Sampling for Estimating Frankliniella Species Flower Thrips and Orius Species Predators in Field Experiments
Summary
Presented here is a protocol to determine the number of thrips and minute pirate bug predators in crops over multiple dates in field experiments. Also illustrated is how to determine the efficacy of management tactics against thrips and evaluate the benefits of predation by minute pirate bugs.
Abstract
The western flower thrips, Frankliniella occidentalis (Pergande), is a polyphagous pest that has been spread worldwide. The extensive use of insecticides in attempts to control its populations eliminates natural enemies and competitor flower thrips species, thereby increasing its populations. An unsustainable situation develops with concomitant resistant pest populations, secondary pest outbreaks, and environmental degradation. Integrated pest management utilizes knowledge of pest and natural enemy relationships to implement tactics that are environmentally friendly and sustainable. Minute pirate bugs are the most important worldwide predators of thrips. They can suppress and ultimately control Frankliniella species flower thrips. Flower samples taken at least weekly are needed to understand predator-prey dynamics. Shown here is the sampling of the flowers of fruiting vegetables and companion plants to estimate the densities of individual thrips and minute pirate bug species. Representative data illustrates how the protocol is used to determine the efficacy of management tactics over time and how to evaluate the benefits of predation by minute pirate bugs. The sampling protocol is similarly adaptable to sampling thrips and minute pirate bugs in other plant species hosts.
Introduction
The western flower thrips, Frankliniella occidentalis (Pergande), was one of the first great pests that was spread worldwide as a result of globalism and the international trade of agricultural products. Economic damage results directly from feeding and oviposition and indirectly through transmission of plant pathogenic viruses. Invasive populations were already largely resistant to most classes of insecticides, and the attempts to control populations with insecticides has only increased damage by eliminating important natural enemies and competitor species. This control approach has destabilized management programs and has resulted in resistant pest populations, secondary pest outbreaks, and environmental degradation1.
Integrated pest management programs have been developed from knowledge of pest and natural enemy relationships and the effects of management tactics on these relationships. The population characteristics of rapid colonization and growth have been long believed to outstrip the capacities of natural enemies to regulate the opportunistic western flower thrips; that is, until it was shown that predation from natural populations of Orius insidiosus (Say) not only resulted in suppression of western flower thrips populations but also a decline of populations toward extinction2. Furthermore, the western flower thrips is mostly flower-inhabiting, in which it competes for pollen and other flower resources with native polyphagous flower thrips.
In most of the eastern United States, the main native competitor is Frankliniella tritici (Fitch), while in southern Florida the main competitor species is Frankliniella bispinosa (Morgan)3. The western flower thrips suffers strong biotic resistance in Florida from the native species of predators and competitor flower thrips species; however, it is the dominant species in habitats disturbed by insecticides and other tactics that exclude competitor thrips and natural enemies. Therefore, a core component of successful integrated pest management programs for fruiting vegetables is increased predation and competition3,4. These programs have been developed from knowledge of predator-prey dynamics and the effectiveness of various tactics to manage thrips and increase biotic resistance. Here, the methodology used to estimate the densities of individual thrips and minute pirate bug species in the flowers of fruiting vegetables and companion plants in Florida are shown. The data are used to determine the efficacy of management tactics and evaluate the benefits of predation by minute pirate bugs.
Designing the flower thrips sampling protocol: background information
When the western flower thrips emerged as a major pest in the 1980s5, it was necessary to develop procedures to accurately, efficiently, and precisely determine the number of individual thrips species in field studies. The procedures described here have been developed from knowledge gained in numerous studies conducted to understand the biology and management of flower thrips. Examples of these studies include work by Funderburk et al.2, Hansen et al.6, Salguero Navas et al.7, Sutherland et al.8, and Tyler-Julian et al.9. The concentrations of Frankliniella species and minute pirate bugs in flowers is behaviorally based and not an artifact of insecticide applications or sampling6. Estimates of populations in flowers over other plant parts usually is sufficient for understanding the local dynamics of predator and prey on a plant host and evaluating the benefits of biological control programs based on the predator to prey ratios. However, the methodology developed for flowers can be adapted to the sampling of other plant parts. The usual sample unit is one or more flowers. The number of samples needed to achieve the desired level of precision is a function of population density and number of flowers in the sample unit.
Species of Frankliniella tend to be an aggregated distribution in flowers, and populations usually are concentrated in the flowers of the upper plant canopy7. For most studies, flowers are randomly selected from the upper half of the plant. Relative techniques to remove thrips from flowers, including liquid washing, mechanical dislodgement, or desiccation, are inaccurate and imprecise8. For this reason, a direct counting, absolute estimate technique is used. Thrips are small organisms about 2 mm in Iength, and microscopy usually is necessary to accurately determine the species. The flowers constituting a sample unit are placed in a vial of 70% alcohol. Once the samples are collected, the vials from each plot are returned to the laboratory for extraction of the thrips and minute pirate bugs and accurate determination of the sex, species, and stage of each. Experiments consist of replicated field plots that are used to evaluate the efficacy of treatments to suppress thrips and the benefits of predation by minute pirate bugs. Flower samples are taken at least weekly during the flowering period of the plant host. Randomized complete block experimental designs are useful in removing from the experimental error differences in thrips and minute pirate bug densities between blocks. Sub-plot treatment arrangements are useful to reduce inter-plot effects of management tactics that affect thrips movement9.
Flower sample processing and analysis: background information
Prior to the 1990s, keys to species of thrips were developed for use by taxonomic specialists, who placed thrips for identification onto microscope slides using one of several mounting media. Researchers studying thrips biology and management were not taxonomic specialists, and there was no involvement by taxonomic specialists in the studies. Typically, the thrips in samples from these studies were lumped into the genus, family, suborder, or order levels of classification. After the spread of the western flower thrips, there was 1) rapid proliferation of research concerning thrips biology and management and 2) recognition by researchers for the need to identify thrips species and develop an efficient system for processing samples.
In studies in the mid-1990s involving thrips population biology, adult thrips from the samples were placed onto microscope slides and identified to species by taxonomic specialist R. J. Beshear (e.g., Salguero Navas et al.7). The larvae were only identified to genus due to a lack of larval identification keys available at that time. The slide mounting was costly and laborious, and a more efficient system was developed2. In subsequent studies, the thrips in the samples were extracted from the flowers in a Petri dish containing 70% alcohol, and the males and females in the Petri dish were identified to species under stereoscopy. Most of our research involves species of Frankliniella. The adults of these species were separated to species under the stereoscope using differences in their chaetotaxy on the dorsal surface of the pronotum, head, and antennae10,11,12.
Additional expertise in thrips taxonomy has been acquired to recognize and identify other thrips genera and species in the samples. There are numerous Orius species worldwide that are important predators of thrips. Two species, O. insidiosus and O. pumilio (Champion), are sympatric throughout much of Florida13. The adults of these species are separated by color characteristics of the basal antennal segment, femora of the hind leg, and cuneus on the wing. Thrips species and genders differ in their biology and behavior; therefore, data for each are typically separately analyzed. Because the thrips populations in flowers have an aggregated pattern of distribution, the data needs transformation to stabilize variances between treatments. Treatment means are compared using analysis of variance as appropriate for the experimental design, and the data is analyzed for each individual date and/or for data pooled over date2,9. The analysis of effects on individual dates is important when treatment differences vary over date. The ratio of the total thrips (adults and larvae) per minute pirate bug (adults and nymphs) is used to evaluate the effectiveness of biological control with minute pirate bugs in Florida field studies suppressing thrips populations at a ratio of about one predator for every 180 thrips2,9.
Protocol
1. Field experiment to determine the effects of UV-reflective mulch, kaolin, and companion plants on flower thrips and their minute pirate bug predator
- Establish a field experiment with a split-split-plot treatment arrangement in a randomized complete block experimental design with mulch type as whole plot treatments, kaolin and no kaolin as subplot treatments, and companion plants and no companion plants as sub-subplot treatments (Figure 1A,B)9,14.
- Layout blocks of tomatoes or pepper that are each at least 6 m wide and 72 m long.
- Randomly lay in each block whole plots of black and UV-reflective mulch, with each whole plot consisting of six raised mulch beds at least 36 m long.
- Plant one linear row of tomatoes every 45 cm or two linear rows of peppers every 30 cm into the four inner beds of each whole plot.
- Kaolin treatment
- Randomly divide each whole plot into equal subplots of kaolin or no kaolin treatments.
- Apply kaolin once or twice weekly at the rate of 7.0 kg/ha to the tomato or pepper plants in the subplots assigned to receive kaolin treatment.
- Companion plants
- Randomly divide each subplot into equal sub-subplots of companion plants or nor companion plant treatments.
- Plant two linear rows of Bidens alba (L.) every 30 cm or one linear row of Helianthus annuus L. every 30 cm into the two outer beds of each sub-subplot treatment with companion plants.

Figure 1: Example experimental field study.
(A) Randomized complete block design to evaluate the separate and interactive effects of companion plants, mulch, and kaolin effects on flower thrips and minute pirate bugs. (B) Bidens alba (L.) evaluated as a companion plant species with tomato as the crop9. Helianthus annuus L. evaluated as a companion plant species with pepper as the crop14. Please click here to view a larger version of this figure.
2. Flower thrips sampling protocol
- Prepare 50 mL sample vials before going to the experimental plots.
- Place a label with the mulch, kaolin, and companion plant treatment, the block number, and the sample date on the outside and inside of each vial.
- Put exactly 30 mL of 70% alcohol in each 50 mL vial.
- Place the vials into a tray.
- Take the trays to the experimental field site.
- Sample the flowers for thrips and minute pirate bugs.
- Randomly assign the tomato or pepper plants to be sampled in each sub-subplot.
- Sample between mid-morning and mid-afternoon.
- Take the samples from the upper half of the plant.
- Remove the vial lid. Using a sharp-edged razor or scissors, carefully remove the flower from the plant. Quickly place the flower into the appropriate pre-labeled vial. Push the flower into the alcohol of the vial (Figure 2). Replace the lid.
- Collect 10 flowers per sample. Make sure each vial is tightly sealed, then shake each vial to ensure that the flowers are within the alcohol.
- Return the trays with samples to the laboratory for storage. To ensure samples do not deteriorate prior to processing, keep samples cool and dry. Refrigerate, if possible, especially for samples that are not processed quickly.
- Repeat the sampling of each sub-subplot at least weekly during the flowering period of the crop.

Figure 2: Sample removal technique.
A sample of 10 tomato flowers being collected from a sub-sub plot in the tomato push-pull experiment9. Please click here to view a larger version of this figure.
3. Sample processing in the laboratory
- Extract the thrips and minute pirate bugs from the flowers in each sample.
- Remove the sample from the refrigerator and tray without disturbing the contents.
- Remove the lid of the vial and carefully extract with a pipette any excess alcohol above the flowers.
- Reseal the vial and shake to dislodge the thrips and minute pirate bugs in the flowers.
- Open the vial and pour the contents into a Petri dish. Rinse the inside of the vial with 70% alcohol and pour the contents into the Petri dish. Make sure all thrips and minute pirate bugs in the vial are flushed into the Petri dish.
- Dissect each flower with forceps and rinse with 70% alcohol to ensure that all the thrips and minute pirate bugs have been dislodged. Remove and discard the flower parts from the Petri dish (Figure 3).
- Transfer the Petri dish to the platform of a stereoscope with 40x–150x magnification.

Figure 3: Extracting thrips and minute pirate bugs from flowers.
A sample of 10 tomato flowers poured into a Petri dish for processing to determine the number of thrips and minute pirate bugs. Please click here to view a larger version of this figure.
- Identify and count the flower thrips in samples.
- Identify and count in each grid the number of adult males and females of each flower thrips species and the number of Frankliniella species larvae.
- Identify the adult flower thrips species in Florida based on the setae on the pronotum, head and second antennal segment10,11,12.
- Separate the adults of F. bispinosa from the adults of F. tritici and F. occidentalis by the extra stoutness of the two setae on the anterior dorsal margin of the second antennal segment (Figure 4).
- Separate the adult F. occidentalis from those of F. bispinosa and F. tritici by the near equal lengths of the anterior marginal and anterior angular major setae on the pronotum and by the longer fourth postocular setae on the head (Figure 4).
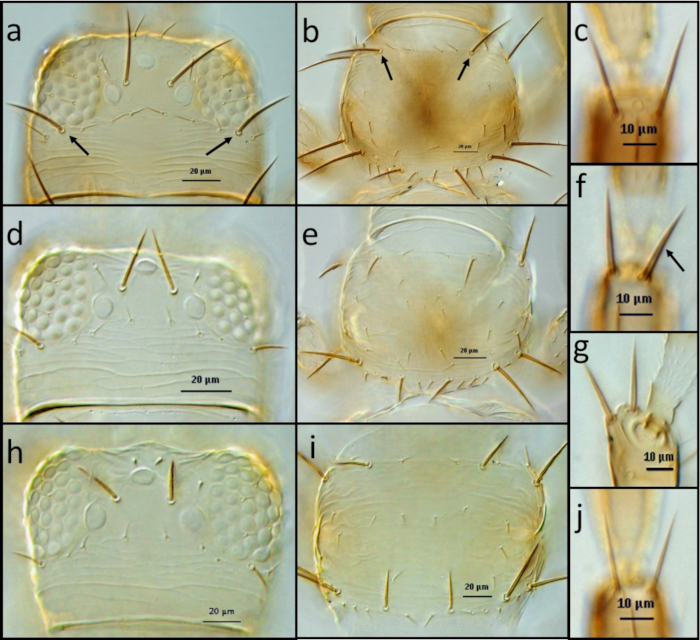
Figure 4: Examples of morphological characters to identify thrips.
(A,B,C) F occidentalis: head (A), arrows indicate postocular setae pair IV; pronotum (B), arrows indicate pair of long anteromarginal setae; distal dorsal setae of antenna segment II (B). (D,E,F,G) . F. bispinosa: head (D); pronotum (E); distal dorsal setae of antenna segment II (F,G), arrow indicates stout setae (F), lateral view of stout setae (G). (H,I,J). F. tritici: head (H); pronotum (I); distal dorsal setae of antenna segment II (J). Please click here to view a larger version of this figure.
- Identify and count the minute pirate bugs in Florida samples.
- Identify and count in each grid the number of adult O. insidiosus and O. pumilio and the number of nymphal Orius species13,15.
- Identify the adult O. insidiosus by the brown basal antennal segments, by the femora that have dark markings, and by the cuneus that is dark brown.
- Identify the adult O. pumilio by the yellow basal antennal segments, the yellow or straw-colored femora, and by the cuneus with pale straw or light-brown color.
- Add the numbers from each grid to determine the total number of adult males and females of each flower thrips species, the number of Frankliniella species larvae, the number of adult minute pirate bug of each species, and the number of minute pirate bug nymphs in the sample.
- Select representative vouchers of flower thrips and minute pirate bug adults from the samples. Label by date, plant host, location, and collector. Curate for long-term preservation.
- Transfer the data from each sample to a spreadsheet that includes the sample date, treatment, and replication.
- Create a data file that contains the data from each sample. Include the experimental location, experimental design, and the amounts and dates of each cultural practice used to establish and maintain the experiment.
- Maintain and manage the datafile with appropriate backup for long-term access.
Representative Results
Data collected in the study by Tyler-Julian et al.9 can be used to demonstrate the separate and combined effects of push factors (i.e., ultraviolet-reflective mulch and kaolin application) and pull factor (i.e., the companion plant Spanish needle, B. alba) on the population dynamics of F. occidentalis adult males and females in tomato flowers (Figure 1A). The agricultural plastic mulch treatments in the experiment were used to form the bed of the raised-bed plastic mulch system that is typical of the production system used to grow high-value vegetables in Florida. The mechanism of the ultraviolet-reflective mulch in pest control is a visual deterrence that disrupts host-finding by the adult thrips. Kaolin application on the tomato plants also reflects enough ultraviolet light to deter the thrips adults. Therefore, a split-split plot randomized complete block design was employed in the experiment to reduce the inter-plot interference on thrips movement resulting from the ultraviolet-reflecting properties of the mulch and kaolin treatments, with mulch treatment (ultraviolet-reflective vs. conventional black mulch) as the whole plot, kaolin treatment (twice weekly kaolin application vs. no kaolin) as the sub-plot, and companion plant treatment (companion plants vs. no companion) as the sub-subplot. Sub-subplot size was six beds by 9 m, with the four inner beds of each sub-subplot consisting of one linear row of tomato with a 45 cm spacing between plants, for a total of 80 plants per sub-subplot. Two rows of Spanish needle were planted into each of the two external beds in the sub-subplots with the companion plant with a 30 cm spacing within and between rows for a total of 128 companion plants per sub-subplot.
Two samples of 10 tomato flowers were collected in each sub-subplot on each of 13 dates in 2011 during the flowering period of the tomato crop, and the number of adult male and female F. occidentalis in each sample were determined (Figure 5). The effects of mulch, kaolin, and companion plant on each gender were analyzed using analysis of variance for a randomized complete block design for a split-split plot treatment arrangement for data across sample date using a mixed model (see Tyler-Julian et al.9 for a complete description of the analysis of variance and results). The main effects of mulch, kaolin, and companion plant were significant for the male western flower thrips (p < 0.01, 0.001, and 0.001, respectively), while the interactive effects of mulch X kaolin, mulch X companion plant, kaolin X companion plant, and mulch X kaolin X companion plant interactions were not significant (p > 0.05). These results showed that each of the main effects reduced the number of adult male F. occidentalis, and that the effects of each tactic were additive when combined with one another.
The main effect of mulch was significant for the female F. occidentalis (p < 0.01), while the main effects of kaolin and companion plants were not significant for the female F. occidentalis (p > 0.05). Therefore, the ultraviolet-reflective mulch reduced the female F. occidentalis in the tomato flowers, but kaolin and the companion plant did not. However, the mulch X kaolin interaction was significant (p < 0.05) showing that the combined effects of ultraviolet-reflective mulch and kaolin reduced the female F. occidentalis more than either tactic alone, while the kaolin applied to tomato on black mulch did not reduce female F. occidentalis numbers. The interactive effects of mulch X companion plant, kaolin X companion plant, and mulch X kaolin X companion plant interactions for female F. occidentalis were not significant (p > 0.05).
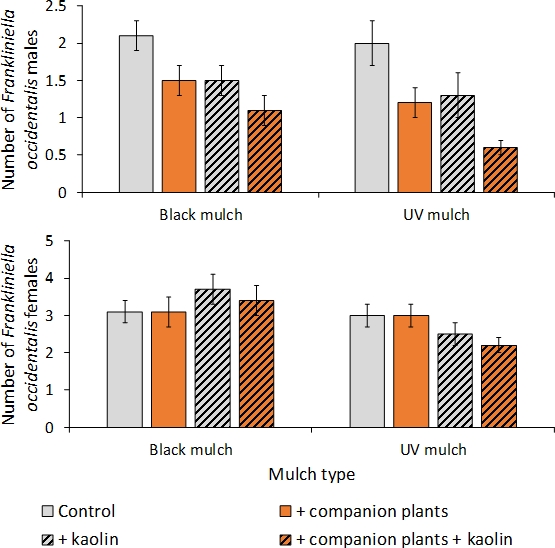
Figure 5: Example of analysis of data over sample date.
The mean number per 10 tomato flowers (SEM) of adult male and female F. occidentalis in mulch, kaolin, and companion plant treatments for sample data pooled across 13 dates in 2011 in a push-pull experiment conducted in Gadsden County, Florida. This figure has been modified from Tyler-Julian et al.9
The interaction of mulch X sample date was significant in the experiment in 2011 for male and female F. occidentalis adults (p < 0.01 and 0.001, respectively)9. This revealed that the ultraviolet-reflective mulch reduced flower thrips numbers on some, but not all, sample dates. Therefore, additional analyses were conducted to evaluate the effects of mulch on individual sample dates. The interaction showed that the ultraviolet-reflective mulch was effective in reducing flower thrips numbers early in the season, but there was no significance on individual sample dates during mid- or late-season (Figure 6).
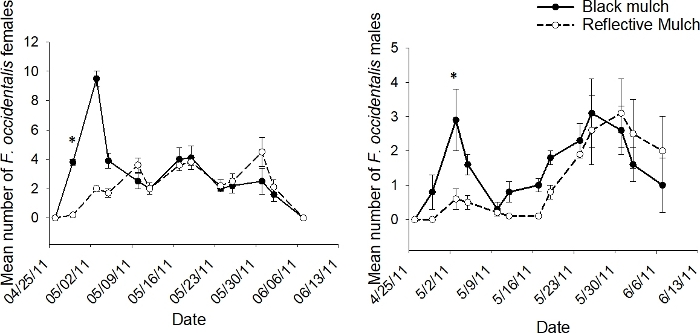
Figure 6: Example of population dynamics for whole plot treatment.
The mean number (+SEM) per 10 tomato flowers (n = 18 samples) of adult male and female F. occidentalis on each 2011 sample date in the whole plot treatment of black and ultraviolet-reflective mulch for data pooled across kaolin and companion plant treatments in the push-pull experiments conducted in Gadsden County, Florida (*indicates significance beyond 95% level of significance according to analysis of variance conducted for individual sample dates; d.f. = 1, 2). This figure has been modified from Tyler-Julian et al.9. Please click here to view a larger version of this figure.
The interaction of kaolin X sample date was not significant in 2011 for male or female F. occidentalis (p > 0.05)9. As previously shown above, the analyses of data pooled over sample date revealed that kaolin did not significantly affect female F. occidentalis numbers, while male F. occidentalis numbers were significantly reduced. The lack of a significant kaolin X sample date interaction in the analyses for data pooled over sample date suggested that the results for each gender were consistent across sample date (Figure 7).
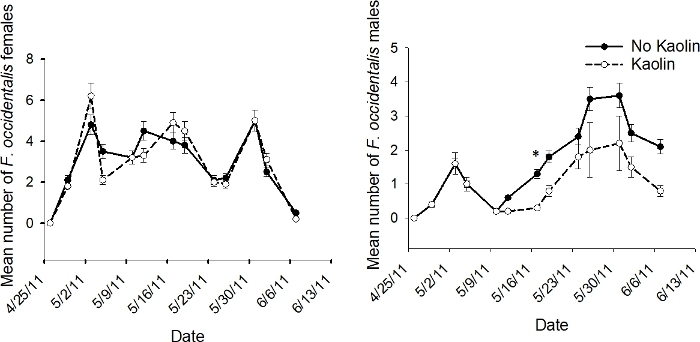
Figure 7: Example of population dynamics for subplot treatment.
The mean number (+SEM) per 10 tomato flowers (n = 12 samples) of adult male and female F. occidentalis on each 2011 sample date in the subplot treatment of kaolin and no kaolin for data pooled across companion plant treatments in the push-pull experiments conducted in Gadsden County, Florida (*indicates significance beyond 95% level of significance according to analysis of variance conducted for individual sample dates; d.f. = 1, 4). This figure has been modified from Tyler-Julian et al.9. Please click here to view a larger version of this figure.
The interaction of companion plant X sample date was significant in 2011 for male F. occidentalis (p < 0.05), but not for female F. occidentalis (p > 0.05)9. The analyses conducted to evaluate the effects of companion plant on individual sample dates revealed that companion plants reduced adult F. occidentalis numbers on late season sample dates, but never on early or mid-season sample dates (Figure 8).
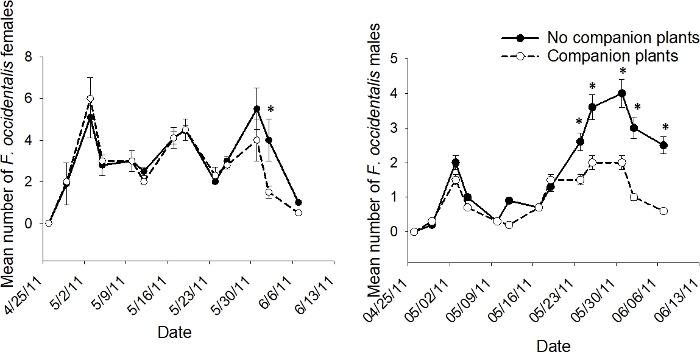
Figure 8: Example of population dynamics for sub-subplot treatment.
The mean number (+SEM) per 10 tomato flowers (n = 6 samples) of adult male and female F. occidentalis on each 2011 sample date in the sub-subplot treatment of companion plant and no companion plant in the push-pull experiments conducted in Gadsden County, Florida (*indicates significance beyond 95% level of significance according to analysis of variance conducted for individual sample dates; d.f. = 1, 8). This figure has been modified from Tyler-Julian et al.9. Please click here to view a larger version of this figure.
Data collected from the flowers of the companion plant in the study of Tyler-Julian et al.14 can be used to demonstrate the dynamic relationship between minute pirate bugs and its thrips prey in flowers (Figure 1B). As in the Tyler-Julian et al.9 study, the objectives were to determine the separate and combined effects of push factors (i.e., ultraviolet-reflective mulch and kaolin application) and a pull factor (i.e., the companion plant), on the population dynamics of Frankliniella species adult males and females in crop flowers. In the Tyler-Julian et al.14 study, the predominant flower thrips species was F. bispinosa in the companion plant H. annuus and in the pepper crop (>99% of the total thrips in the flowers). The thrips rapidly colonized the sunflowers and the pepper flowers, and their numbers were greatest soon after flowering began (Figure 9). Populations of thrips declined over time as the numbers of minute pirate bugs increased. The predator-prey ratio illustrated the ability of the predator to suppress the thrips populations with near extinction of the thrips populations occurring at ratios of >1 predator per 40 thrips.
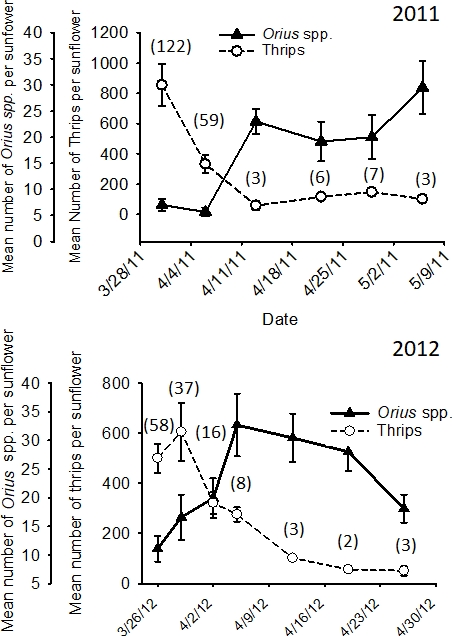
Figure 9: Example of evaluating the benefits of predation.
Mean number (+SEM) of total thrips (adults and larvae) and total Orius spp. (adults and nymphs) per Helianthus annuus flower head in experiments conducted in 2011 and 2012 in Palm Beach County, Florida (the number of total thrips prey per predator on each date shown in parenthesis). This figure has been adapted from data reported in Tyler-Julian et al.14 with permission from Oxford University Press.
Discussion
Sampling protocols with desired levels of precision to estimate population densities of flower thrips were developed for Florida crops over more than three decades of field research. Studies were conducted to understand important aspects of flower thrips biology that affect population estimates. For example, studies were conducted to understand the effects on estimates of time of day when sampling16, sample location within the field16, sample location on individual plants6,16, patterns of aggregation in flowers7, and flower color17. These factors were found to influence population estimates; so, the decisions of where, when, and how are critically important when designing the sampling protocol in future research studies.
Minute pirate bug adults and nymphs are highly anthophilous, and the predator aggregates with its prey in a density-dependent manner by preferring the same flowers also preferred by the thrips17. They also exploit cues from prey or from plants damaged by prey in locating thrips. The adults move rapidly between flowers, a behavior that enhances their ability to track local populations of thrips prey in space and time18. Therefore, sampling protocols developed for estimating populations of thrips should be used in future studies when estimating populations of minute pirate bugs. Minute pirate bugs are efficient predators of the adults and larvae of different Frankliniella species flower thrips19. The number of the predator relative to the number of total thrips prey provides the best estimate of the ability of the minute pirate bugs to suppress and control mixed populations of thrips in the flowers. This should be considered when analyzing the data in future studies.
The Frankliniella species adults rapidly colonize host crops once flowering begins, and rapid population growth follows in the absence of mortality from natural enemies2,18,19. On a good plant host for both predator and thrips prey such as sunflower, numbers of thrips are greatest soon after flower initiation, followed by declines in populations as minute pirate bugs increase (Figure 9). Populations of minute pirate bugs remain even though thrips are nearly extinct. To fully understand this predator-prey dynamic relationship, it is necessary to sample frequently throughout the flowering period of the crop. The same is true when investigating the efficacy of other types of tactics, as they may be efficacious on some dates and not others. Once or twice weekly sampling over the entire period of flowering was employed to evaluate the effects of multiple tactics in the push-pull system under investigation in Tyler-Julian et al.9,14.
Frankliniella is the second largest genus in the family Thripidae, and there is a large amount of literature describing the adult life stages20. A complex of species inhabits flowers that is specific to different plant host species and geographic locations. Therefore, expert identification of slide-prepared specimens from a subset of the initial samplings is critical. Then, in any given plant host and geographic location, taxonomic characters unique to the adults of each species can be chosen so that the species can be determined in future studies without going to the laborious and costly procedure of placing each onto microscope slides for viewing under a compound microscope. They simply can be viewed and identified under a stereoscope. (In some unusual situations, the morphological characters separating two species are so similar that they cannot be separated under the stereoscope.) Methods described here for the flower thrips species common in most crops in Florida should be adapted and used at other geographic locations when processing the large numbers of samples needed in field studies to determine the efficacy of management tactics and to evaluate the benefits of predation by minute pirate bugs.
Divulgaciones
The authors have nothing to disclose.
Acknowledgements
Support was provided by Specialty Crop Block Grants from the Florida Department of Agriculture and Consumer Services Numbers 01856 and 024049. Additional support came from cooperative agreements between the USDA-ARS and the University of Florida Numbers 58-6618-2-096 and 58-6618-4-035. We thank the previous students, postdocs, and collaborators who have contributed in so many ways to our research to understand the factors affecting the population dynamics of flower thrips.
Materials
| Alcohol | Any source | 70% ethanol or isopropyl | |
| Centrifuge tube | Fisher Scientific Co. | 06-443-18 | Flat cap and trayed |
| Forceps | Fisher Scientific Co. | 08-885 | Medium point |
| Kaolin clay | Novasource | Surround WP | 95% kaolin |
| Pasteur pipet | Fisher Scientific Co. | 13-678-6A | 5 ¾ inch disposable |
| Petri dish | Fisher Scientific Co. | FB0875711A | With grid |
| Probes/seekers | Fisher Scientific Co. | 08-995 | 6 inch bent end |
| Scalpel | Fisher Scientific Co. | 14-840-00 | Excel international |
| Stereomicroscope | Leica Microsystems | M Series | 40X and greater |
| UV-reflective mulch | Intergro | Metalized |
Referencias
- Morse, J. G., Hoddle, M. S. Invasion biology of thrips. Annual Review of Entomology. 51, 67-89 (2006).
- Funderburk, J., Stavisky, J., Olson, S. Predation of Frankliniella occidentalis (Thysanoptera: Thripidae) in field peppers by Orius insidiosus (Hemiptera: Anthocoridae). Environmental Entomology. 29, 376-382 (2000).
- Funderburk, J., Frantz, G., Mellinger, C., Tyler-Julian, K., Srivastava, M. Biotic resistance limits the invasiveness of the western flower thrips, Frankliniella occidentalis (Thysanoptera: Thripidae), in Florida. Insect Science. 23, 175-182 (2016).
- Demirozer, O., Tyler-Julian, K., Funderburk, J., Leppla, N., Reitz, S. Frankliniella occidentalis (Pergande) integrated pest management programs for fruiting vegetables in Florida. Pest Management Science. 68, 1537-1545 (2012).
- Kirk, W. D. J., Terry, L. I. The spread of the western flower thrips Frankliniella occidentalis (Pergande). Agricultural and Forest Entomology. 5, 301-310 (2003).
- Hansen, E. A., et al. Within-plant distribution of Frankliniella species (Thysanoptera: Thripidae) and Orius insidiosus (Heteroptera: Anthocoridae) in field pepper. Environmental Entomology. 32, 1035-1044 (2003).
- Salguero Navas, V. E., Funderburk, J. E., Mack, T. P., Beshear, R. J., Olson, S. M. Aggregation indices and sample size curves for binomial sampling of flower-inhabiting Frankliniella species (Thysanoptera: Thripidae) on tomato. Journal of Economic Entomology. 87, 1622-1626 (1994).
- Sutherland, A. M., Parrella, M. P. Accuracy, precision, and economic efficiency for three methods of thrips (Thysanoptera: Thripidae) population density assessment. Journal of Economic Entomology. 104, 1323-1328 (2011).
- Tyler-Julian, K., Funderburk, J., Srivastava, M., Olson, S., Adkins, S. Evaluation of a push-pull system for the management of Frankliniella species (Thysanoptera: Thripidae) in tomato. Insects. 9, 187 (2018).
- Arthurs, S. P., Kok-Yokomi, M. L., Smith, H. Florida flower thrips (suggested common name) Frankliniella bispinosa Morgan (Insecta: Thysanoptera: Thripidae). University of Florida, IFAS, EDIS Document EENY639. , (2018).
- Cluever, J. D., Smith, H. A., Funderburk, J. E., Frantz, G. Western flower thrips (Frankliniella occidentalis [Pergande). University of Florida, IFAS, EDIS Document ENY883. , (2018).
- Sprague, D., Funderburk, J., Lucky, A. Flower thrips, Frankliniella tritici (Fitch). University of Florida, IFAS, EDIS Document EENY720. , (2018).
- Herring, J. L. The genus Orius of the Western Hemisphere (Hemiptera: Anthocoridae). Annals of the Entomological Society of America. 59, 1093-1109 (1966).
- Tyler-Julian, K., Funderburk, J., Frantz, G., Mellinger, C. Evaluation of a push-pull strategy for the management of Frankliniella bispinosa (Thysanoptera: Thripidae) in bell pepper. Environmental Entomology. 43, 1364-1378 (2014).
- Shapiro, J. P., Shirk, P. D., Kelley, K., Lewis, T. M., Horton, D. R. Identity of two sympatric species of Orius (Hemiptera: Heteroptera: Anthocoridae). Journal of Insect Science. 10, 189 (2010).
- Salguero Navas, V. E., Funderburk, J. E., Beshear, R. J., Olson, S. M., Mack, T. P. Seasonal patterns of Frankliniella spp. (Thysanoptera: Thripidae) in tomato flowers. Journal of Economic Entomology. 84, 1818-1822 (1991).
- Funderburk, C., et al. Population dynamics of Frankliniella bispinosa (Thysanoptera: Thripidae) and the predator Orius insidiosus (Hemiptera: Anthocoridae) as influenced by flower color of Lagerstroemia (Lythraceae). Environmental Entomology. 44, 668-679 (2015).
- Ramachandran, S., Funderburk, J. E., Stavisky, J., Olson, S. Population abundance and movement of Frankiliniella thrips and Orius insidiosus in field pepper. Agricultural and Forest Entomology. 3, 129-137 (2001).
- Baez, I., Reitz, S., Funderburk, J. Predation by Orius insidiosus (Heteroptera: Anthocoridae) on life stages and species of Frankliniella flower thrips (Thysanoptera: Thripidae) in pepper flowers. Environmental Entomology. 33, 662-670 (2004).
- Nakahara, S. Annotated list of the Frankliniella species of the world (Thysanoptera: Thripidae). Contributions on Entomology, International. 2, 355-389 (1997).

