Evaluation of Hemisphere Lateralization with Bilateral Local Field Potential Recording in Secondary Motor Cortex of Mice
Summary
We present in vivo electrophysiological recording of the local field potential (LFP) in bilateral secondary motor cortex (M2) of mice, which can be applied to evaluate hemisphere lateralization. The study revealed altered levels of synchronization between the left and right M2 in APP/PS1 mice compared to WT controls.
Abstract
This article demonstrates complete, detailed procedures for both in vivo bilateral recording and analysis of local field potential (LFP) in the cortical areas of mice, which are useful for evaluating possible laterality deficits, as well as for assessing brain connectivity and coupling of neural network activities in rodents. The pathological mechanisms underlying Alzheimer's disease (AD), a common neurodegenerative disease, remain largely unknown. Altered brain laterality has been demonstrated in aging people, but whether or not abnormal lateralization is one of the early signs of AD has not been determined. To investigate this, we recorded bilateral LFPs in 3-5-month-old AD model mice, APP/PS1, together with littermate wild type (WT) controls. The LFPs of the left and right secondary motor cortex (M2), specifically in the gamma band, were more synchronized in APP/PS1 mice than in WT controls, suggesting a declined hemispheric asymmetry of bilateral M2 in this AD mouse model. Notably, the recording and data analysis processes are flexible and easy to carry out, and can also be applied to other brain pathways when conducting experiments that focus on neuronal circuits.
Introduction
Alzheimer's disease (AD) is the most common form of dementia1,2. Extracellular beta amyloid protein (β-amyloid protein, Aβ) deposition and intracellular neurofibrillary tangles (NFTs) are the main pathological features of AD3,4,5, but the mechanisms underlying AD pathogenesis remain largely unclear. Cerebral cortex, a key structure in cognition and memory, is impaired in AD6, and motor deficits such as slow walking, difficulty navigating the environment and gait disturbances occur with advancing age7. Aβ deposition and neurofibrillary tangles have also been observed in the premotor cortex (PMC) and supplementary motor area (SMA) in AD patients8 and cognitively impacted older adults9, indicating the involvement of an impaired motor system in AD pathogenesis.
The brain is formed by two distinct cerebral hemispheres that are divided by a longitudinal fissure. A healthy brain exhibits both structural and functional asymmetries10, which is called "lateralization", allowing the brain to efficiently deal with multiple tasks and activities. Aging results in a deterioration in cognition and locomotion, together with a reduction in brain laterality11,12. The motor abilities of the left hemisphere are readily apparent in the healthy brain13, but in the AD brain aberrant laterality occurs as a consequence of the failure of left hemisphere dominance associated with left cortical atrophy14,15,16. Therefore, an understanding of a possible alteration of brain lateralization in AD pathogenesis and the underlying mechanisms may provide new insights into AD pathogenesis and lead to identification of potential biomarkers for treatment.
Electrophysiological measurement is a sensitive and effective method of evaluating changes in the neuronal activities of animals. The reduction of hemispheric asymmetry in elders (HAROLD)17 has been documented by electrophysiological research with synchronized interhemispheric transfer time, which shows weakening or absence of hemispheric asymmetry to monaurally presented speech stimuli in the elderly18. Utilizing APP/PS1, one of the most commonly used AD mouse models19,20,21,22, in combination with in vivo bilateral extracellular recording of LFPs in both left and right M2, we evaluated possible laterality deficits in AD. In addition, with simple parameter settings, the built-in function of data analysis software (see the Table of Materials) provides a faster and more straightforward way to analyze the synchronization of electrical signals than mathematically complex programming language, which is friendly to beginners with in vivo electrophysiology.
Protocol
All animals were paired-housed under standard conditions (12 h light/dark, constant temperature environment, free access to food and water) according to the Chinese Ministry of Science and Technology Laboratory Animals Guidelines and experiments were approved by the local ethical committee of Guangzhou University. This is a non-survival procedure.
NOTE: For data shown in the representative results, APP/PS1 (B6C3-Tg (APPswe, PSEN1dE9) 85Dbo/J) double-transgenic mice and littermate wild-type (WT) controls at 3-5 months of age, were used for recordings (n = 10, per group).
1. Animal anesthesia and surgery
- Weigh and anesthetize the mouse by your approved anesthesia regimen from your local animal care committee.
- Perform a tail or toe pinch with forceps to confirm deep anesthesia prior to surgery.
- Position the mouse in a stereotaxic apparatus and fix its head.
- Apply eye ointment on both eyes to keep moist. Follow your local animal care guidelines regarding pre- and postoperative analgesia.
- Shave the hair using surgical clippers. Make a small incision (12-15 mm) in the middle of the exposed surgical area with scissors. Using forceps, gently pull the scalp away from the midline.
- Separate the skin gently and remove residual tissue. Clean the skull using hydrogen peroxide-coated cotton buds.
- Drill two small holes of radii 1.0-1.5 mm on both left and right sides of the skull to allow insertion of the recording microelectrodes into the M2 regions under a stereomicroscope (Figure 1A).
NOTE: Stereotaxic locations of bilateral M2: 1.94 mm anterior to the bregma, 1.0 mm lateral to the midline, and 0.8-1.1 mm ventral to the dura. - Remove the dura mater carefully with a tungsten needle.
- Pull glass borosilicate micropipettes (outer diameter: 1.0 mm) as recording microelectrodes with resistance of 1-2 MΩ.
- Insert two separate recording microelectrodes filled with 0.5 M NaCl into the holes using mechanical micromanipulators (at 60°, Figure 1B).
2. LFP recordings in bilateral M2 of mice
- Lower the left and right glass electrodes slowly into appropriate coordinates of bilateral M2 (Figure 1C).
- For quality control, test the resistance of each electrode using the differential amplifier before capturing LFPs.
- Set the recording process at 0.1 Hz high-pass and 1,000 Hz low-pass with 1,000x amplification.
- Collect digitized raw LFP data of at least 60 s spontaneous activities in stable state, with mice breathing evenly at a respiratory rate of 2 breaths per second under anesthesia.
- After recording, slowly raise the electrodes out of the brain, then euthanize the mice by fast cervical dislocation.
- Save the data and analyze offline.
3. Cross-correlation analysis
- Click Analysis – Waveform correlation in the analysis software and import the data.
- Parameter settings
- Define one waveform channel signal as the first channel and the other as the reference. Set width as 2 and offset as 1 (Figure 2A).
- Set the duration of both LFPs for 100 s by selecting the start time and end time. Press the Process button to perform cross-correlation analysis (Figure 2B).
NOTE: Simultaneous bilateral signals with such durations would be long enough to show neuronal spontaneous activities, thereby revealing the basic properties of synchronization.
- Click File – Export As, then save the cross-correlation results corresponding to the resulting pop-up chart in .txt format.
- Open the .txt file (Figure 2C), remove the correlation values at time lags ranged 0 ± 0.01 s (since two continuous gamma waves have at least 0.01 s interval), then average the rest of the cross-correlation data in the negative time lag part or average the rest of the cross-correlation data in the positive time lag part.
4. Coherence analysis
- Import and run the data in the analysis software.
- Assign the two LFP signals to be the first and second waveform channels separately. Then set the block size value (Figure 3A).
NOTE: Block size means the number of data points used in the FFT. The larger the block size, the better the frequency resolution. Here we recommend setting it as 4096. - Move the dotted lines manually to ensure the time accuracy for signals in both channels are being set as the same period (Figure 3B). Press the Add Area button to load the area and perform coherence analysis.
- Click File – Save As to save the coherence results corresponding to the resulting pop-up chart in .txt format (Figure 3B).
Representative Results
To see whether early AD pathology impairs the capacity of hemisphere lateralization, we conducted bilateral extracellular LFP recordings in the left and right M2 of APP/PS1 mice and WT controls (aged 3-5 months), and analyzed the cross-correlation of these left and right LFPs. In WT mice, the results demonstrated that the mean correlation between left and right LFPs at positive time lags differed significantly from that at negative time lags, implicating the existence of hemispheric asymmetries in M2 areas of WT controls (Figure 4C; WT-positive, 0.08161 ± 0.01246; WT-negative, 0.0206 ± 0.01218; p = 4.74531E-4 < 0.001 by a two sample t-test). In comparison, the left and right LFPs of APP/PS1 mice showed higher synchronized in time domain, suggesting a reduction of asymmetry between the left and right M2 (Figure 4C; APP/PS1-positive, 0.13336 ± 0.0105 APP/PS1-negative, 0.12635 ± 0.01066; p = 0.64157 > 0.05 by a two sample t-test).
We then filtered gamma oscillations from the LFPs (Figure 5A) and performed a coherence analysis as described in the protocol to measure the similarity of electrical signals in the gamma frequency range. The result showed that the gamma coherence between left and right M2 in APP/PS1 was significantly higher than that in WT mice (Figure 5B,C; WT, 0.13267 ± 0.00598; APP/PS1, 0.17078 ± 0.0072; p = 0.00550 < 0.01 by two sample t-test), indicating a higher synchronization, and consequently reduced lateralization, between left and right M2 in APP/PS1 mice.
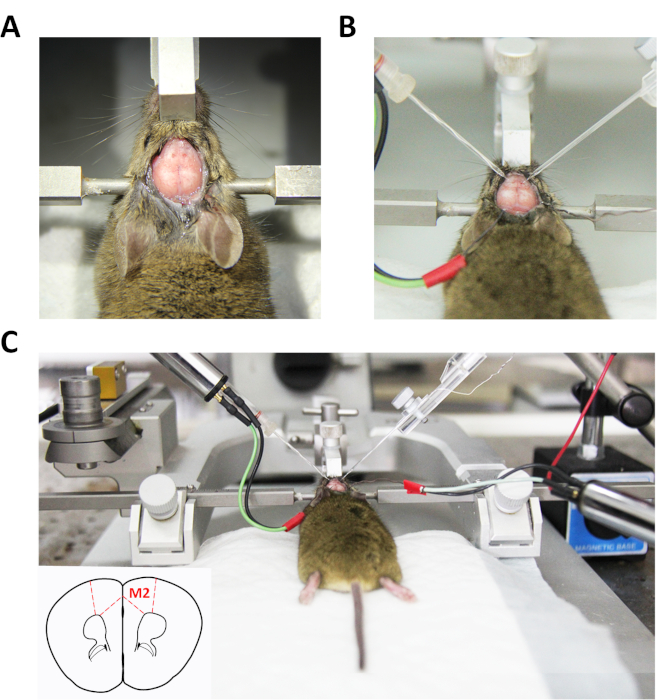
Figure 1: Diagram of the simultaneous LFP recording procedure. (A) Stereotaxic mouse with skull exposed and dura mater removed for in vivo bilateral recording of LFPs in left and right M2. (B) Two glass microelectrodes in touch with the cortical surface in the hole drilled simultaneously. (C) Recording microelectrodes along with the Ag/AgCl wires as reference electrodes positioned at appropriate sites. Please click here to view a larger version of this figure.
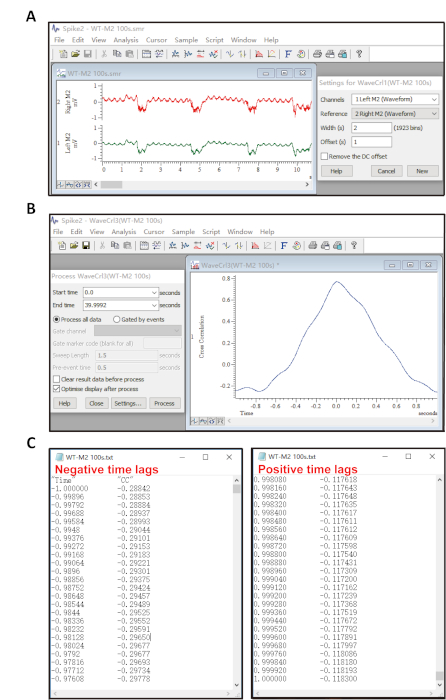
Figure 2: Illustration of cross-correlation analysis. (A) Settings for the waveform correlation dialog box. This provides options for choosing which waveform channel is the reference and for analyzing the correlation of two signals. (B) The process dialog box. This provides options for setting the time length of the reference waveform and the duration of another waveform will be appended. The analysis is only done for regions of data in which both waveform channels exist. (C) Example .txt file with values of cross-correlation at negative and positive time lag ranges separately for statistics. Please click here to view a larger version of this figure.
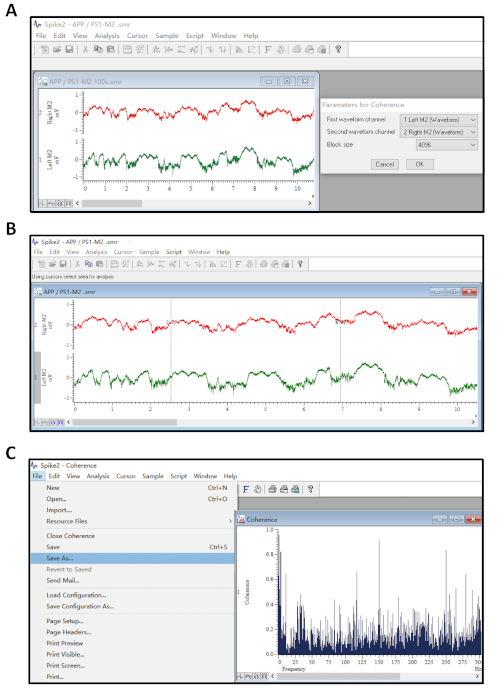
Figure 3: Illustration of coherence analysis. (A) Parameter settings for the coherence dialog box. The block size determines the number of data points used in the analysis, and the frequency resolution. (B) The dotted lines are adjustable for operator to move manually in order to set the duration of signals for analyzing. (C) After the software has created a chart, click File – Save As to save the coherence results as a file with a .txt filename extension . Please click here to view a larger version of this figure.
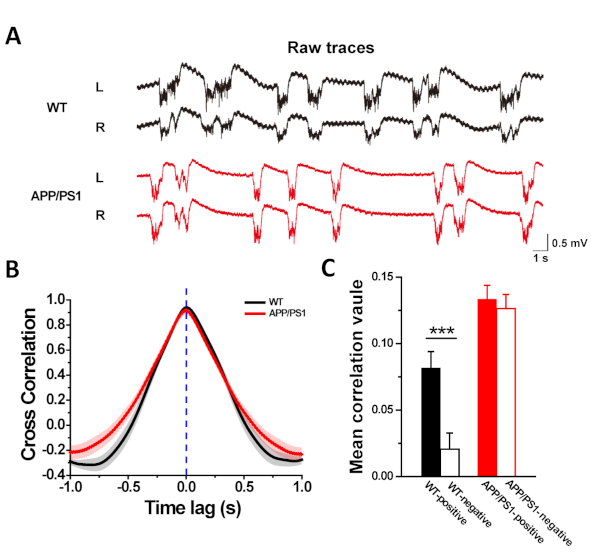
Figure 4: Cross-correlation indicates the declined hemisphere lateralization between left and right M2 of APP/PS1 mice. (A) Representative raw traces of LFPs recorded simultaneously in bilateral M2 of WT and APP/PS1 mice using extracellular recording method (L: left M2; R: right M2). (B) The cross correlation curve shows correlation of bilateral LFP signals at different time lags. (C) Between left and right M2, WT controls showed significantly higher cross-correlation value at positive time lag ranges than negative ones. In contrast, the cross-correlation value of APP/PS1 mice has a similarity, indicating a decline of asymmetry (n = 10, per group). Value represents mean ± standard error of the mean. ***p < 0.001; two sample t-test. Please click here to view a larger version of this figure.

Figure 5: Coherence of gamma oscillations between left and right M2 of WT and APP/PS1 mice. (A) Representative traces of gamma oscillations filtered from LFPs in left and right M2. (B) Coherence distribution between LFPs simultaneously recorded in bilateral M2. APP/PS1 mice differ largely from WT controls in gamma frequency range. (C) The coherence between gamma oscillations of bilateral M2 in
APP/PS1 mice are significantly higher than WT controls (n = 10, per group). Value represents mean ± standard error of the mean. **, p < 0.01; two sample t-test. Please click here to view a larger version of this figure.
Discussion
We report here the procedure for in vivo bilateral extracellular recording, along with analyzing the synchronization of dual-region LFP signals, which is both flexible and easy to conduct for estimating brain hemisphere lateralization, as well as the connectivity, directionality or coupling between neural activities of two brain areas. This can be widely used to reveal not only group-neuronal activities, but also some basic properties of interregional electrophysiology, especially for labs which are interested in screening oscillatory activities or labs which do not have systems for multi-channel recording in behaving animals23.
In general, a series of techniques are available to monitor brain activities, including electroencephalography (EEG), magnetoencephalography (MEG), and functional magnetic resonance imaging (fMRI). Such methods have relatively lower temporal and spatial resolution in comparison with our presented recordings. For example, EEG is one of the oldest and most commercially available instruments for investigating extracellular activity of the brain. Although there are studies using "high density" EEG in freely moving rodents to improve the insufficient spatial resolution24,25,26, the skull always generates more noise and thus reduces the signal-to-noise ratio of cortical gamma oscillation, especially for small-sized mice. Our method with glass microelectrodes would be a good choice to prevent researchers from that "distorting noise" since microelectrodes could be inserted into the brain structure directly. Moreover, the recording glass pipettes used here are inexpensive, highly maneuverable, and can be applied to explore deeper brain areas not limited to cortical areas.
Close attention should be paid to the following. First, it is mandatory to carry out anesthesia strictly based on the body weight, and to test the depth of anesthesia hourly. This is because the physiological state of the mouse plays an important role in the quality of the LFP recorded, and any movement of the referencing sites caused by, e.g., sudden awakening of the animal, could generate background electrophysiological noise that would depreciate the availability. Second, because microelectrode resistance varies with the shape and diameter of the glass pipette tip, the heating must be carefully adjusted within the range for appropriate impedance when pulling microelectrodes. As described earlier in the protocol section, we found that the electrodes with impedance ranging from 1 to 2 MΩ captured high qualitied cortical oscillatory activities.
Gamma oscillations reflect the neuronal synchronization of different brain regions when animals are engaged in learning or stimulation-cued tasks27,28,29. The synchronization of gamma-band modulates excitation rapidly to activate postsynaptic neurons effectively30. It is worth noting that although the gamma oscillation was defined in the present study as oscillatory activity with frequency in the range 25-80 Hz as shown by several groups28,31,32, there are studies that describe 30-70 Hz as low gamma and 70-100 Hz as high gamma33,34,35. Regardless of the definition, the principles for data analysis remain similar. In signal processing, cross-correlation is used for determining the time delay between electrical signals of two brain regions36. For signals under stimulation conditions, the durations selected for cross-correlation analysis could be shorter37.
Though there are limitations in the use of LFP recording for the evaluation of neural activities; for instance, it can neither distinguish between pre- and post-synaptic activities nor detect resting membrane potentials of the neurons recorded23, the approach introduced here serves as a useful tool for the measurement of activities of a group of neurons from different brain areas of mice, allowing the investigation of brain-area functional connectivity and the coupling of electrical signals before and after drug infusion.
Several explanations have been proposed for the emergence of hemispheric asymmetry, e.g., asymmetry enhances an individual's ability to perform two different tasks at the same time38; or asymmetry increases neural capacity, avoiding unnecessary duplication of neural networks39; or two different cognitive processes may be more readily performed simultaneously if they are lateralized to different hemispheres40. Hemisphere lateralization is assumed to provide cognitive advantages, but it changes with age12,41. Neuroimaging studies have shown consistently that prefrontal activation tends to be less lateralized in older adults than in younger individuals42,43. AD patients with early unilateral or bilateral pathological changes develop brain abnormalities, including lateralization associating with forgetfulness, slow responses to sound stimulation and cognitive decline11,44. We observed, in the present study, a disrupted level of hemisphere lateralization between left and right M2 of APP/PS1 mice at 3-5 months, which is the period when such mice do not aggregate apparent deposition of beta amyloid plaques45,46, implying that toxicity induced by soluble beta amyloid oligomers may contribute, at least in part, to aberrant cortical hemisphere lateralization, which could accelerate brain deterioration in AD pathogenesis16,47.
Divulgaciones
The authors have nothing to disclose.
Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (31771219, 31871170), the Science and Technology Division of Guangdong (2013KJCX0054), and the Natural Science Foundation of Guangdong Province (2014A030313418, 2014A030313440).
Materials
| AC/DC Differential Amplifier | A-M Systems | Model 3000 | |
| Analog Digital converter | Cambridge Electronic Design Ltd. | Micro1401 | |
| Glass borosilicate micropipettes | Nanjing spring teaching experimental equipment company | 161230 | Outer diameter: 1.0mm |
| Microelectrode puller | Narishige | PC-10 | |
| NaCl | Guangzhou Chemical Reagent Factory | 7647-14-5 | |
| Pin microelectrode holder | World Precision Instruments, INC. | MEH3SW10 | |
| Spike2 | Cambridge Electronic Design Ltd. | ||
| Stereomicroscope | Zeiss | 435064-9020-000 | |
| Stereotaxic apparatus | RWD Life Science | 68045 | |
| Urethane | Sigma-Aldrich | 94300 |
Referencias
- Goedert, M., Spillantini, M. G. A century of Alzheimer’s disease. Science. 314 (5800), 777-781 (2006).
- Perrin, R. J., Fagan, A. M., Holtzman, D. M. Multimodal techniques for diagnosis and prognosis of Alzheimer’s disease. Nature. 461 (7266), 916-922 (2009).
- Cummings, B. J., Pike, C. J., Shankle, R., Cotman, C. W. Beta-amyloid deposition and other measures of neuropathology predict cognitive status in Alzheimer’s disease. Neurobiology of aging. 17 (6), 921-933 (1996).
- Gordon, M. N., et al. Correlation between cognitive deficits and Abeta deposits in transgenic APP+PS1 mice. Neurobiology of aging. 22 (3), 377-385 (2001).
- Fitzpatrick, A. W. P., et al. Cryo-EM structures of tau filaments from Alzheimer’s disease. Nature. 547 (7662), 185-190 (2017).
- Shankar, G. M., et al. Amyloid-beta protein dimers isolated directly from Alzheimer’s brains impair synaptic plasticity and memory. Nature medicine. 14 (8), 837-842 (2008).
- Buchman, A. S., Bennett, D. A. Loss of motor function in preclinical Alzheimer’s disease. Expert review of neurotherapeutics. 11 (5), 665-676 (2011).
- Arnold, S. E., Hyman, B. T., Flory, J., Damasio, A. R., Van Hoesen, G. W. The topographical and neuroanatomical distribution of neurofibrillary tangles and neuritic plaques in the cerebral cortex of patients with Alzheimer’s disease. Cerebral cortex. 1 (1), 103-116 (1991).
- Giannakopoulos, P., Hof, P. R., Michel, J. P., Guimon, J., Bouras, C. Cerebral cortex pathology in aging and Alzheimer’s disease: a quantitative survey of large hospital-based geriatric and psychiatric cohorts. Brain research. Brain research reviews. 25 (2), 217-245 (1997).
- Renteria, M. E. Cerebral asymmetry: a quantitative, multifactorial, and plastic brain phenotype. Twin research and human genetics : the official journal of the International Society for Twin Studies. 15 (3), 401-413 (2012).
- Derflinger, S., et al. Grey-matter atrophy in Alzheimer’s disease is asymmetric but not lateralized. Journal of Alzheimer’s disease : JAD. 25 (2), 347-357 (2011).
- Abdul Manan, H., Yusoff, A. N., Franz, E. A., Sarah Mukari, S. Z. Early and Late Shift of Brain Laterality in STG, HG, and Cerebellum with Normal Aging during a Short-Term Memory Task. ISRN neurology. 2013, 892072 (2013).
- Kim, S. G., et al. Functional magnetic resonance imaging of motor cortex: hemispheric asymmetry and handedness. Science. 261 (5121), 615-617 (1993).
- Bartolomeo, P., D’Erme, P., Perri, R., Gainotti, G. Perception and action in hemispatial neglect. Neuropsychologia. 36 (3), 227-237 (1998).
- Bartolomeo, P., et al. Right-side neglect in Alzheimer’s disease. Neurology. 51 (4), 1207-1209 (1998).
- Thompson, P. M., et al. Tracking Alzheimer’s disease. Annals of the New York Academy of Sciences. 1097, 183-214 (2007).
- Cabeza, R., Anderson, N. D., Locantore, J. K., McIntosh, A. R. Aging gracefully: compensatory brain activity in high-performing older adults. NeuroImage. 17 (3), 1394-1402 (2002).
- Bellis, T. J., Nicol, T., Kraus, N. Aging affects hemispheric asymmetry in the neural representation of speech sounds. The Journal of neuroscience : the official journal of the Society for Neuroscience. 20 (2), 791-797 (2000).
- Jankowsky, J. L., et al. Co-expression of multiple transgenes in mouse CNS: a comparison of strategies. Biomolecular engineering. 17 (6), 157-165 (2001).
- Venegas, C., et al. Microglia-derived ASC specks cross-seed amyloid-beta in Alzheimer’s disease. Nature. 552 (7685), 355-361 (2017).
- Busche, M. A., et al. Tau impairs neural circuits, dominating amyloid-beta effects, in Alzheimer models in vivo. Nat Neurosci. 22 (1), 57-64 (2019).
- Velazquez, R., et al. Maternal choline supplementation ameliorates Alzheimer’s disease pathology by reducing brain homocysteine levels across multiple generations. Molecular Psychiatry. , (2019).
- Huo, Q., et al. Prefrontal Cortical GABAergic Dysfunction Contributes to Aberrant UP-State Duration in APP Knockout Mice. Cerebral Cortex. 27 (8), 4060-4072 (2017).
- Palop, J. J., et al. Aberrant excitatory neuronal activity and compensatory remodeling of inhibitory hippocampal circuits in mouse models of Alzheimer’s disease. Neuron. 55 (5), 697-711 (2007).
- Ang, G., et al. Absent sleep EEG spindle activity in GluA1 (Gria1) knockout mice: relevance to neuropsychiatric disorders. Translational Psychiatry. 8 (1), 154 (2018).
- Funk, C. M., Honjoh, S., Rodriguez, A. V., Cirelli, C., Tononi, G. Local Slow Waves in Superficial Layers of Primary Cortical Areas during REM Sleep. Current Biology. 26 (3), 396-403 (2016).
- Gregoriou, G. G., Gotts, S. J., Zhou, H., Desimone, R. High-frequency, long-range coupling between prefrontal and visual cortex during attention. Science. 324 (5931), 1207-1210 (2009).
- Zheng, C., Bieri, K. W., Hsiao, Y. T., Colgin, L. L. Spatial Sequence Coding Differs during Slow and Fast Gamma Rhythms in the Hippocampus. Neuron. 89 (2), 398-408 (2016).
- Freeman, W. J., Holmes, M. D., West, G. A., Vanhatalo, S. Fine spatiotemporal structure of phase in human intracranial EEG. Clinical neurophysiology : official journal of the International Federation of Clinical Neurophysiology. 117 (6), 1228-1243 (2006).
- Fries, P. Rhythms for Cognition: Communication through Coherence. Neuron. 88 (1), 220-235 (2015).
- Cardin, J. A., et al. Driving fast-spiking cells induces gamma rhythm and controls sensory responses. Nature. 459 (7247), 663-667 (2009).
- Verret, L., et al. Inhibitory interneuron deficit links altered network activity and cognitive dysfunction in Alzheimer model. Cell. 149 (3), 708-721 (2012).
- Ahlbeck, J., Song, L., Chini, M., Bitzenhofer, S. H., Hanganu-Opatz, I. L. Glutamatergic drive along the septo-temporal axis of hippocampus boosts prelimbic oscillations in the neonatal mouse. Elife. 7, (2018).
- Spellman, T., et al. Hippocampal-prefrontal input supports spatial encoding in working memory. Nature. 522 (7556), 309-314 (2015).
- Vandecasteele, M., et al. Optogenetic activation of septal cholinergic neurons suppresses sharp wave ripples and enhances theta oscillations in the hippocampus. Proceedings of the National Academy of Sciences of the United States of America. 111 (37), 13535-13540 (2014).
- Seidenbecher, T., Laxmi, T. R., Stork, O., Pape, H. C. Amygdalar and hippocampal theta rhythm synchronization during fear memory retrieval. Science. 301 (5634), 846-850 (2003).
- Zitnik, G. A., Curtis, A. L., Wood, S. K., Arner, J., Valentino, R. J. Adolescent Social Stress Produces an Enduring Activation of the Rat Locus Coeruleus and Alters its Coherence with the Prefrontal Cortex. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 41 (5), 1376-1385 (2015).
- Rogers, L. J., Zucca, P., Vallortigara, G. Advantages of having a lateralized brain. Proceedings. Biological sciences / The Royal Society. 271, 420-422 (2004).
- Vallortigara, G. The evolutionary psychology of left and right: costs and benefits of lateralization. Developmental psychobiology. 48 (6), 418-427 (2006).
- MacNeilage, P. F., Rogers, L. J., Vallortigara, G. Origins of the left, right brain. Scientific American. 301 (1), 60-67 (2009).
- Habas, P. A., et al. Early folding patterns and asymmetries of the normal human brain detected from in utero MRI. Cerebral cortex. 22 (1), 13-25 (2012).
- Dennis, N. A., Kim, H., Cabeza, R. Effects of aging on true and false memory formation: an fMRI study. Neuropsychologia. 45 (14), 3157-3166 (2007).
- Cabeza, R., et al. Task-independent and task-specific age effects on brain activity during working memory, visual attention and episodic retrieval. Cerebral cortex. 14 (4), 364-375 (2004).
- Cherbuin, N., Reglade-Meslin, C., Kumar, R., Sachdev, P., Anstey, K. J. Mild Cognitive Disorders are Associated with Different Patterns of Brain asymmetry than Normal Aging: The PATH through Life Study. Frontiers in psychiatry / Frontiers Research Foundation. 1, 11 (2010).
- Jankowsky, J. L., et al. Mutant presenilins specifically elevate the levels of the 42 residue beta-amyloid peptide in vivo: evidence for augmentation of a 42-specific gamma secretase. Human molecular genetics. 13 (2), 159-170 (2004).
- Radde, R., et al. Abeta42-driven cerebral amyloidosis in transgenic mice reveals early and robust pathology. EMBO reports. 7 (9), 940-946 (2006).
- Lacor, P. N., et al. Abeta oligomer-induced aberrations in synapse composition, shape, and density provide a molecular basis for loss of connectivity in Alzheimer’s disease. The Journal of neuroscience : the official journal of the Society for Neuroscience. 27 (4), 796-807 (2007).

