Technical Aspect of the Automated Synthesis and Real-Time Kinetic Evaluation of [11C]SNAP-7941
Summary
Here, we represent a protocol for the fully automated radiolabelling of [11C]SNAP-7941 and the analysis of the real-time kinetics of this PET-tracer on P-gp expressing and non-expressing cells.
Abstract
Positron emission tomography (PET) is an essential molecular imaging technique providing insights into pathways and using specific targeted radioligands for in vivo investigations. Within this protocol, a robust and reliable remote-controlled radiosynthesis of [11C]SNAP-7941, an antagonist to the melanin-concentrating hormone receptor 1, is described. The radiosynthesis starts with cyclotron produced [11C]CO2 that is subsequently further reacted via a gas-phase transition to [11C]CH3OTf. Then, this reactive intermediate is introduced to the precursor solution and forms the respective radiotracer. Chemical as well as the radiochemical purity are determined by means of RP-HPLC, routinely implemented in the radiopharmaceutical quality control process. Additionally, the molar activity is calculated as it is a necessity for the following real-time kinetic investigations. Furthermore, [11C]SNAP-7941 is applied to MDCKII-WT and MDCKII-hMDR1 cells for evaluating the impact of P-glycoprotein (P-gp) expression on cell accumulation. For this reason, the P-gp expressing cell line (MDCKII-hMDR1) is either used without or with blocking prior to experiments by means of the P-gp substrate (±)-verapamil and the results are compared to the ones observed for the wildtype cells. The overall experimental approach demonstrates the importance of a precise time-management that is essential for every preclinical and clinical study using PET tracers radiolabelled with short-lived nuclides, such as carbon-11 (half-life: 20 min).
Introduction
[11C]SNAP-7941 was evolved as the first positron emission tomography (PET)-tracer targeting the melanin-concentrating hormone receptor 1 (MCHR1) – a receptor mainly involved in the central regulation of appetite and food intake1. Carbon-11 labelling of SNAP-7941, a well characterised MCHR1 antagonist, yielded the authentic PET-tracer2,3,4,5. However, fully automated radiosynthesis is highly challenging in terms of time efficacy and reproducibility with the short-lived radionuclide carbon-11 offering a half-life of 20 min6. The overall synthesis time should be kept to a minimum, and as a rule of thumb should not exceed 2-3 half-lives (i.e., around 40-60 min for carbon-11)7. Especially, synthesis procedures for radiotracers targeting receptor systems with low expression densities must be extensively optimized to obtain sufficient yields and consequently high molar activity8. The synthetic strategy often follows the radionuclide production within a cyclotron and release of [11C]CO2 to the synthesizer. There, [11C]CO2 is first reduced to [11C]CH4 and subsequently reacted with iodine to yield [11C]CH3I via the gas-phase method9,10. Further treatment with silver triflate yields [11C]CH3OTf directly on-line. Afterwards, this reactive carbon-11 labelled intermediate is introduced into a solution containing the precursor molecule. An automated radiosynthesis additionally involves a purification process with semi-preparative RP-HPLC including subsequent formulation of the product suitable for preclinical and clinical studies.
Regardless of the half-life of the radionuclide and the time effort of the radiosynthesis, the pharmacokinetic of a radiopharmaceutical is the most critical part to be evaluated during PET-tracer development. In terms of neuroimaging, brain entry of the PET-tracer is the main prerequisite. However, the blood brain barrier (BBB), a "security border" of the brain, highly expresses efflux transporters that can unload small molecules (e.g., PET-tracers) and efficiently hamper their applicability.
A huge drawback during preclinical evaluation are unexpected interactions towards these efflux transporters, which are often unrecognized in in vitro experiments and leading to failure of the PET-tracer in vivo, as observed for [11C]SNAP-7941. µPET imaging in rats demonstrated low brain accumulation, which increased dramatically after administration of the P-gp inhibitor tariquidar11. These data suggested that [11C]SNAP-7941 is a substrate of this efflux transporter system impeding ligand binding to central MCHR1. Unfortunately, there is still a lack of adequate in vitro models enabling the prediction of BBB penetration in an early stage of tracer development.
Here, we describe the automated synthesis of [11C]SNAP-7941 using a synthesizer for carbon-11 methylations. The emphasis of this work is to give an overview on how to organize a consecutive experimental approach including the automated synthesis, quality control as well as successive in vitro evaluation with the very short-lived nuclide carbon-11.
First, the key steps for a successful radiosynthesis with minimal time expenditure and maximal yield are described. Then, a reliable quality control procedure is set up making the radiotracer available for potential clinical studies and meeting the criteria of the European Pharmacopoeia12. Quantification of the molar concentration and calculation of the respective molar activity is an essential requirement for the successive kinetic measurements.
Finally, a new and straightforward in vitro method evaluating [11C]SNAP-7941's interactions towards the efflux transporter, P-gp (hMDR1), is presented. The proposed kinetic model uses an easy-to-handle device that allows an immediate data interpretation and requires minimal cell culture effort13.
Protocol
CAUTION: In the following protocol are multiple steps involved that require handling and manipulation of radioactivity. It is important that every step is in agreement with the Radiation Safety Department of the institute and the respective national legislature. It is mandatory to minimise exposure to ionizing radiation for the operators involved following the ALARA (“As Low As Reasonably Achievable”) principle.
1. Time management and planning of the experiment
NOTE: The short half-life of carbon-11 requires an accurate time management to minimize loss of radioactivity (Figure 1). It is important that any person involved knows their area of responsibility and the time point of the respective action. For the set up of a real-time kinetic experiment of [11C]SNAP-7941, around four persons are necessary for a smooth process.
- Organize a producer for [11C]SNAP-7941.
- Inform the quality control operator performing the specification evaluation, calculation of mass concentration and molar activity of the synthesis start time.
- Hold the real-time kinetic experimenter ready for dilution calculation and performing the experiment.
- Instruct the runner for transferring the radioactivity to the respective place at the respective time point.
2. Automated synthesis of [11C]SNAP-7941 for preclinical use
- Preparation of the synthesizer (Figure 2).
- Turn on the synthesizer, required technical gases (helium and hydrogen) and the synthesizer control software Tracerlab. Select the respective synthesis sequence snap.
- Wash V1-V3 once with water and twice with acetone via the reactor and HPLC valve either on load or inject position into the loop waste bottle.
- Dry all associated lines into and out of the reactor via the injection valve with a continuous helium flow activated via V18.
- Heat the [11C]CH3I trap as well as the [11C]CH3OTf trap under a helium stream of 100 mL·min-1 up to 200 °C via V24, V25, V29, V15, V9, V17 and V32/V33 with detached reactor.
- Check the same pathway for leakages (with attached reactor) with a helium flow of 100 mL·min-1. Tightness is guaranteed when the flow falls below 4 mL·min-1.
- Turn on the HPLC pump (5-10 mL·min-1) and wash the HPLC lines and injection valve with acetonitrile/water (50/50, v/v) as well as the HPLC lines with the HPLC solvent via V14 into the bulb.
- Empty the bulb via V11 and V12 into the waste bottle with a helium stream via V19. Wash the respective lines with water from V6 again via V11 and V12 with a helium stream via V18.
- Wash V5 with ethanol and V4 with water via V11 and V12 into the product collection vial (PCV) using a helium stream via V18, then empty the PCV via V13 in to the waste bottle with a helium stream via V20.
- Turn the HPLC pump off, switch to the solvent for the synthesis ((aqueous ammonium acetate (2.5 g·L-1)/acetic acid 97.5/2.5 v/v; pH 3.5)/acetonitrile 75/25 v/v), attach the semi-preparative HPLC column and equilibrate the column (flow of 5 mL·min-1).
- Fill in the reagents for the synthesis and purification: 1.5 mL of water (V2), 4.5 mL of 0.9% NaCl (V4), 0.5 mL of 96% ethanol (V5), 10 mL of water (V6), and 90 mL of water (bulb).
- Attach a new loop waste vial; a product vial (labelled and equipped with an aeration needle) to V13 and the liquid nitrogen.
- Precondition a SPE (solid phase extraction) cartridge with 10 mL of 96% ethanol and 20 mL of water and attach it between V11 and V12.
- Start the pre-run sequence 20 min before starting the synthesis, consisting of heating the [11C]CO2 trap to 400 °C and flushing the trap with a helium stream of 50 mL·min-1 via V27 (2 min). Then, pre-flush the [11C]CO2 trap for 3 min with hydrogen gas (120 mL·min-1) and finally cool down to below 40 °C.
- Dissolve 1 mg of precursor in 400 µL of acetonitrile (for DNA synthesis, < 10 H2O), transfer the solution to the reaction and add 5.5 µL of TBAH (264 mg·mL-1 in methanol). Attach the reactor to its respective position.
- Close the hot cell and turn on the under pressure of the hot cell.
- Confirm the start of the cooling process of the CH4 trap with liquid nitrogen to -75 °C.
- Release the radioactivity when the temperature is reached.
- Cyclotron production of [11C]CO2
NOTE: Steps 2.2.1-2.2.4 are conducted simultaneously to the preparation of the radiosynthesis module (see also Figure 1).- Turn on the magnet of the cyclotron.
- Select the target 11C-CO2 and start the beam 65 µA.
- Confirm the start of the irradiation by pushing the button Start irradiation
- Stop the beam and confirm the release of radioactivity into the synthesizer by pushing the button Delivery, after the desired amount of radioactivity is reached (approx. 120 GBq after 30 min).
- Execution of the fully-automated radiosynthesis
- Confirm that the system is ready to receive the radioactivity and start the automated process
- Monitoring the following automated process.
- Check if the [11C]CO2 is automatically transferred from the cyclotron into the synthesis unit via V26 (approx. 4 min) and that the [11C]CO2 is trapped on the molecular sieve impregnated with nickel as a catalyst ([11C]CO2 trap) at room temperature.
- Track if the [11C]CO2 trap is automatically flushed first with helium (50 mL·min-1) and then with hydrogen gas (120 mL·min-1). Then, observe that V27 and V25 are closed and the trapped [11C]CO2 including the hydrogen gas is heated to 400 °C and that the formed [11C]CH4 is further transported to the pre-cooled [11C]CH4 trap and trapped there.
- Monitor that V10 is closed, V9 opened (towards the iodine column) and [11C]CH4 is again released by heating the [11C]CH4 trap slowly to 80 °C and transported into the circulation unit with a helium stream via V10 (exhaust exit). And further check if [11C]CH4 is converted to [11C]CH3I within the circulation unit, which last for approx. 3 min and the formed [11C]CH3I is continuously trapped on the [11C]CH3I trap.
- Make sure that the exhaust valve V16 is opened and the [11C]CH3I trap is heated to 90 °C with a stream of helium (20 mL·min-1) via V24. Hereby, possible byproducts are removed and then V16 is closed.
- Confirm that the [11C]CH3I is ready to be released into the reactor.
- Monitor the following automated process: Check if the [11C]CH3I trap is heated to 190 °C and the formed [11C]CH3I is released into the reactor via the [11C]CH3OTf trap (V32 and V33, still at 200 °C), V7, and V8 under a continuous helium stream (10-25 mL·min-1, V24). During this process, V21 and V23 (exhaust exit) have to be open.
- Confirm that the radioactivity arrived in the reactor, once the amount of radioactivity in the reactor is not raising anymore.
- Monitoring the following automated process and preparing for manual intervention, if necessary
- Check if V23, V21, and V8 are automatically closed and the reaction mixture is heated to 75 °C. After 3 min, observe if the reactor is cooled with liquid nitrogen until the temperature is below 35 °C. Subsequently, make sure that the reaction mixture is quenched with 1.5 mL of water via V2. During this process, V21 and V23 (exhaust exit) have to be open.
- Track if V21 and V23 are closed and the reaction mixture is transferred to the HPLC injection valve by passing through a fluid detector into the HPLC loop (5 mL). Track if the reaction mixture is automatically injected onto the HPLC column.
- Observe the chromatogram and cut manually the product peak via the button Peak start. Press Peak end when the product peak signal falls below approximately 400 cps after a retention time of around 8-10 min (flow: 5 mL·min-1, Figure 3).
- Turn on an additional helium stream to accelerate the bulb emptying.
- Monitor whether the bulb is emptying and observe the waste bottle: Check if the bulb is emptied via V11, the SPE cartridge and V12 into the waste with a helium stream via V19 and an additional helium line and if the product retains on the SPE cartridge.
- Confirm that the bulb is completely empty.
- Monitoring the automated process of SPE purification and product transfer
- Check if the SPE cartridge is washed with 10 mL of water via V6, V11, and V12 into the waste and if ethanol elutes the product into the PCV via V5, V11, and V12. Finally, observe if 0.9% NaCl is added into the PCV via V4, V11 and V12 to dilute the product.
- Make sure that the product solution is transferred via V13 and a sterile filter into the final product vial with a helium stream via V20.
- Confirm that the product has been completely transferred.
3. Quality control (QC)
NOTE: The quality control of radiopharmaceuticals includes measuring the following parameters:
- Radiochemical purity and molar activity (RP-HPLC)
- Residual solvents (gas chromatography, GC)
- Osmolality (vapor pressure osmometer)
- pH (pH-meter)
- γ spectrum/radionuclide purity (γ-spectrometer)
- Half-life/radionuclide purity (dose calibrator)
All physico-chemical parameters are determined prior to release of the product and the values have to be in the defined quality parameter range.
- Preparation of the quality control equipment
- Start the preparation 30 min prior the end of the synthesis.
- Prepare a conical vial in a lead shielded container and a shielded 1 mL syringe with an attached needle.
- Prepare a reference standard solution of SNAP-7941 (10 µg·mL-1) in water for HPLC.
- Turn on the pH meter, the gas chromatograph and the used gases, the osmometer and all components of the HPLC.
- Turn on the HPLC controlling software and select the dedicated column and the respective method for [11C]SNAP-7941 (mobile phase: (water/acetic acid 97.5/ 2.5 v/v; 2.5 g.L-1 ammonium acetate; pH 3.5)/acetonitrile 70/30 v/v; flow: 1 mL·min-1) and write a sample list with two measurements (standard and product). Start the method for conditioning of the column.
- Inject 20 µL of the SNAP-7941 reference solution with a Hamilton syringe after 10 min conditioning and start the analysis run.
- Wash the Hamilton syringe two times with acetonitrile and water after injection.
- Start the GC control software and write the sample list.
- Performing quality control
- Measure the absolute radioactive yield of the product after the end of synthesis and transfer 100 µL of the product into a reaction vial with the prepared lead-shielded syringe for quality control.
NOTE: All data obtained from the quality control has to be documented in accordance with the national regulations. - Analytical HPLC: Measure the radiochemical purity and chemical purity and inform the person in charge for the cell culture experiments immediately of the results.
- Draw 40 µL of the QC-sample and inject to the HPLC. Start the run by moving the arm of the injection valve from Load a Inject (Figure 3).
NOTE: During the run (8 min), other measurements of the protocol can be performed to avoid as much decay as possible. - Open the chromatogram and integrate all peaks in the radioactivity channel. Compare the retention time of the product (5.0-7.0 min) with the retention time of the reference standard. Compare the area under the curve in percent of all integrated peaks. The product peak has to be more than 95% (radiochemical purity) of the total integrated areas (radio channel).
- Integrate the signal in the UV-channel to determine chemical purity. The main impurity is usually the precursor SNAP acid at 2.8-3.5 min. The concentration of [natC]SNAP-7941 is calculated from the calibration curve after integration. Calculate the concentration of SNAP-7941 in µmol·mL-1 and determine the molar activity by means of the following equation:
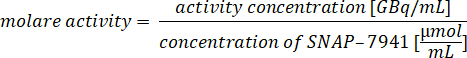
- Draw 40 µL of the QC-sample and inject to the HPLC. Start the run by moving the arm of the injection valve from Load a Inject (Figure 3).
- For gas chromatography, transfer 20 µL of the quality control sample in a dedicated glass vial. Place it in the autosampler and start the measurement. After the measurement is finished, open the chromatogram and write down the numbers of automatically integrated peaks. The sample should not contain more than 410 ppm acetonitrile and 10% ethanol.
- Osmolality: Put a paper sample disc on the dedicated hollow and apply 10 µL of the sample. Press the Close button for measuring the osmolality. After 3 min, the device displays the osmolality that has to be in a range of 190-500 mosm·kg-1.
- pH: Wash the electrode with water. Measure the pH by putting the electrode in the sample. The pH has to be in a range of 5.0-8.5. Wash the electrode again after measurement and place the electrode in the reference pH solution.
- γ-spectrum: Put the QC sample in the γ-spectrometer, open the controlling software and start the measurement. Stop the measurement and write down the measured energy that has to be 511 keV (range 420-520 keV). Open the file menu and safe the file with the respective batch number. Recall the saved spectrum in the dedicated software and print the spectrum.
- Half-life: Measure the activity of the QC sample two times by using the dose calibrator. Write down the respective times and calculate the half-life of the exponential.
- Measure the absolute radioactive yield of the product after the end of synthesis and transfer 100 µL of the product into a reaction vial with the prepared lead-shielded syringe for quality control.
- Release
- After all parameters have been tested and the results are in accordance with the guidelines of the Austrian authorities, release the radiotracer. The operator has to sign the correctness of the data.
4. Evaluation of interactions towards the P-gp transporter
- Cell Culture
- Start the laminar air flow (LAF) of the workbench and clean the bench at least 15 min before starting to work.
- Mix the cell culture medium under sterile conditions in the LAF with 10% of FCS (50 mL) and 0.5% of antibiotics (2.5 mL) with a sterile volumetric pipette using an automated pipetting aid.
- Thaw MDCKII-hMDR1 and MDCKII-WT cells and cultivate in a T75 or T175 cell culture flask 10-14 days in advance of experiments under aseptic conditions (LAF).
- Change the medium on the next day to remove all not adherent and apoptotic cells.
- Replace every three days the medium with fresh one (12 mL for the T75 and 23 mL for the T175 cell culture flask, respectively).
- Split the cells according to the time schedule of experiments to fresh flasks or to cell culture dishes upon a confluence of 80% to avoid bilayer growing.
- Cell preparation for experiments
- Split the cells two days before experiments and seed in a concentration of 2.5 x 105 cells per 2 mL in an oblique plane of a cell culture dish (Figure 4). Use an automatic cell counter device or a Neubauer counting chamber to count the cells and calculate the appropriate splitting ratio.
- Remove the medium one day before experiments, wash the cells on the culture dish with 4 mL of DPBS and add fresh 5 mL of cell culture medium. Place the dish horizontally in the incubator.
- Wash the cells with DPBS at least 0.5 h before experiments and replace with 2 mL of FBS free medium. Examine the confluence and cell morphology with a microscope.
- Performing the experiments in three different setups.
- Use the Petri dish for the experiments as described in 4.2.3.
- Treat the cells for 0.5 h before experiments with 10 µM (±)-verapamil hydrochloride.
- Add 0.98 µL of verapamil stock solution (20.4 mM (±)-verapamil hydrochloride in DMSO). Final DMSO content is ≤0.05% during treatment.
- Incubate the cells for 0.5 h with the vehicle control (DMSO). Use the same volume as used for the drug treatment.
- Experiments of the real-time assay (for all three setups)
- Turn on the device, the computer and make sure that they are correctly connected, then open the control software.
- Choose the One target option, unlock the template and adjust the following setting:
Number of positions: 2 (two)
Detection time (sec): 3 (three)
Detection delay time (s): 2 (two)
Name of the first phase: baseline - Insert the cell culture dish into the inclined support of the device, making sure that the cell pole is on the bottom side and covered with cell culture medium.
- Start the run via Start button. The system is asking to save the file. Choose a file name and a saving path. The control center pops up and the baseline measurement begins (the cell culture dish support starts to rotate).
- Select under Other options:
Time scale: minutes
Nuclide correction: carbon-11 - Check all the boxes under Curves in graph (background (red), target (blue) and target minus background (black)).
- Obtain quality control parameters: molar activity [GBq.µmol-1] and activity concentration [MBq.mL-1] at the end of synthesis.
- Calculate the respective tracer volume for 15 nM of [natC]SNAP-7941 in the assay volume of 2 mL.
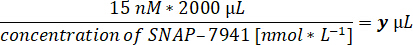
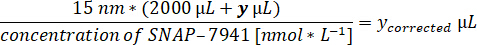
- Prepare the pipette to the respective volume and an aliquot of the [11C]SNAP-7941 product in a lead shielding.
- Click Pause run in the control center window. Wait until dish rotation stops and the side bar with the title Paused and next phase occur.
- Open the lid of the real-time kinetic device. Turn the culture dish support 90° to the left. Add the calculated volume of tracer and turn back to initial position. Then, close the lid of the device. Press immediately the Continuar button for starting the experiment.
- End the experiment after 20 min by selecting pause and subsequently terminate the run (press End run in the side bar).
- Data analysis and processing using a statistic software
- Open the real-time control software. Click on Open files to recall the required kinetics. The kinetics is illustrated in the control center window.
- Choose by right-click directly on the kinetics Export curves. Save the text file containing the raw data. Open the statistics program and paste the raw data file of real-time experiments.
- Start with the time (x-axis) at addition of radiotracer (exclude background measurement) and normalize to percent signal of the maximum CPS (counts per second).
- Load all normalized data to a new GraphPad Prism file.
- Calculate mean values and standard deviation.
- Illustrate the obtained data as graph showing the deviation (rate of increase) of tracer uptake of all three set ups.
Representative Results
The fully automated radiosynthesis of [11C]SNAP-7941 yielded 5.7 ± 2.5 GBq (4.6 ± 2.0% at EOB, 14.9 ± 5.9% based on [11C]CH3OTf; n = 10) of formulated product. The overall synthesis lasted around 40 min, where 15 min were required for preparation of [11C]CH3OTf via the gas phase method, another 5 min were necessary for radiolabelling of the precursor, followed by 10 min of semi-preparative RP-HPLC purification and 10 min for the C18 cartridge solid phase extraction and formulation. Then, a small aliquot (approx. 100-200 µL) was delivered to the person responsible for the quality control, whereas the original product vial containing the ready-to-use tracer was passed to the experimenter of the real-time kinetic analysis.
Quality control was completed within 10 min after the end of synthesis. The molar activity was in a range of 72 ± 41 GBq/µmol (n = 10) and the radiochemical purity was always > 95%. All other parameters (pH, osmolality, residual solvents) fulfilled the release criteria. For the real-time kinetic assay, three different experimental setups were chosen: (A) treated and non-treated (vehicle) MDCKII-WT cells, (B) non-treated or treated with vehicle MDCKII-hMDR1 cells and (C) the latter cell line with blocking prior the real-time kinetic assay of the transporter using (±)-verapamil. Applying [11C]SNAP-7941 to the wildtype cell line MDCKII-WT (P-gp non-expressing) and MDCKII-hMDR1 (P-gp expressing) shows a different kinetic behavior, as there is a fast accumulation to the wildtype cell line, whereas no accumulation was observed for the MDCKII-hMDR1 cells. However, blocking the P-gp efflux transporter inMDCKII-hMDR1 cells with (±)-verapamil led to a comparable real-time kinetic as already seen for the wildtype cell line (Figure 5).
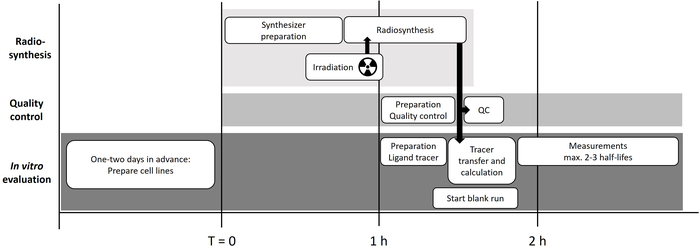
Figure 1: Overview of the work-flow. Work-flow of the radiosynthesis, quality control, and performance of the real-time kinetic measurement of [11C]SNAP-7941. The black arrows indicate the transport way of the radioactivity. Please click here to view a larger version of this figure.
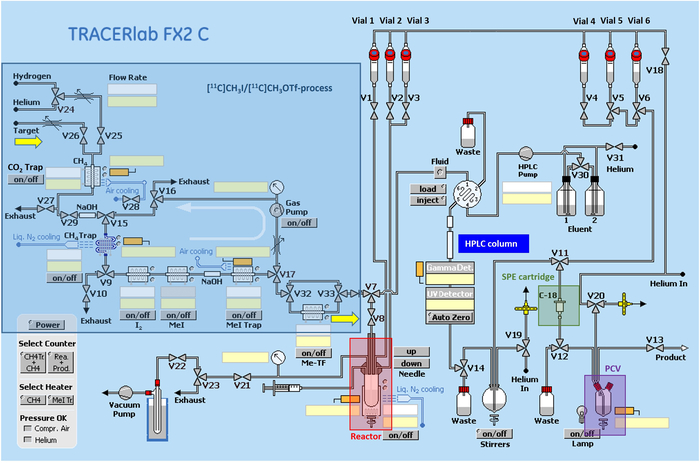
Figure 2: Radiosynthetic scheme of the automated synthesizer. Switching circuit of the automated synthesizer, starting with the circulation unit for [11C]CH3I/[11C]CH3OTf production, reactor for introduction of the activity into the precursor solution and SPE purification (SPE = solid phase extraction; PCV = product collection vial). Please click here to view a larger version of this figure.
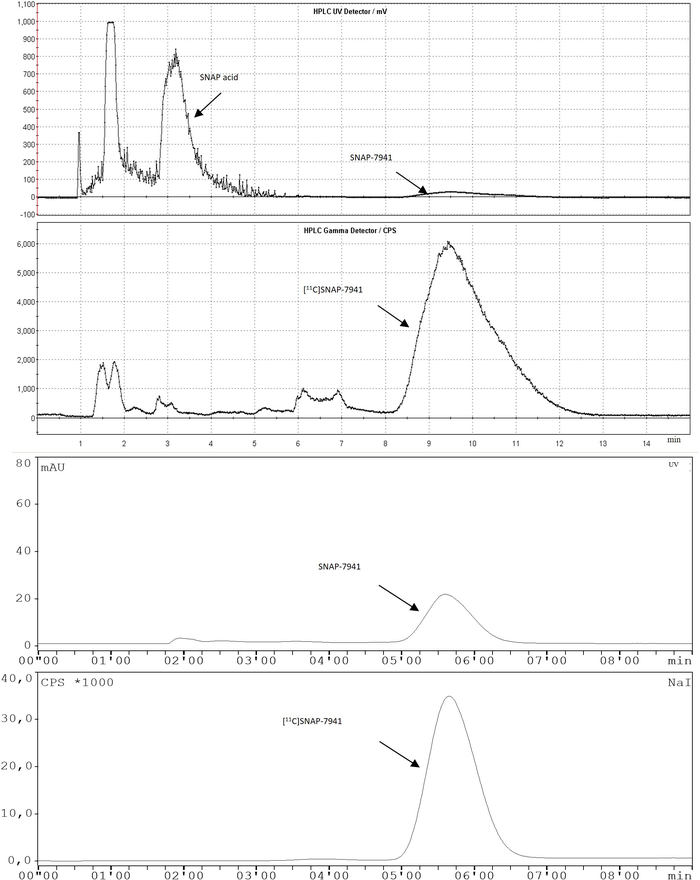
Figure 3: Representative chromatograms of the fully automated radiosynthesis of [11C]SNAP-7841. The semi-preparative RP-HPLC chromatogram is illustrated at the top and the analytical one after purification on the bottom. Please click here to view a larger version of this figure.
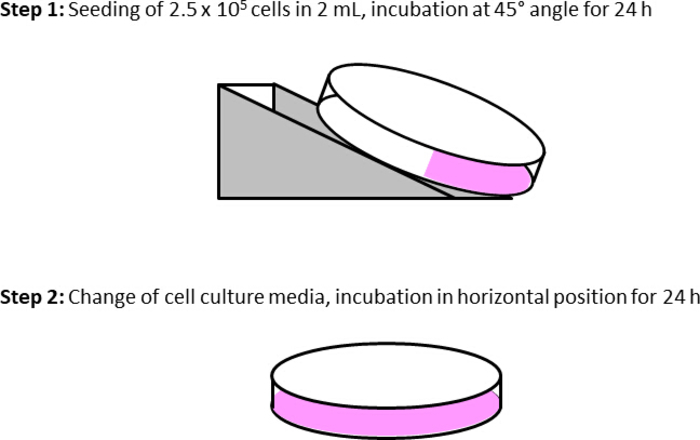
Figure 4: Cell culture dish preparation for real-time experiments. Step 1 includes the seeding of 2.5 x 105 up to 1 x 106 depending on the cell type and their growth rate. Subsequently, the culture dish is placed in an oblique plane (approximately 30-45°). Therefore, the supplied metal apparatus or the lid of a cell culture dish can be used in the incubator to stabilize the slanting position of the dish. The day after (24 h) the dish is adjusted horizontally, and fresh cell culture medium is added to completely cover the cell surface. On the day of experiment, cell viability and confluence are examined. According to the experimental protocol, the cells are washed with DPBS, the medium is replaced by serum-free medium (2 mL), and the culture dish is repositioned to an oblique position till the start of experiments. Henceforth, the cells can be treated with the inhibitor or vehicle. Please click here to view a larger version of this figure.
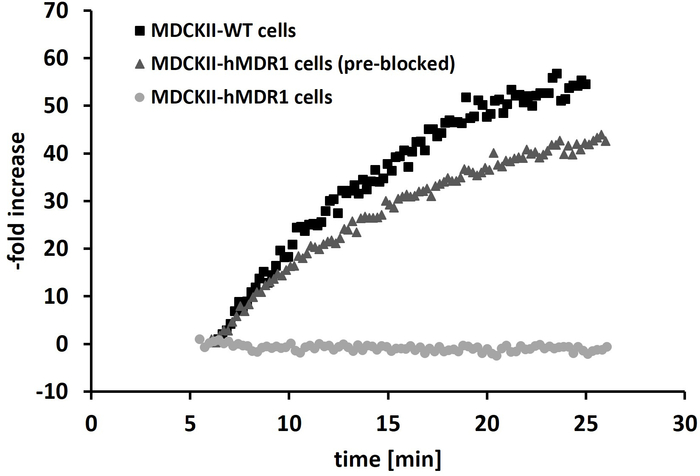
Figure 5: Real-time kinetic measurements of [11C]SNAP-7941. Representative real-time kinetics of [11C]SNAP-7941 are shown in three different set ups: using MDCKII-WT cells; MDCKII-hMDR1 cells pre-blocked with (±)-verapamil as P-gp inhibitor and MDCKII-hMDR1 cells without blockage. The y-axis illustrates the rate of increase of the pre-blocked MDCKII-hMDR1 and WT cells compared to the results of the untreated or vehicle-treated MDCKII-MDR1 cells, respectively (no uptake, 0%). Please click here to view a larger version of this figure.
Discussion
The radiosynthesis of [11C]SNAP-7941 was established on a commercial synthesis module. Due to the possibility to fully automate the preparation procedure, the radiosynthesis proofed to be reliable, and improvements regarding radiation protection of the operator were achieved. The preparation of the synthesizer has an enormous impact on the quality of the radiotracer, especially in terms of molar activity. Thus, it is essential to constantly work under inert conditions (e.g., helium atmosphere), and to flush all lines located prior to the reaction vessel (target line, [11C]CH3I production cycle and reactor (see Figure 2)). Moreover, heating the respective traps and ovens before the start of the synthesis to remove moisture and atmospheric carbon increases the molar activity advantageously. Especially the AgOTf column, impregnated with graphitized carbon, is extremely sensitive to humidity. Even minor amounts of any source of moisture disturb the conversion of [11C]CH3I to [11C]CH3OTf. Before starting the synthesis, the [11C]CO2 trap and the [11C]CH3I trap have to be cooled down to room temperature again in order to enable subsequent trapping.Furthermore, it is recommended to dissolve the precursor shortly before starting the synthesis and to add the base directly into the precursor solution.
The quality control for carbon-11 radiotracers has to be rationally designed for a continuous and fast workflow. However, the most important parameters for cell culture studies are radiochemical purity and molar activity to obtain valid results. Correct evaluation of the molar activity requires a robust analytical HPLC method and the calibration curve has to cover the concentration range of the final product. The challenging part for radiotracers is to achieve a concentration above the limit of quantification (LOQ) due to small amounts, which are produced during radiosynthesis. Hence, the art is to find the balance between high molar activities to avoid receptor saturation and high enough concentrations to still be able to quantify the non-radioactive signal.
[11C]SNAP-7941 was confirmed to be a potent substrate of the human P-gp transporter, as no accumulation was observed in the untreated or vehicle-treated MDCKII-hMDR1 cells due to rapid efflux. In contrast, both experimental set ups (MDCKII-WT or pre-blocked MDCKII-hMDR1 cells) provided similar results (accumulation of [11C]SNAP-7941), supporting the versatility of this in vitro assay. MDCKII-hMDR1 cells are highly suitable for LigandTracer experiments due to their stable transfection, fast growth and persistent against shear stress caused by the rotating cell culture dish. The lack of [11C]SNAP-7941 uptake in the rat and mice brain might therefore occur caused by efflux through the P-gp transporter. Owing to the transfection of canine kidney cells with the human multi drug resistance protein 1 (hMDR-1, P-gp), the predictive value of this method for efflux transporter binding in humans is high, which is favorable in terms of a future clinical application. However, so far, the selectivity against other efflux transporter was not verified. Therefore, other cell lines can be used, expressing different prominent efflux transporters as the breast cancer resistance protein (BCRP) or multiple resistance protein-1 (MRP-1), to study interactions towards these transporters. The method is in comparison to classical accumulation or transport assays very simple and gives immediately qualitative results. Moreover, the greatest advantage is that this technology enables evaluation of direct interaction of the PET tracer and the target in real-time, in contrast to the conventional experiment using indirect quantification (mostly displacement). Additionally, the real-time radioassay software provides experimental flexibility (e.g., nuclide decay correction, measuring time and positions, etc.) and therefore, high freedom for users. On the other side, limitations of the method include a low sample throughput, as only one cell dish can be measured at a time. Furthermore, a few other technical and operational issues should be taken into account: the described technology is very sensitive to background radiation; thus, radiation sources should be kept at distance and emphasis should be placed on the background measurement prior to the experiment. Another issue concerning experiments at higher temperatures than room temperature, is the heating of the inclined support: evaporation of the cell culture medium might affect the detector. Instead of heating, the whole device is preferably placed into the incubator. Moreover, the method is limited to adherent cell lines. Through the rotation of the cell culture dish, shear stress sensitive cells might detach from the dish, which can lead to invalid results.
Nevertheless, if the experimenter pays attention to these minor drawbacks the method delivers fast and reliable results for the analysis of the kinetic behavior of preclinical PET-tracers.
Divulgaciones
The authors have nothing to disclose.
Acknowledgements
This work was supported by the Austrian Science Fund (FWF P26502-B24, M. Mitterhauser). We are grateful for the technical support of T. Zenz and A. Krcal. Furthermore, we thank K. Pallitsch for preparation of the AgOTf and H. Spreitzer for distributing the precursor.
Materials
| Table 1: List of materials and instrumentation of the fully automated radiosynthesis of [11C]SNAP-7941 | |||
| Ni catalyst | Shimadzu, Kyoto, Japan | Shimalilte Ni reduced, 80/100 mesh | |
| Iodine | Merck, Darmstadt, Germany | 1.04761.0100 | |
| Acetonitrile | Merck, Darmstadt, Germany | for DNA synthesis, < 10 ppm H2O | |
| Acetonitrile | Sigma-Aldrich, St. Louis, MO, USA | HPLC grade | |
| Ammonium acetate | Merck, Darmstadt, Germany | ||
| Acetic acid | Merck, Darmstadt, Germany | glacial | |
| Ethanol | Merck, Darmstadt, Germany | 96% | |
| NaCl | B. Braun, Melsungen, Germany | 0.9% | |
| Tetrabutylammonium hydroxide | Sigma-Aldrich, St. Louis, MO, USA | ||
| Methanol | Sigma-Aldrich, St. Louis, MO, USA | HPLC grade | |
| SPE cartridge | Waters, Milford, MA, USA | SepPak C18plus | |
| Semi-preparative RP-HPLC column | Merck, Darmstadt, Germany | Chromolith SemiPrep RP-18e, 100-10 mm | |
| Precolumn | Merck, Darmstadt, Germany | Chromolith Guard RP-18e, 5-4.6 mm | |
| Precursor | University of Vienna, Austria | SNAP-acid | |
| Reference compound | University of Vienna, Austria | SNAP-7941 | |
| Silver trifluoromethanesulfonate | Sigma-Aldrich, St. Louis, MO, USA | ||
| Graphpa GC | Alltech, Deerfield, IL, USA | 80/100 mesh | |
| PET trace 860 cyclotron | GE Healthcare, Uppsala, Sweden | ||
| [11C]CO2 high pressure target | Air Liquide, Vienna, Austria | ||
| TRACERlabFX2 C | GE Healthcare, Uppsala, Sweden | ||
| N2 + 1% O2 | Air Liquide, Vienna, Austria | Target gas | |
| Name | Company | Catalog Number | Comments |
| Table 2: List of materials and instrumentation of the quality control of [11C]SNAP-7941. | |||
| Merck Hitachi LaChrom, L-7100 | Hitachi Vantara Austria GmbH (Vienna, Austria) | HPLC pump | |
| Merck Hitachi, L7400 | Hitachi Vantara Austria GmbH (Tokyo, Japan) | UV-detector | |
| NaI-radiodetector | Raytest (Straubenhardt, Germany) | NaI-radiodetector | |
| Chromolith Performance RP-18e, 100-4.6 mm | Merck (Darmstadt, Germany) | HPLC column | |
| 430-GC | Bruker (Bremen, Germany) | Gas chromatograph | |
| Capillary column ID-BP20; 12 mx0.22 mmx0.25 mm | SGE Ananlytical Science Pty. Ltd. (Victoria, Australia) | Gas capillary | |
| Wesco, osmometer Vapro 5600 | Sanoya Medical Systems (Vienna, Austria) | Osmometer | |
| g-spectrometer | g-spectrometer | ||
| Gas chromatography controlling software | VARIAN (Palo Alto, California, U.S.A) | Galaxie Version 1.9.302.952 | |
| Gamma spectrometer controlling software | ORTEC (Oak Ridge, Tenessee, U.S.A.) | Maestro for windows Version 6.06 | |
| Gamma spectrum recalling software | ORTEC (Oak Ridge, Tenessee, U.S.A.) | Winplots version 3.21 | |
| HPLC controlling software | Raytest (Straubenhardt, Germany) | Gina Star Version 5.9 | |
| inolab 740 | WTW (Weilheim, Germany) | pH meter | |
| Name | Company | Catalog Number | Comments |
| Table 3: List of materials and instrumentation for the evaluation of the real-time kinetic behaviour of [11C]SNAP-7941. | |||
| Madin-Darby Canine Kidney cell line (MDCKII-hMDR1) | Netherlands Cancer Institute (NKI, Amsterdam, Netherlands) | Expressing the human P-glycoprotein (hMDR1) | |
| Madin-Darby Canine Kidney cell line (MDCKII-WT) | Netherlands Cancer Institute (NKI, Amsterdam, Netherlands) | Wildtype (WT) | |
| DMEM GlutaMAX | VWR International GmbH, Vienna, Austria | Gibco 61965-026 | |
| Fetal Calf Serum (FCS) | VWR International GmbH, Vienna, Austria | Gibco 10270-106 | |
| Penicillin/Streptomycin | VWR International GmbH, Vienna, Austria | Gibco 15140 | |
| Cell culture dish | Greiner Bio-One GmbH, Frickenhausen, Germany | Cellstar 100 mm x 20 mm, Mfr.No. 664160 | |
| In vitro experiments | |||
| DMEM GlutaMAX | VWR International GmbH, Vienna, Austria | Gibco 61965-026 | |
| (±)-Verapamil hydrochloride | Sigma Aldrich (St. Louis, Missouri, USA) | ||
| DMSO | Sigma Aldrich (St. Louis, Missouri, USA) | 276855-100 mL | |
| Cell culture dish | Greiner Bio-One GmbH, Frickenhausen, Germany | Cellstar 100 mm x 20 mm, Mfr.No. 664160 | |
| Sterile disposable plastic pipettes | VWR International GmbH, Vienna, Austria | Sterilin, 5 mL – 25 mL | |
| Sterile pipette tips | VWR International GmbH, Vienna, Austria | Eppendorf epT.I.P.S. Biopur 20 µL – 200 µL | |
| Cell culture flasks | Greiner Bio-One GmbH, Frickenhausen, Germany | Cellstar 250 mL, 75 cm2 red filter screw cap, Mfr.No.658175 | |
| LigandTracer control Version 2.2.2 | Ridgeview Instruments AB, Uppsala, Sweden. | ||
| LigandTracer Yellow | Ridgeview Instruments AB, Uppsala, Sweden. | ||
| LigandTracer White | Ridgeview Instruments AB, Uppsala, Sweden. | ||
| GraphPad Prism 6.0 | GraphPad Software, Inc. | ||
| Handheld automated Cell Counter | Millipore Corporation Billerica MA01821 | Scepter (Cat.No. PHC00000) | |
| Cell Counter Sensors | Millipore Corporation Billerica MA01821 | Scepter Sensor 60 µm (Cat.No. PHCC60050) |
Referencias
- Saito, Y., Maruyama, K. Identification of melanin-concentrating hormone receptor and its impact on drug discovery. Journal of Experimental Zoology. 305, 761-768 (2006).
- Philippe, C., et al. Preclinical in vitro & in vivo evaluation of [11C]SNAP-7941 – the first PET tracer for the melanin concentrating hormone receptor 1. Nuclear Medicine and Biology. 40, 919-925 (2013).
- Philippe, C., et al. Radiosynthesis of [ 11C]SNAP-7941-the first PET-tracer for the melanin concentrating hormone receptor 1 (MCHR1). Applied. Radiation and Isotopes. 70, 2287-2294 (2012).
- Philippe, C., et al. SNAPshots of the MCHR1 : a Comparison Between the PET-Tracers [ 18 F ] FE @ SNAP and [ 11 C ] SNAP-7941. Molecular Imaging Biology. , 1-12 (2018).
- Schirmer, E., et al. Syntheses of precursors and reference compounds of the melanin- concentrating hormone receptor 1 (MCHR1) Tracers [11C]SNAP-7941 and [18F]FE@SNAP for positron emission tomography. Molecules. 18, 12119-12143 (2013).
- Miller, P. W., Long, N. J., Vilar, R., Gee, A. D. Synthese von 11C-, 18F-, 15O- und 13N-Radiotracern für die Positronenemissionstomographie. Angewandte Chemie. 120, 9136-9172 (2008).
- Pichler, V., et al. An overview on PET radiochemistry: part 1 – covalent labels – 18 F, 11 C, and 13 N. Journal of Nuclear Medicine. 59, 1350-1354 (2018).
- Pichler, V., et al. Molar activity – The keystone in 11C-radiochemistry: An explorative study using the gas phase method. Nuclear Medicine and Biology. 67, 21-26 (2018).
- Larsen, P., Ulin, J., Dahlstrøm, K., Jensen, M. Synthesis of [11C]iodomethane by iodination of [11C]methane. Applied Radiation and Isotopes. 48, 153-157 (1997).
- Dahl, K., Halldin, C., Schou, M. New methodologies for the preparation of carbon-11 labeled radiopharmaceuticals. Clinical and Translational Imaging. 5, 275-289 (2017).
- Raaphorst, R. M., et al. Radiopharmaceuticals for assessing ABC transporters at the blood-brain barrier. Clinical. Pharmacollogy & Therapeutics. 97, 362-371 (2015).
- Nics, L., et al. Speed matters to raise molar radioactivity: Fast HPLC shortens the quality control of C-11 PET-tracers. Nuclear Medicine and Biology. 57, 28-33 (2018).
- Zeilinger, M., et al. New approaches for the reliable in vitro assessment of binding affinity based on high-resolution real-time data acquisition of radioligand-receptor binding kinetics. European Journal of Nuclear Medicine and Molecular Imaging (Research). 7, 22 (2017).

