 25 g yeast extract and water to 375 mL. Autoclave. Add 125 mL of 40% galactose to give 5x yeast peptone (YP) solution containing 10% galactose.
- Add, to a 250 mL flask, 0.04 g CSM-His, 0.335 g YNB + nitrogen, and water to 47.5 mL. Autoclave. Add 2.5 mL of 40% glucose to get 50 mL of CSM-His + YNB + 2% glucose.
- Prepare 1 L of 1M Tris pH 7.0 by mixing 121.14 g of Tris base with 800 mL of water. Add concentrated hydrochloric acid until the pH reaches 7.0. Add water to a final volume of 1 L and pass through a 0.2 µm filter.
- Prepare 500 mL of 0.5 M ethylenediaminetetraacetic Acid (EDTA) pH 8.0 by mixing 73.06 g of EDTA with 400 mL water. Add sodium hydroxide pellets until the EDTA has dissolved and the pH reaches 8.0. Add water to a final volume of 500 mL and pass through a 0.2 µm filter.
- Prepare 100 mL of 100 mM phenylmethanesulfonyl fluoride (PMSF) by mixing 1.74 g of PMSF with 200 proof ethanol. Store at -20 °C.
- Prepare 225 mL of 2x bead wash buffer consisting of 50 mM Tris pH 7.0, 1.4 M NaCl, 20% glycerol, and 1 mM EDTA pH 8.0.
- Prepare 100 mL of the size exclusion chromatography buffer (S200 buffer) consisting of 20 mM 4-Morpholinoethanesulfonic acid hydrate (MES) pH 6.5, 100 mM NaCl, 2% glycerol, and 0.03% n-Dodecyl-beta-D-Maltopyranoside (DDM).
- Prepare 100 mL of membrane resuspension buffer consisting of 50 mM Tris pH 7.0, 500 mM NaCl, and 10% glycerol.
- Prepare 10 mL of a 3x SDS-PAGE loading dye by mixing 3 mL 20% sodium dodecyl sulfate (SDS), 3 mL glycerol, 2.4 mL 1M Tris pH 6.8, 0.03 g bromophenol blue, and 1.6 mL 2-mercaptoethanol.
2. Overexpression of Borate Transporter in S. cerevisiae
- Inoculate three transformed yeast colonies in 50 mL of CSM-His + YNB + 2% glucose media and shake overnight at 190 rpm at 30 °C.
- The following day use a spectrophotometer to determine the optical density at a wavelength of 600 nm (OD600) and inoculate to an OD600 of 0.01 each of four 2 L flasks that each contain 500 mL of CSM-His + YNB + 2% glucose media.
- Shake at 190 rpm at 30 °C for 30 h to allow the cells to consume all glucose and grow to a high density.
NOTE: A longer growth time results in larger cell yields, larger harvested membrane yields, and ultimately larger purified protein yields. - Induce expression by adding 125 mL of 5x YP media supplemented with 10% galactose for a final induction concentration of 2% galactose. Shake at 190 rpm at 30 °C for 16 h.
- Harvest cells by spinning at 4,000 x g for 15 min. Resuspend the cells in 100 mL cold water.
NOTE: At this point cells may be frozen at -80 °C indefinitely, or the prep may continue into cell lysis and membrane harvesting. We typically harvest 40-45 g of cells.
3. Harvesting of Yeast Membranes
- Add to the cell resuspension 11.25 mL of 1M Tris pH 7.0, 0.45 mL of 0.5 M EDTA, and 2.25 mL of 100 mM PMSF, the latter two of which act as protease inhibitors. Add water to a final volume of 225 mL, which results in a cell resuspension buffer of 50 mM Tris pH 7.0, 1 mM EDTA, and 1 mM PMSF.
NOTE: If using frozen cells allow cells to thaw thoroughly, about 1 h at room temperature, because if they remain partially frozen, they will resist lysis by bead beating. - Add the cell resuspension into a 450 mL metal canister for bead beating. Top off the remaining volume with cold 0.5 mm glass beads.
- Assemble a bead-beating chamber with the rotor and immerse in an ice bath. Perform six 1 min pulses, separated by 2 min rest periods to prevent overheating the lysate.
- Assemble a vacuum filtration apparatus by using a plastic disposable bottle top filter screwed into a glass bottle. Remove the filtration membrane, because the remaining plastic device can capture the beads while allowing lysate to pass. Separate the beads from the lysate by pouring the contents of the bead beating chamber onto the assembly while applying the vacuum.
- Wash the bead beating chamber with 225 mL of a 2x wash buffer that contains 50 mM Tris pH 7.0, 1 mM EDTA, 1 mM PMSF, 1.4 M NaCl, and 20% glycerol. Empty the contents of the chamber onto the beads to wash them.
NOTE: The wash gives a final volume of ~450 mL lysed cells and significantly increases harvested membrane yields. The final concentrations are 50 mM Tris pH 7.0, 1 mM EDTA, 1 mM PMSF, 10% glycerol, and 700 mM NaCl, and the primary purpose of elevating the salt concentration is to help dissociate peripheral membrane proteins. - Spin down the cell lysate for 15 min at 15,000 x g. Pour the supernatant into polycarbonate bottles and spin for 1 h at 135,000 x g in an ultracentrifuge to collect membranes.
NOTE: If an ultracentrifuge is not available, membranes may be collected by spinning for 3 h at 53,000 x g, a force that is achievable in floor model centrifuges. - Discard the supernatant and weigh the bottles with the membrane pellets. Resuspend the membrane pellets in approximately 35 mL membrane resuspension buffer containing 50 mM Tris pH 7.0, 500 mM NaCl, and 10% glycerol and add to a glass douncer. Weigh the empty centrifuge tubes to determine the mass of membranes harvested.
NOTE: In an efficient membrane harvest, the weight of membranes will be equal to approximately 20% the weight of cells used for that membrane harvest. This protocol gives typical cell yields of 40-45 g, and thus we likewise expect a yield of 8-9 g membranes. - Dounce homogenize the membranes, and aliquot into 50 mL conical tubes that may now be stored indefinitely at -80 °C.
NOTE: For this experiment, typically two aliquots are made, to allow two separate protein purifications to be performed from one original cell preparation.
4. Solubilization and Purification of Protein
- To a beaker add a stir bar and 150 mg of n-Dodecyl-β-D-Maltopyranoside (DDM) per g membrane being used. Keep protein on the ice or at 4 °C at all times.
NOTE: DDM is hygroscopic and should be stored in a glass jar with desiccant at -20 °C when not in use. Also, it is typical to use between 4-5 g of membranes in a purification to obtain an appreciable amount of pure protein. - Thaw membranes and bring to a final volume of 15 mL per g membrane in membrane resuspension buffer supplemented with 1 mM PMSF and 20 mM imidazole pH 8.0. Add membranes to the beaker with DDM and stir for 1 h at 4 °C.
NOTE: The DDM concentration will be 1% w/v, but for the solubilization of membrane proteins it is more critical to be aware of the mass of detergent added per g membrane. - Spin for 25 min at 135,000 x g at 4 °C to pellet non-solubilized material. Filter the supernatant through a 5 µm syringe filter.
- Load the sample by the peristaltic pump set to a flow rate of 1 mL/min onto a 1 mL immobilized nickel affinity column equilibrated in 20 mM Tris pH 7.0, 500 mM NaCl, 10% glycerol, 20 mM imidazole pH 8.0, and 0.05% DDM.
NOTE: For lower expressing proteins, improved purity and yields were observed by using a 1 mL instead of 5 mL column, and nickel instead of cobalt ions for affinity chromatography. - Wash the column with 10 column volumes of a wash buffer containing 20 mM Tris pH 7.0, 500 mM NaCl, 10% glycerol, 80 mM imidazole pH 8.0, and 0.05% DDM.
NOTE: The imidazole concentration that can be used during washes for a 10-His-tagged protein is higher than is commonly appreciated and results in improved purity. - Elute the protein in the buffer containing 20 mM Tris pH 7.0, 200 mM NaCl, 10% glycerol, 300 mM imidazole pH 8.0, and 0.05% DDM. Collect the eluate in ten 1 mL fractions and run on a 4-20% Tris-glycine SDS-PAGE gel along with solubilized lysate and wash fractions.
- Pool peak fractions, usually about 5-6 mL, and concentrate to 500 µL or less volume in a 50 kDa cutoff concentrator in a benchtop centrifuge refrigerated at 4 °C.
NOTE: 500 µL is a typical maximum volume injected into size exclusion chromatography (SEC) columns. At this point the protein may be stored indefinitely at -80 °C or continue onto SEC. - Filter the protein through a 0.2 µm spin column filter and inject onto a size exclusion column (e.g., Superdex-200) equilibrated in S200 buffer: 20 mM Mes pH 6.5, 100 mM NaCl, 2% glycerol, and 0.03% DDM.
NOTE: For the subsequent glutaraldehyde cross-linking assay, the buffering agent must not contain a primary amine. SEC is an opportunity to exchange buffers if necessary, as done here when exchanging Tris for Mes. - Run the peak fractions on a 4-20% Tris-glycine SDS-PAGE gel. Stain the gel, collect pure peak fractions, and concentrate in a 50 kDa-cutoff concentrator at 4 °C.
- Determine the protein concentration by measuring the absorbance at a wavelength of 280 nm, and store indefinitely at -80 °C.
5. Glutaraldehyde Cross-linking Assay
- Prepare a 10 µL reaction by mixing 3 µL of 0.5 mg/mL protein in S200 buffer, 5 µL of S200 buffer, 1 µL of either water or 20% sodium dodecyl sulfate (SDS), followed by 1 µL of 1.5% glutaraldehyde.
NOTE: This will give a 1x reaction of 0.15 mg/mL protein and 0.15% glutaraldehyde. Samples containing a pre-treatment of SDS are important negative controls and should be mixed and incubated at room temperature for 5 min before adding glutaraldehyde. - Incubate the reaction for 30 min at room temperature. Terminate the reaction by adding 5µL of 3x SDS-PAGE gel loading dye, which contains an excess of Tris buffer that quenches the glutaraldehyde.
- Load all 15 µL onto 4-20% Tris-glycine SDS-PAGE gel, which will load 1.5 µg protein per lane. Run the gel at 200 V for 30 min. Stain and determine the extent of dimer cross-linking by evidence of a band that runs at twice the size of the denatured monomer.
NOTE: A glutaraldehyde titration and time course may be performed first to find the best conditions for a homomeric transporter.
Representative Results
Typical gels for the eluted fractions from performing nickel affinity chromatography show all three proteins partially purified (Figure 1A). To the right of the ladder is the lysate, which in each case does not show a significant band corresponding to the borate transporter which is typical for proteins that do not overexpress well. The 80 mM wash lane shows minimal loss of 10-His-tagged protein despite the relatively high imidazole concentration. Bands corresponding to the borate transporters are readily apparent in eluted fractions, and fractions deemed to contain the bulk of the eluted protein are concentrated before injecting onto the S200 gel filtration column. At this stage, the proteins are insufficiently purified for many downstream applications, as indicated by extra bands in the concentrated sample before injecting onto the S200 column (Figure 1B). However, injecting the sample onto the size exclusion column reveals eluted fractions of high purity (Figure 1B-C). Chromatograms and their corresponding gels for each of the borate transporters are presented. Despite the moderate purity of the samples upon elution from affinity chromatography, the protein is highly pure upon eluting from the gel filtration column. Critically, the chromatograms reveal each protein to migrate primarily as a single monodisperse peak with little protein retained in the void volume. A stable and folded protein generally gives a monodisperse and symmetrical peak, while unstable, misfolded, or aggregated protein will generally give either multiple asymmetric peaks or a large peak in the void volume. It is typical to obtain a final yield of approximately 2 mg purified protein per L of yeast culture for ScBor1, and approximately 1 mg each of purified AtBor1 and OsBor3 per L culture. These numbers are the amount of protein purified from one aliquot of 4-5 g of membranes, which originates from one half of our original cell preparation.
The cross-linking experiment shows that purified homomeric transporters can have their assembly in solution readily assessed (Figure 2). The purpose of the samples containing a pre-treatment of SDS is to show that cross-linking is dependent on a folded state in solution and that cross-linking therefore does not occur when multimerization is disrupted by a harsh detergent. The results shown distinguish that one of the three borate transporters, ScBor1, is not dimerized when purified in DDM in these conditions, while the other two, AtBor1 and OsBor3, cross-link and show dimerization. It is possible to see that not all protein may cross-link. In this example, trace amounts of OsBor3 monomer are visible, while AtBor1 cross-links to completion. The extent of cross-linking will depend on the number of lysine residues in proximity to one another, and it is possible that other cross-linking reagents may efficiently cross-link different membrane proteins.
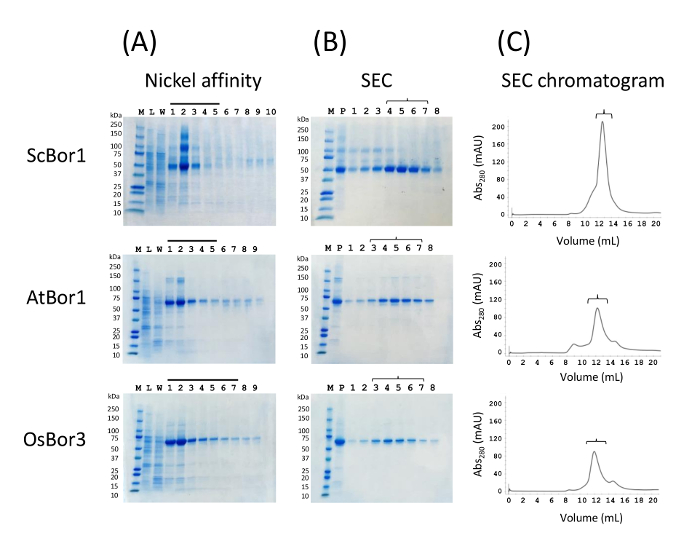
Figure 1. Nickel affinity and size exclusion chromatography purification for three borate transporters. Each vertical panel (A), (B), and (C) contains data for ScBor1, AtBor1, and OsBor3. (A) Nickel affinity column. M is a ladder of molecular weight markers with weights in kDa specified to the left. L is the crude lysate, and W is the 80 mM imidazole wash. All other numbered lanes correspond to 1 mL fractions eluted with 300 mM imidazole. Bars above fractions indicate which were selected to be combined and concentrated for injections onto the column. (B) P indicates concentrated protein before injecting onto the S200 column. Eluted SEC fractions are to the right, with brackets used to match gel lanes with their chromatogram fractions in (C). The void volume is just after 8 mL. Please click here to view a larger version of this figure.
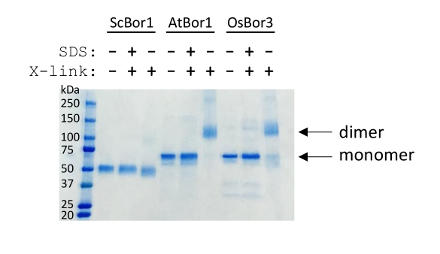
Figure 2. Cross-linking assay assesses purified transporters for multimeric assembly. A ladder with molecular weights is indicated on the left. Above all other lanes is shown which protein is present and whether the sample has had glutaraldehyde added or a pre-treatment of SDS before glutaraldehyde addition. Arrows indicate the positions of monomer and dimer for AtBor1 and OsBor3. ScBor1 is a smaller protein than AtBor1 and OsBor3 and runs as expected. Please click here to view a larger version of this figure.
Discussion
Here we have shared detailed protocols that result in the purification to homogeneity of three distinct eukaryotic borate transporters. The protocols presented here are derived from other protocols for the expression of integral membrane proteins in S. cerevisiae1,14, and our optimizations result in both improved purity and improved yields. The parameters optimized here include cell culture growth volumes and times, bead-beating lysis procedures, buffer composition during cell lysis and protein purification, amount of detergent used per gram membrane, metal ion identify in affinity purification, volume of the affinity column, and the implementation of a cross-linking assay to evaluate homolog and detergent suitability by assessing the multimeric state of oligomeric transporters. The protocols are successful for multiple SLC4 homologs from the plant and fungal species. A limitation of all strategies for the purification of integral membrane proteins is that there exists no universal membrane protein purification method that is guaranteed to work. It is possible that our protocols are more likely to be successful for proteins closer in sequence identity and structure to SLC4 transporters, and thus members of the SLC4, SLC23, and SLC26 families could be promising targets15. Likewise, the more evolutionarily distant from the borate transporters a membrane transporter might be, the more likely the protocol will have to be different, such as by varying the expression system, detergent, or other key parameters4.
The protocol takes advantage of the most commonly used affinity tag in protein purification, the His-tag. Despite the presence of impurities in the initial eluted fractions, the combinations of nickel affinity, extensive washing, and subsequent SEC purification results in the highly purified protein. A 10-His-tag allows for the more stringent imidazole washes and thus can remove more background binding proteins than can be removed in washes permitted by 8-His- or 6-His-tags. Our selections for pure fractions err on the conservative side of the most highly pure gel fractions, which correspond to the peak SEC fractions. Final protein yields can be increased by pooling and concentrating more fractions, albeit with the trade-off of slightly less pure protein. The methods presented here enabled the purification of AtBor1 in quantities that led to the determination of its crystal structure7, with purification differences consisting of cutting off the His-tag and exchanging the transporter into a different detergent to improve crystal diffraction7.
Our protocol raises important considerations for the selection of homologs and the detergent used to solubilize and purify them. DDM is a common first choice for detergent because it is relatively mild, often successful at solubilizing, and has been used in structural and functional studies of a wide variety of membrane proteins. In determining whether a detergent is a poor selection for a membrane, a common method of evaluation is whether the protein gives a single monodisperse peak on an SEC chromatogram, rather than a large peak in the void volume or a range of polydisperse peaks, which indicate misfolded or unstable protein. A more subtle consideration is raised by our purification of ScBor1. It purifies in large quantities and looks favorable and monodisperse on an SEC chromatogram, which suggests it could be an attractive target for structural studies. However, our cross-linking assay reveals that it is a monomer when purified. While it is possible that ScBor1 could natively exist as a monomer in the cell, studies indicate that SLC4 transporters and their homologs are likely to be dimers7,8,9,10. Our development of the cross-linking assay occurred after crystallizing and solving the structure of AtBor1. However, had we been able to evaluate ScBor1 in comparison with AtBor1 with the cross-linking assay, valuable time and research efforts could have been redirected to the pursuit of AtBor1 instead of ScBor1, the latter of which ultimately was not successful in crystallization and diffraction experiments. This assay can thus help researchers distinguish between either homologs or detergents to use when pursuing structural studies in order to prioritize conditions in which the protein maintains its suspected native conformation. Additionally, the assay can be used to probe which amino acids are critical for multimerization, in order to find amino acid substitutions that destabilize the multimerization interface and lead to obligate monomers. Such an approach has been used to probe the functional significance of homomeric assembly in membrane transporters16,17,18.
A general advantage of our method is that yeast has been shown to enable the expression of many challenging membrane proteins of diverse function1. Additionally, its low cost and growth times promote its accessibility for many research efforts that may be less able to use expression strategies that require tissue culture or expensive growth media. The procedures presented here are relatively inexpensive and can be performed in one week which underscores the feasibility of the approach. Implementation of these protocols can help enable structural and functional studies of other challenging membrane proteins.
Divulgaciones
The authors have nothing to disclose.
Acknowledgements
This research was supported by startup funds at Davidson College.
Materials
| 2-mercaptoethanol | Sigma-Aldrich | M3148 | |
| 4-Morpholinoethanesulfonic acid hydrate (MES) | Acros | 172591000 | |
| Äkta Pure 25 L FPLC | GE Healthcare | 29018224 | |
| Amicon Ultra 15 mL, 50 kDa MWCO concentrator | Millipore | UFC905024 | for concentrating nickel column fractions |
| Amicon Ultra 4 mL, 50 kDa MWCO concentrator | Millipore | UFC805024 | for concentrating S200 fractions |
| Bacto Peptone | BD Diagnostics | 211677 | |
| Bacto Yeast Extract | BD Diagnostics | 288620 | |
| Glass Erlenmeyer flask, 2L | Sigma-Aldrich | CLS44442L | |
| Benchtop centrifuge | Sorvall | Legend RT | |
| Bottle top filter | Nalgene | 595-4520 | the membrane is removed and used to filter glass beads |
| Bromophenol blue | Sigma-Aldrich | 114391 | |
| Complete supplement mixture without histidine | Sunrise Science | 1006-100 | |
| D-Galactose | Sigma-Aldrich | G0750 | |
| D-Glucose | Sigma-Aldrich | RDD016 | |
| Ethanol, 200 proof | Pharmco-Aaper | 111000200 | |
| Ethylenediaminetetraacetic Acid (EDTA) | Sigma-Aldrich | EDS-500G | |
| Gel tank SDS-PAGE system | Bio-Rad | 1658004 | |
| Glass bead-beating cell disruptor | BioSpec | 1107900 | |
| Glass beads, 0.5mm | BioSpec | 11079105 | |
| Glutaraldehyde | Electron Microscopy Sciences | 16019 | sent as an 8% solution under nitrogen |
| Glycerol | Sigma-Aldrich | G7893 | |
| HiTrap IMAC FF column, 1 mL | GE Healthcare | 17-0921-02 | charged with nickel |
| Hydrochloric acid | Sigma-Aldrich | 258148 | |
| Imidazole | Acros | 12202 | |
| Instant Blue gel stain | Expedeon | ISB1L | |
| JA-10 rotor | Beckman | 369687 | |
| JA-14 rotor | Beckman | 339247 | |
| Microcentrifuge tubes, 1.5mL | Thermo Scientific | 3451 | |
| Microcentrifuge, refrigerated | Fisher | 13-100-676 | |
| Mini-Protean Tris-glycine gels, 4-20% | Bio-Rad | 456-1096 | |
| Minipuls 3 peristaltic pump | Gilson | F155005 | used for loading lysate onto affinity column |
| n-Dodecyl-beta-D-Maltopyranoside (DDM) | Inalco | 1758-1350 | |
| Nickel(II) Sulfate Hexahydrate | Sigma-Aldrich | 227676 | |
| Orbital shaking incubator with temperature control | New Brunswick | C24 | |
| p423 GAL1 plasmid with borate transporter insert | available from authors | ||
| Phenylmethanesulfonyl fluoride (PMSF) | Acros | 215740050 | |
| Polycarbonate ultracentrifuge tubes | Beckman | 355618 | |
| Polypropylene bottles, 250mL | Beckman | 356011 | |
| Polypropylene bottles, 500mL | Beckman | 355607 | |
| Precision Plus Protein Kaleidoscope Standards | Bio-Rad | 1610375 | |
| S. cerevisiae expression strain DSY-5 | available from authors | ||
| Sodium chloride | Sigma-Aldrich | S7653 | |
| Sodium dodecyl sulfate | Sigma-Aldrich | L3771 | |
| Sodium hydroxide | Sigma-Aldrich | S8045 | |
| Spin column with 0.2 µm filter, 0.5mL | Millipore | UFC30GV0S | for filtering protein before injecting onto S200 column |
| Sterile Falcon tubes, 15mL | Lab Depot | TLD431696 | |
| Sterile Falcon tubes, 50mL | Lab Depot | TLD431698 | |
| Superdex 200 10/300 GL column | GE Healthcare | 17-5175-01 | used for SEC purification |
| Syringe filter, 5µm | Pall | 4650 | for filtering lysate before loading affinity column |
| Tris-base | Sigma-Aldrich | T1503 | |
| Type 70 Ti rotor | Beckman | 337922 | |
| Ultracentrifuge | Beckman | L8-70M | |
| YNB+Nitrogen without amino acids | Sunrise Science | 1501-500 | |
Referencias
- Drew, D., et al. GFP-based optimization scheme for the overexpression and purification of eukaryotic membrane proteins in Saccharomyces cerevisiae. Nature Protocols. 3 (5), 784-798 (2008).
- Goehring, A., et al. Screening and large-scale expression of membrane proteins in mammalian cells for structural studies. Nature Protocols. 9 (11), 2574-2585 (2014).
- Andréll, J., Tate, C. G. Overexpression of membrane proteins in mammalian cells for structural studies. Molecular Membrane Biology. 30 (1), 52-63 (2013).
- Lyons, J. A., Shahsavar, A., Paulsen, P. A., Pedersen, B. P., Nissen, P. Expression strategies for structural studies of eukaryotic membrane proteins. Current Opinion in Structural Biology. 38, 137-144 (2016).
- Kawate, T., Gouaux, E. Fluorescence-detection size-exclusion chromatography for precrystallization screening of integral membrane proteins. Structure. 14 (4), 673-681 (2006).
- Fairbanks, G., Steck, T. L., Wallach, D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Bioquímica. 10 (13), 2606-2617 (1971).
- Thurtle-Schmidt, B. H., Stroud, R. M. Structure of Bor1 supports an elevator transport mechanism for SLC4 anion exchangers. Proceedings of the National Academy of Sciences of the United States of America. 113 (38), 10542-10546 (2016).
- Coudray, N., et al. Structure of the SLC4 transporter Bor1p in an inward-facing conformation. Protein Science. 26 (1), 130-145 (2016).
- Arakawa, T., et al. Crystal structure of the anion exchanger domain of human erythrocyte band 3. Science. 350 (6261), 680-684 (2015).
- Huynh, K. W., et al. CryoEM structure of the human SLC4A4 sodium-coupled acid-base transporter NBCe1. Nature Communications. 9 (1), (2018).
- Zhao, R., Reithmeier, R. A. Expression and characterization of the anion transporter homologue YNL275w in Saccharomyces cerevisiae. American Journal of Physiology Cell Physiology. 281 (1), 33-45 (2001).
- Takano, J., et al. Arabidopsis boron transporter for xylem loading. Nature. 420, 337-340 (2002).
- Nakagawa, Y., et al. Cell-type specificity of the expression of Os BOR1, a rice efflux boron transporter gene, is regulated in response to boron availability for efficient boron uptake and xylem loading. Plant Cell. 19 (8), 2624-2635 (2007).
- Hays, F. A., Roe-Zurz, Z., Stroud, R. M. Overexpression and purification of integral membrane proteins in yeast. Methods in Enzymology. 470, (2010).
- Chang, Y. -. N., Geertsma, E. R. The novel class of seven transmembrane segment inverted repeat carriers. Biological Chemistry. 398 (2), 165-174 (2017).
- Robertson, J. L., Kolmakova-Partensky, L., Miller, C. Design, function and structure of a monomeric ClC transporter. Nature. 468 (7325), 844-847 (2010).
- Last, N. B., Miller, C. Functional Monomerization of a ClC-Type Fluoride Transporter. Journal of Molecular Biology. 427 (22), 3607-3612 (2015).
- Yu, X., et al. Dimeric structure of the uracil:proton symporter UraA provides mechanistic insights into the SLC4/23/26 transporters. Cell Research. 27 (8), 1020-1033 (2017).

