Stochastic Noise Application for the Assessment of Medial Vestibular Nucleus Neuron Sensitivity In Vitro
Summary
Galvanic vestibular stimulation in humans exhibits improvements in vestibular function. However, it is unknown how these effects occur. Here, we describe how to apply sinusoidal and stochastic electrical noise and evaluate appropriate stimulus amplitudes in individual medial vestibular nucleus neurons in the C57BL/6 mouse.
Abstract
Galvanic vestibular stimulation (GVS) has been shown to improve balance measures in individuals with balance or vestibular impairments. This is proposed to be due to the stochastic resonance (SR) phenomenon, which is defined as application of a low-level/subthreshold stimulus to a non-linear system to increase detection of weaker signals. However, it is still unknown how SR exhibits its positive effects on human balance. This is one of the first demonstrations of the effects of sinusoidal and stochastic noise on individual neurons. Using whole-cell patch clamp electrophysiology, sinusoidal and stochastic noise can be applied directly to individual neurons in the medial vestibular nucleus (MVN) of C57BL/6 mice. Here we demonstrate how to determine the threshold of MVN neurons in order to ensure the sinusoidal and stochastic stimuli are subthreshold and from this, determine the effects that each type of noise has on MVN neuronal gain. We show that subthreshold sinusoidal and stochastic noise can modulate the sensitivity of individual neurons in the MVN without affecting basal firing rates.
Introduction
The vestibular (or balance) system controls our sense of balance by integrating auditory, proprioceptive, somatosensory and visual information. Degradation of the vestibular system has been shown to occur as a function of age and can result in balance deficits1,2. However, therapies targeting the functioning of the vestibular system are scarce.
Galvanic Vestibular Stimulation (GVS) has been shown to improve balance measures, autonomic functioning and other sensory modalities within humans3,4,5,6. These improvements are said to be due to the Stochastic Resonance (SR) phenomenon, which is the increase in the detection of weaker signals in non-linear systems via the application of subthreshold noise7,8. These studies have shown improvements in static9,10 and dynamic11,12 balance, and vestibular output tests such as Ocular Counter Roll (OCR)13. However, many of these studies have used different combinations of stimulus parameters such as white noise9, colored noise13, different stimulus frequency ranges and thresholding techniques. Therefore, optimal stimulus parameters remain unknown and this protocol can assist with determining the most effective parameters. Besides stimulus parameters, the type of stimulus is also important in therapeutic and experimental efficacy. The above work in humans was performed using electrical noise stimuli, whilst much of the in vivo animal work has used mechanical14,15 or optogenetic16 noise stimuli. This protocol will use electrical noise to examine the effects on vestibular nuclei.
Previously, application of GVS to stimulate primary vestibular afferents was been performed in vivo in squirrel monkeys17, chinchillas18, chicken embryos15 and guinea pigs14. However, only two of these studies examined the effect GVS has on the gain of primary vestibular afferents14,15. These experiments were performed in vivo meaning that the precise patterns of stimulation imposed on vestibular nuclei cannot be determined. To our knowledge, only one other study has applied stochastic noise to individual enzymatically dissociated neurons in the central nervous system19. However, no experiments have been performed in the central vestibular nuclei to assess appropriate stimulus parameters and thresholding techniques, making this protocol more precise in determining stimulus effects on individual neurons within the vestibular nuclei.
Here, we describe how to apply sinusoidal and stochastic (electrical) noise directly to individual neurons in the medial vestibular nucleus (MVN), determine neuronal threshold and measure changes in gain/sensitivity.
Protocol
All experimental protocols described were approved by the University of Sydney Animal Ethics Committee (approved protocol number: 2018/1308).
1. Animals
NOTE: Mice were obtained from the Australian Rodent Centre (ARC; Perth, Australia) and held at the Medical Foundation Building Animal Facility at the University of Sydney.
- Maintain the mice on a normal 12 h light/dark cycle with environmental enrichment.
- Use male and female C57BL/6 mice (3–5 weeks old) for all experiments.
2. Preparation of Solutions
- Prepare 1 L of artificial cerebrospinal fluid (ACSF) composed of 29 mM NaHCO3, 11 mM glucose, 120 mM NaCl, 3.3 mM KCl, 1.4 mM NaH2PO4, 2.2 mM MgCl2, 2.77 mM CaCl2.
- Prepare 200 mL of sucrose-ACSF (sACSF) containing 29 mM NaHCO3, 11 mM glucose, 241.5 mM sucrose, 3.3 mM KCl, 1.4 mM NaH2PO4, 2.2 mM MgCl2, 2.77 mM CaCl2. Prior to the inclusion of CaCl2 to the ACSF and sACSF, gas the solutions with carbogen (95 % O2 and 5 % CO2) to establish a pH of 7.4 and avoid calcium precipitation (cloudiness).
- Prepare K+-based intracellular solution composed of 70 mM potassium gluconate, 70 mM KCl, 2 mM NaCl, 10 mM HEPES, 4 mM EGTA, 4 mM Mg2-ATP, 0.3 mM Na3-GTP; with a final pH of 7.3 (adjusted using KOH).
NOTE: It is recommended to filter intracellular solutions with 0.22 µm filters and store 0.5 mL aliquots of the solution at -20 °C.
3. Preparation of the Brainstem
- Prior to brainstem extraction, equilibrate the sACSF with carbogen and cool at -80 °C for 25 min so that an ice slurry is formed.
- Anaesthetize the mouse with isoflurane (3–5 %) saturated in oxygen (3 mL/min). Once the hind paw reflexes are absent, decapitate the mouse with sharp stainless-steel scissors.
- Expose the skull by making a sagittal incision in the skin using a razor blade (#22 rounded).
- Using the pointed end of a pair of standard pattern scissors make a small incision at the lambda and cut along the longitudinal fissure.
- Carefully reflect away the paired parietal bones and the occipital bones using a pair of shallow-bend Pearson rongeurs.
NOTE: During this whole procedure the brain is continuously bathed in situ using the previously prepared ice-cold sACSF slurry. - Isolate the brainstem from the forebrain and its bony encasing using a razor blade (#11 straight) to cut down the parieto-occipital sulcus and at the caudal medulla.
- Mount the isolated brainstem ventral end down on a previously cut trapezoidal polystyrene block. Remove excess fluid around the dissected tissue with a wick of tissue paper to ensure good tissue adhesion to the cutting stage.
NOTE: The polystyrene block is cut in a trapezoidal shape, to ensure the rostral end of the midbrain fits and tapers into the spinal cord. - Use cyanoacrylate glue to fix the polystyrene block with the attached brainstem rostral end down to the cutting stage.
- Using an advance speed of 0.16 mm/s and vibration amplitude of 3.00 mm, prepare 200 µm transverse slices of the MVN.
NOTE: Location of the MVN is determined using the Paxinos and Franklin mouse brain atlas (Figures 79–89)20. The MVN (listed as MVe in atlas) lies immediately ventrolateral to the 4th ventricle and is largest right before the attachment of the cerebellum (between the inferior colliculi and the obex). - Use a plastic-trimmed pipette to transfer slices onto a filter paper disc sitting in carbogenated ACSF at 25 °C for at least 30 min prior to recording.
4. Instruments
- Use a standard electrophysiological setup to perform whole-cell patch clamp techniques21.
- Prepare micropipettes using a two-step protocol (heat step 1: 70; heat step 2: 45) on a micropipette puller (see the Table of Materials). Micropipettes should have a final resistance ranging 3–5 MΩ with internal solution when placed in the bath.
NOTE: Settings used may vary depending on the temperature within room and can change quite frequently.
5. Whole-cell Patch Clamp Electrophysiology
- To obtain whole-cell patch clamp recordings from individual neurons in the MVN, a K+-based internal solution is used within the recording pipette.
- Transfer a single tissue slice from the incubation chamber to the recording chamber and secure the slice using a nylon thread on a U-shaped weight. Continuously perfuse the recording chamber with carbogenated-ACSF at 25 °C at a flow rate of 3 mL/min.
- After filling a micropipette with internal solution, locate the MVN using a low power (10x) objective lens. Using a high-power (40x) objective, individual neurons within the MVN can be located.
NOTE: Cell quality is essential in ensuring quality recordings and durability of the cell when attempting to achieve the whole-cell configuration. A good cell will demonstrate spherical shape, a reflective cell membrane and an invisible nucleus. A bad cell will have a large visible nucleus (egg-like) and a swollen/shrunken appearance. - Before breaching the tissue with the pipette, apply a small amount of positive pressure to push debris away from the pipette tip.
- Move the pipette using the micromanipulator towards the chosen neuron and a small dimple should form on the neuronal membrane. Release positive pressure and apply a small amount of negative pressure.
- Once a 1 GΩ seal is achieved, apply gentle short and sharp positive pressure to the pipette holder through the suction port to rupture the membrane and create a whole-cell configuration.
- Make whole-cell current clamp recordings using standard techniques21,22.
6. Applying Sinusoidal and Stochastic Noise to Individual Medial Vestibular Nucleus Neurons
- Apply the stochastic and sinusoidal noise at a range of amplitudes from 3 to 24 pA to determine neuronal threshold and firing rate.
- Determine the sensory threshold by grouping lower and higher stimulus intensities and perform an ANOVA to observe any differences (as shown in Supplementary Figure 1).
- Calculate the average firing rate over the 10 s period where the depolarizing current step was/will be injected for each individual current level (i.e., 7 total episodes; Figure 1).
- Use the average firing rate values to generate a firing rate versus current plot and perform a linear regression analysis to determine the gradient of the line of best fit. The gradient of the line of best fit is indicative of the neuronal gain22.
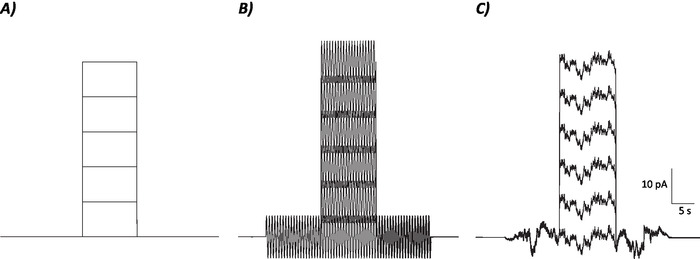
Figure 1: Diagrammatic profiles of control, sinusoidal and stochastic noise protocols. (A) Control (no noise) protocols applied to MVN neurons. (B) Sinusoidal noise protocol with a frequency of 2 Hz. (C) Stochastic noise protocols where majority of the power spectrum is ≤2 Hz. Each protocol presented here has an amplitude of ±6 pA with a 10 s depolarizing current increasing by 10 pA up to 50 pA. The true stimulus does not have a depolarizing current step and is therefore the first episode of these protocols to determine neuronal gain changes. Please click here to view a larger version of this figure.
Representative Results
Initial recordings can provide information about the effects that sinusoidal and stochastic noise have on basal firing rates of individual MVN neurons and how the stimuli effect the gain of neurons. Figure 2 shows that neither sinusoidal nor stochastic noise change basal firing rates of MVN neurons when compared to control (no noise) recordings. This information is crucial for determining the threshold of the individual neurons. During the application of galvanic vestibular stimulation to humans, a sensory thresholding task is performed to ensure that the stimulus is subthreshold13. The subthreshold stimulus is an important component of the stochastic resonance (SR) phenomenon7,8. In vitro, this thresholding task needs to be performed differently and the activity or basal firing rate of neurons has been chosen for this. This ensures that the stimuli are as close to subthreshold as possible and therefore comparable to human studies. Figure 2B highlights that the selected noise level (6 pA) is subthreshold, as it can be observed that average firing rate begins to increase from 12 pA (experimental threshold). This threshold was determined objectively by grouping stimulus levels above (18 and 24 pA) and below (3 and 6 pA) the 12 pA threshold and is shown in Supplementary Figure 1.
Next, neuronal gain was evaluated by subjecting neurons to a suite of depolarizing current steps (0-50 pA, increasing by 10 pA) with and without (control) noise (Figure 1). These results are crucial to determining the effect that stochastic noise may have on neurons in the central vestibular system and thus, potentially how GVS is eliciting its effects on human balance. Figure 3 shows that sinusoidal (Figure 3B) and stochastic (Figure 3A) noise applied at subthreshold amplitudes of 6 pA can alter the gain of MVN neurons. These results were assessed by measuring the firing rate during each 10 s current step and performing a linear regression analysis to calculate the gain (gradient) from the line of best fit.
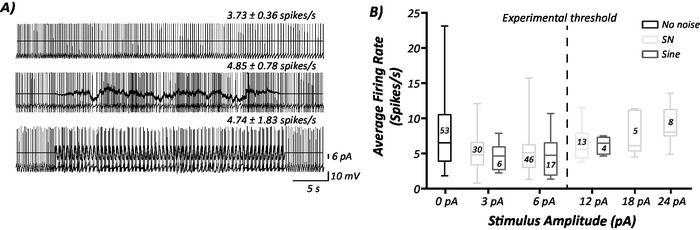
Figure 2: The effect of sinusoidal and stochastic noise on MVN neuronal firing rate. (A) Stochastic (SN; middle trace) and sinusoidal noise (bottom trace) at a 6 pA amplitude show no significant effect on basal firing rate of an individual MVN neuron in comparison to control (no noise; top trace). (B) Firing rate of MVN neurons in response to control (n = 53), stochastic and sinusoidal noise protocols (with no current steps) of amplitudes 3 (SN, n = 30; sine, n = 6), 6 (SN, n = 46; sine, n = 17), 12 (SN, n = 13; sine, n = 4), 18 (SN, n = 5; sine, n = 0) and 24 (SN, n = 8; sine, n = 0) pA. Lines/whiskers indicate the maximum and minimum values, the box indicates the 25th-75th percentiles and the line within the box indicates the mean firing rate (spikes/s). The dashed line indicates experimental threshold, as chosen by pooling the mean firing rates within 3 and 6 pA (below 12 pA) and 18 and 24 pA (above 12 pA) shown in Supplementary Figure 1. Please click here to view a larger version of this figure.
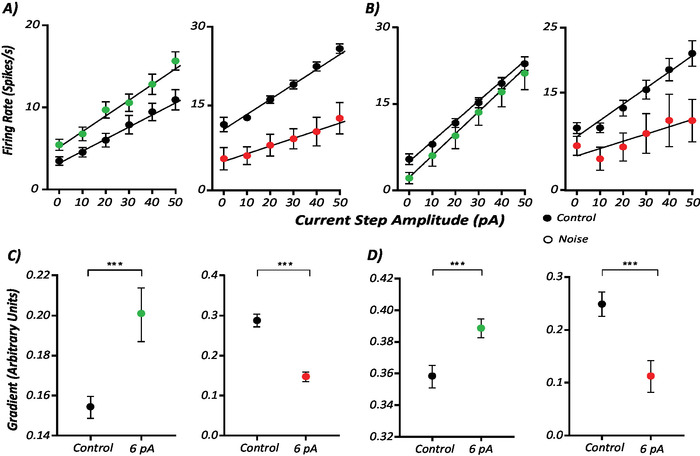
Figure 3: Sinusoidal and stochastic noise alter MVN neuronal gain. (A) MVN neuronal firing rate at each depolarizing current step and the corresponding gain calculation in response to stochastic noise. (B) The data presented were generated the same way as in Figure 3A but during the application of sinusoidal noise. (C,D) Graphs represent the gains calculated from the lines of best fit of A and B. Error bars indicate S.D. Statistical significance was determined by linear regression analysis comparing the gradients of the lines of best fit between control and experimental condition. **p < 0.02; ***p < 0.01. Please click here to view a larger version of this figure.
Supplementary Figure 1: Objective determination of 12 pA threshold. Firing rates for less than 12 pA (3 and 6 pA) and more than 12 pA (18 and 24 pA) were pooled and averaged. These averages were then analyzed using an ANOVA and statistical significance between sham and >12 pA and between <12 pA and >12 pA. *p < 0.05. Please click here to download this file.
Discussion
The effects of galvanic vestibular stimulation (GVS) on the vestibular system has been highlighted in vivo in humans3,13,23, guinea pigs14, rodents18 and non-human primates24. However, none of these studies have assessed the direct impact of electrical noise on the sensitivity of individual neurons in the vestibular system. Here we demonstrate the first in vitro application of stochastic noise directly to individual medial vestibular nucleus (MVN) neurons.
The primary objective of applying stochastic noise directly to individual MVN neurons, is to determine whether the noise is exhibiting an effect on neuronal sensitivity directly. Thus, establishing how Stochastic Resonance (SR) impacts balance in humans. For SR to be evident, the stimulus needs to be subthreshold to ensure that individual neurons are not being overtly activated7 (Figure 2). Therefore, the in vitro neuronal firing rate must remain comparable to control (no stimulus) conditions. This step is critical for the protocol in order to highlight the SR phenomenon, and may be different for other neuronal populations and therefore performed slightly differently.
Although this preparation provides clear advantages over previous in vivo work in animals14,15,17,18, there are still some caveats. First, the stimuli are applied to individual neurons and therefore the thresholding of stochastic and sinusoidal noise may not represent what is occurring at a population level. However, using this protocol we are able to analyze changes at a single neuron level and use this information to subsequently model what may happen in behavioral studies. Second, these electrophysiological recordings are limited to neurons that display spontaneous activity or responses to direct current injection to simulate natural activity. This is one of the reasons for choosing the MVN as a target for testing the effects of these electrical stimuli, as it exhibits spontaneous neuronal activity21.
An advantage of using whole-cell patch clamp recordings of individual MVN neurons is that the response can be more reliably linked to a specific output of the vestibular system. Behavioral studies are able to provide such information regarding the otolith-ocular pathway through the measurement of ocular vestibular-evoked myogenic potentials (oVEMPs) and ocular counter-rolls (OCRs) at a more macro level13. Through electrophysiological recordings, information regarding specific nuclei involvement and thus, the specific pathways involved can be elucidated. Further, previous work in stimulating primary vestibular afferents in vivo has provided important information into how GVS may be working but cannot directly assess how the central vestibular nuclei respond14,15,17,18. Therefore, highlighting the sensitivity and precision of whole-cell patch clamp recordings helps in elucidating how GVS may improve vestibular functioning.
Future studies could apply this protocol to other neuronal populations displaying spontaneous activity. One study has applied stochastic noise to a non-spontaneously active neuronal population within the somatosensory and auditory cortices of rats19. However, this was performed in a cell suspension of enzymatically dissociated pyramidal neurons and was recording Na+ currents specifically, which are taken from postsynaptic cells using voltage clamp experiments. In this protocol the spontaneous activity of MVN neurons was recorded from individual neurons within transverse slices of the brainstem using current clamp experiments.
Divulgaciones
The authors have nothing to disclose.
Acknowledgements
SPS was supported by the University of Sydney postgraduate research scholarship.
Materials
| CaCl | Scharlau | CA01951000 | Used for ACSF and sACSF |
| D-(+)-Glucose | Sigma | G8270 | Used for ACSF and sACSF |
| EGTA | Sigma | E0396-25G | Used for K-based intracellular solution |
| HEPES | Sigma | H3375-25G | Used for K-based intracellular solution |
| KCl | Chem-supply | PA054-500G | Used for ACSF, sACSF and intracellular solution |
| K-gluconate | Sigma | P1847-100G | Used for K-based intracellular solution |
| Mg-ATP | Sigma | A9187-500MG | Used for K-based intracellular solution |
| MgCl | Chem-supply | MA00360500 | Used for ACSF and sACSF |
| Na3-GTP | Sigma | G8877-100MG | Used for K-based intracellular solution |
| NaCl | Chem-supply | SO02270500 | Use for ACSF and intracellular solution |
| NaH2PO4.2H2O | Ajax | AJA471-500G | Used for ACSF and sACSF |
| NaHCO3 | Sigma | S5761-1KG | Used for ACSF and sACSF |
| Sucrose | Chem-supply | SA030-500G | Used for sACSF |
| Isoflurane | Henry Schein | 1169567762 | Used for anaesthetising mice |
| EQUIPMENT | |||
| Borosilicate glass capillaries | Warner instruments | GC150T-7.5 | 1.5mm OD, 1.16mm ID, 7.5cm length |
| Data acquisition software | Axograph | Used for electrophysiology and analysis | |
| Friedmen-Pearson Rongeurs | World precision instruments | 14089 | Used for dissection |
| Micropipette puller | Narishige | PP-830 | Used for micropipette |
| Multiclamp amplifier | Axon instruments | 700B | Used for electrophysiology |
| pH meter | Sper scientific | 860033 | Used for internal solution |
| Standard pattern scissors | FST | 14028-10 | Used for dissection |
| Sutter micromanipulator | Sutter | MP-225/M | Used for electrophysiology |
| Upright microscope | Olympus | BX51WI | Used for electrophysiology |
| Vibratome | Leica | VT1200 | Used for slicing brain tissue |
Referencias
- Amiridis, I. G., Hatzitaki, V., Arabatzi, F. Age-induced modifications of static postural control in humans. Neuroscience Letters. 350 (3), 137-140 (2003).
- Iwasaki, S., Yamasoba, T. Dizziness and imbalance in the elderly: age-related decline in the vestibular system. Aging and disease. 6 (1), (2015).
- Fujimoto, C., et al. Noisy galvanic vestibular stimulation induces a sustained improvement in body balance in elderly adults. Scientific Reports. 6, 37575 (2016).
- Breen, P. P., et al. Peripheral tactile sensory perception of older adults improved using subsensory electrical noise stimulation. Medical Engineering & Physics. 38 (8), 822-825 (2016).
- Yamamoto, Y., Struzik, Z. R., Soma, R., Ohashi, K., Kwak, S. Noisy vestibular stimulation improves autonomic and motor responsiveness in central neurodegenerative disorders. Annals of Neurology. 58 (2), 175-181 (2005).
- Soma, R., Nozaki, D., Kwak, S., Yamamoto, Y. 1/f noise outperforms white noise in sensitizing baroreflex function in the human brain. Physical Review Letters. 91 (7), 078101 (2003).
- Wiesenfeld, K., Moss, F. Stochastic resonance and the benefits of noise: from ice ages to crayfish and SQUIDs. Nature. 373 (6509), 33-36 (1995).
- Moss, F., Ward, L. M., Sannita, W. G. Stochastic resonance and sensory information processing: a tutorial and review of application. Clinical Neurophysiology. 115 (2), 267-281 (2004).
- Goel, R., et al. Using low levels of stochastic vestibular stimulation to improve balance function. PloS one. 10 (8), e0136335 (2015).
- Inukai, Y., et al. Effect of noisy galvanic vestibular stimulation on center of pressure sway of static standing posture. Brain Stimulation: Basic, Translational, and Clinical Research in Neuromodulation. 11 (1), 85-93 (2018).
- Mulavara, A. P., et al. Using low levels of stochastic vestibular stimulation to improve locomotor stability. Frontiers in Systems Neuroscience. 9, 117 (2015).
- Iwasaki, S., et al. Noisy vestibular stimulation increases gait speed in normals and in bilateral vestibulopathy. Brain stimulation. 11 (4), 709-715 (2018).
- Serrador, J. M., Deegan, B. M., Geraghty, M. C., Wood, S. J. Enhancing vestibular function in the elderly with imperceptible electrical stimulation. Scientific Reports. 8 (1), 336 (2018).
- Kim, J., Curthoys, I. S. Responses of primary vestibular neurons to galvanic vestibular stimulation (GVS) in the anaesthetised guinea pig. Brain Research Bulletin. 64 (3), 265-271 (2004).
- Flores, A., et al. Stochastic resonance in the synaptic transmission between hair cells and vestibular primary afferents in development. Neurociencias. 322, 416-429 (2016).
- Huidobro, N., et al. Brownian Optogenetic-Noise-Photostimulation on the Brain Amplifies Somatosensory-Evoked Field Potentials. Frontiers in Neuroscience. 11, 464-464 (2017).
- Goldberg, J., Ferna, C., Smith, C. Responses of vestibular-nerve afferents in the squirrel monkey to externally applied galvanic currents. Brain Research. 252 (1), 156-160 (1982).
- Baird, R., Desmadryl, G., Fernandez, C., Goldberg, J. The vestibular nerve of the chinchilla. II. Relation between afferent response properties and peripheral innervation patterns in the semicircular canals. Journal of Neurophysiology. 60 (1), 182-203 (1988).
- Remedios, L., et al. Effects of Short-Term Random Noise Electrical Stimulation on Dissociated Pyramidal Neurons from the Cerebral Cortex. Neurociencias. 404, 371-386 (2019).
- Paxinos, G., Franklin, K. B. . The mouse brain in stereotaxic coordinates. , (2004).
- Camp, A. J., Callister, R. J., Brichta, A. M. Inhibitory synaptic transmission differs in mouse type A and B medial vestibular nucleus neurons in vitro. Journal of Neurophysiology. 95 (5), 3208-3218 (2006).
- Camp, A., et al. Attenuated glycine receptor function reduces excitability of mouse medial vestibular nucleus neurons. Neurociencias. 170 (1), 348-360 (2010).
- Iwasaki, S., et al. Effect of Noisy Galvanic Vestibular Stimulation on Ocular Vestibular-Evoked Myogenic Potentials to Bone-Conducted Vibration. Front in Neurology. 8, 26 (2017).
- Goldberg, J., Smith, C. E., Fernandez, C. Relation between discharge regularity and responses to externally applied galvanic currents in vestibular nerve afferents of the squirrel monkey. Journal of Neurophysiology. 51 (6), 1236-1256 (1984).

