Targeting Drugs to Larval Zebrafish Macrophages by Injecting Drug-Loaded Liposomes
Summary
Here, we describe the synthesis of drug-loaded liposomes and their microinjection into larval zebrafish for the purpose of targeting drug delivery to macrophage-lineage cells.
Abstract
Zebrafish (Danio rerio) larvae have developed into a popular model to investigate host-pathogen interactions and the contribution of innate immune cells to inflammatory disease due to their functionally conserved innate immune system. They are also widely used to examine how innate immune cells help guide developmental processes. By taking advantage of the optical transparency and genetic tractability of larval zebrafish, these studies often focus on live imaging approaches to functionally characterize fluorescently marked macrophages and neutrophils within intact animals. Due to their diverse functional heterogeneity and ever-expanding roles in disease pathogenesis, macrophages have received significant attention. In addition to genetic manipulations, chemical interventions are now routinely used to manipulate and examine macrophage behavior in larval zebrafish. Delivery of these drugs is typically limited to passive targeting of free drug through direct immersion or microinjection. These approaches rely on the assumption that any changes to macrophage behavior are the result of a direct effect of the drug on the macrophages themselves, and not a downstream consequence of a direct effect on another cell type. Here, we present our protocols for targeting drugs specifically to larval zebrafish macrophages by microinjecting drug-loaded fluorescent liposomes. We reveal that poloxamer 188-modified drug-loaded blue fluorescent liposomes are readily taken up by macrophages, and not by neutrophils. We also provide evidence that drugs delivered in this way can impact macrophage activity in a manner consistent with the mechanism of action of the drug. This technique will be of value to researchers wanting to ensure targeting of drugs to macrophages and when drugs are too toxic to be delivered by traditional methods like immersion.
Introduction
The mononuclear phagocyte system provides a first line of defense against invading pathogens. This system consists of monocytes, monocyte-derived dendritic cells and macrophages, which actively phagocytoze foreign pathogens, thereby limiting pathogen spread. In addition to these phagocytic and microbicidal effector functions, dendritic cells and macrophages are also capable of cytokine production and antigen-presentation to activate the adaptive immune system1. Of these cells, macrophages have received particular attention due to their diverse functional heterogeneity and involvement in multiple inflammatory diseases, from autoimmunity and infectious diseases to cancer2,3,4,5,6,7. The plasticity of macrophages and their ability to functionally adapt to the needs to their tissue environment necessitates experimental approaches to directly observe and interrogate these cells in vivo.
Larval zebrafish are an ideal model organism by which to study the function and plasticity of macrophages in vivo8. The optical transparency of larval zebrafish provides a window to directly observe the behavior of macrophages, especially when coupled with macrophage-marking transgenic reporter lines. Exploiting the live imaging potential and experimental tractability of larval zebrafish has led to many significant insights into macrophage function that have direct relevance to human disease9,10,11,12,13,14,15. Many of these studies have also taken advantage of the high conservation of drug activity in zebrafish (an attribute that underpins their use as a whole animal drug discovery platform16,17,18), by utilizing chemical interventions to pharmacologically manipulate macrophage function. To date, these pharmacological treatments have been mostly delivered either through immersion, which requires the drug to be water soluble, or by direct microinjection of free drug (Figure 1A). Limitations of these passive delivery strategies include off-target effects and general toxicity that may preclude assessing any impact on macrophage function. Additionally, when investigating drug effects on macrophages it is unknown whether the drugs are acting on the macrophages themselves or through more indirect mechanisms. When performing similar chemical intervention studies to investigate macrophage function, we recognized there was an unmet need to develop an inexpensive and straightforward delivery method to target drugs specifically to macrophages.
Liposomes are microscopic, biocompatible, lipid bilayered vesicles that can encapsulate proteins, nucleotides and drug cargo19. The unilamellar or multilamellar lipid bilayer structure of liposomes forms an aqueous inner lumen where water-soluble drugs can be incorporated while hydrophobic drugs can be integrated into the lipid membranes. In addition, the physicochemical properties of liposomes, including size, charge and surface modifications can be manipulated to tailor their targeting to specific cells20,21. These features of liposomes have made them an attractive vehicle to deliver drugs and enhance the precision of current treatment regimens20. As liposomes are naturally phagocytozed by macrophages (a feature exploited by their routine use in delivering clodronate specifically to macrophages for ablation experiments22), they present as an attractive option for macrophage-specific drug delivery (Figure 1B).
This protocol describes the formulation of drugs into blue fluorescent liposomes coated with the hydrophilic polymer poloxamer 188, that forms a protective layer on the liposome surface and has been shown to enhance drug retention and have superior biocompatibility23. Poloxamer was chosen for surface coating of liposomes as our previous research had shown that, when compared to polyethylene glycol modified liposomes, poloxamer modified liposomes showed better biocompatibility following subcutaneous injection of rat paws and similar pharmacokinetics in rabbits following intravenous infusion23. Protocols are also described for their microinjection into larval zebrafish and live imaging to assess their macrophage-targeting ability and localization to intracellular compartments necessary for liposome degradation and cytoplasmic drug delivery. As a proof-of-concept we have previously used this technique to target two drugs to macrophages to suppress their activation in a larval zebrafish model of acute gouty inflammation24. This drug delivery technique expands the chemical "toolkit" available to zebrafish researchers wanting to ensure macrophage-targeting of their drugs of interest.
Protocol
1. Preparation of Drug-loaded Marina Blue-labeled Liposomes
NOTE: Liposomes carrying the blue fluorescent dye, Marina Blue and drug are prepared using a thin film hydration method with post insertion of poloxamer 188. All procedures are performed at room temperature unless otherwise specified. Control liposomes only carry Marina blue and PBS. The example here describes loading liposomes with a mitochondria-targeting antioxidant drug25 that is used in the representative results as a proof-of-concept.
- To prepare liposomes, dissolve 16.4 mg (22.2 µmol) of the phospholipids 1,2-dipalmitoyl-sn-glycero-3-phosphocholine (see Table of Materials), 4.3 mg (11.1 µmol) cholesterol (see Table of Materials) and 2.8 mg (3.7 µmol) 1,2-diseteroyl-sn-glycero-3-phosphocholine (see Table of Materials, in 3 mL of chloroform:methanol (3:1 v/v) in a 25 mL round-bottom flask.
- To the above mixture, add 31 nmol of Marina Blue 1,2-dihexadecanoyl-sn-glycero-phosphoethanolamine (see Table of Materials) and 300 nmol of a mROS inhibiting drug (see Table of Materials) and sonicate briefly to fully dissolve. For control liposomes, only add Marina Blue 1,2-dihexadecanoyl-sn-glycero-phosphoethanolamine.
NOTE: To avoid photobleaching of the light-sensitive Marina Blue fluorescent dye, protect all procedures from light, where possible. This is also important if the drug to be loaded is light-sensitive. - Remove the solvent slowly on a rotary evaporator (see Table of Materials) over a temperature controlled water bath set to 45 °C by rotating at 75 rpm under vacuum pressure (200 mbar for 15 min then 0 mbar for 20 min). This forms a dry thin lipid film within the glass walls of the flask that is further dried for 45 min under N2 to facilitate complete removal of the organic solvent.
- Following formation of the thin film, set the vacuum pressure to 40 mbar for 15 min, then purge with nitrogen gas for 30 min to ensure removal of residue solvent.
- Hydrate the thin film using 1 mL of phosphate-buffered saline (PBS, pH 7.4) and seal with parafilm before agitating the flask using a rotary evaporator over a 60 °C water bath for 20 min in order to form vesicles.
- Subject the hydrated suspension to 7 cycles of freeze and thaw by freezing in liquid N2 for 3 min followed by immersion in a 45 °C water bath for 7 min.
- Extrude liposomes through a membrane using a mini-extruder (see Table of Materials) and 1.0 µm track-etched polycarbonate membranes (see Table of Materials) for 2-6 cycles in order to obtain large unilamellar vesicles.
NOTE: In order to achieve more favorable targeting towards macrophages, manipulate extrusion cycles until the diameter of liposomes is approximately 1 µm26,27,28. - Condense the fresh liposomes into a pellet by ultracentrifugation at 186,000 x g and incubate with 1 mL of 1% poloxamer 188 (see Table of Materials) solution (10 mg/mL in PBS) at 45 °C and 750 rpm for 30 min using a thermomixer with a 2 mL microcentrifuge tube attachment (see Table of Materials).
- Ultracentrifuge the mixture at 186,000 x g for 1 h at 4 °C and remove the supernatant in order to remove any unbound polymer or drug. Store the liposomes pellets at 4 °C, protected from light.
2. Characterization of Liposome Size and Zeta Potential
- Dilute liposome formulation with ultrapure water (1:100) and measure the particle size, polydispersity index (PDI) and zeta potential of the liposomes using a dynamic light scattering analyzer (see Table of Materials), in triplicate at 25 °C.
NOTE: The PDI is a measure of the size distribution of the nanoparticle population. PDI values < 0.05 indicates a more uniform distribution while values closer to 1 indicate a larger size distribution. The zeta potential is the overall surface charge.
3. Calculation of Entrapment Efficiency and Drug Loading in Liposomes
NOTE: To determine the entrapment efficiency of drug in liposomes, the drug content contained in the supernatant and liposome pellet are measured.
- Resuspend the liposome pellet in 1 mL of PBS.
- Remove the supernatant, dilute 10 x with ultrapure water and analyze by high-performance liquid chromatography (HPLC).
- To determine the drug concentration within the liposomes, add 100 µL of liposome suspension to 900 µL of 10% triton-X100 solution (to destroy the lipid membrane, releasing the entrapped drug and preventing lipid build up within the HPLC column) and vortex for 3 min before analysis.
- Inject 20 μL of sample into an HPLC system consisting of a quaternary pump, thermostat auto-sampler and a 3 μm 250 x 4.6 mm column. Set the mobile phase (10 mM PBS pH 2.5, acetonitrile and methanol (30:40:30, v/v/v)) flow rate to 0.9 mL/min and obtain spectral profiles using a diode array detector set at 230 mM.
- Calculate the entrapment efficiency (EE) and drug loading (DL) using the following equations:
EE (%) = [Me / Mt] x 100
DL (%) = [Me / Mlip] x 100
Where Me is the mass of drug encapsulated, Mt is the total mass of drug used during formulation and Mlip is the total mass of the lipids (including cholesterol) and encapsulated drug.
4. Preparation and Injection of Liposomes
- Prepare borosilicate microinjection needles by inserting a thin wall borosilicate glass capillary (1 mm O.D. x 0.78 mm I.D. x 10 cm length) into a micropipette puller device (see Table of Materials) with the following generic puller settings (heat 680, pull 75, velocity 40, time 55 and pressure 530) to produce a tapered microinjection needle.
- Place the needles into a Petri dish onto the modeling clay and arrange in a way that the pulled end is not touching the bottom of the dish. Keep the Petri dish lid closed to avoid dust contamination until ready for use.
- Dilute freshly-prepared liposome formulations 1:1 in sterile PBS (pH 7.4) and briefly vortex (2-3 s) to mix.
NOTE: Protect liposomes from light until larvae are ready for microinjection. - Set up adult zebrafish breeding pairs the night before and collect embryos as previously described by Rosen et al.29.
- Transfer the embryos into a Petri dish (100 mm x 20 mm style with approximately 60 embryos per dish) containing E3 medium (5 mM NaCl, 0.17 mM KCl, 0.33 mM CaCl2, 0.33 mM MgSO4) supplemented with 0.1% methylene blue to inhibit fungal growth. Remove all dead or unfertilized embryos and incubate overnight at 28.5 °C.
- To facilitate live imaging, add 0.003% N-Phenylthiourea (PTU) to the E3 media when the embryos reach 24 h of development to prevent pigment formation (melanization).
- Manually dechorionate the embryos at approximately 30 h post fertilization (hpf) using fine tip forceps.
NOTE: Do not use enzymatic treatment to dechorionate embryos as this can cause epithelial damage and local inflammation thereby influencing immunological studies - Let the embryos develop at 28.5 °C until 2 days post fertilization (dpf).
- Prepare an injection plate by pouring enough 3% methyl cellulose (in E3 media) into the lid of a small 35 mm culture dish to form a thin film covering the entire surface.
NOTE: When preparing 3% methyl cellulose in E3 media, it takes several days for the methyl cellulose to dissolve with gentle agitation on an orbital shaker. - Anaesthetize the larvae by incubating in E3 media supplemented with 200 µg/mL of Tricaine.
- Collect a pool of approximately 20-25 larvae using a plastic transfer pipette and allow the embryos to concentrate at the tip of the pipette by gravity. Taking care to transfer the larvae with minimal liquid, place them in the middle of the 35 mm culture dish lid.
- Using a microcapillary loader, arrange the larvae in the following ways: anterior towards the top of the injection plate with the dorsal surface facing toward the microinjection needle, to inject into the hindbrain ventricle (Figure 2A-D); or, anterior towards the left of the injection plate (when injecting from the right) with the ventral surface facing towards the microinjection needle to inject into the sinus venosus (Figure 2I).
NOTE: When injected this way, hindbrain ventricle-resident (Figure 2E) and caudal hematopoietic tissue(CHT)-resident (Figure 2J) macrophages can be targeted, respectively. The CHT region of larval zebrafish is a hematopoietic site analogous to the mammalian fetal liver. Pay extra attention not to damage the embryos while arranging as this may cause local inflammation thereby influencing immunological studies. - Take freshly prepared liposomes (from step 4.3) and back fill an injection needle with approximately 5 μL of the liposome injection mix using an microcapillary loader (see Table of Materials).
NOTE: When assessing the localization of liposomes to the phagolysosomal compartments of macrophages (Figure 2H), supplement this injection mix with 200 μg/mL of a red fluorescent marker of phagosomes (see Table of Materials) and 100 nM of a far red fluorescent marker of lysosomes (see Table of Materials) to mark phagosomes30,31 and lysosomes32, respectively. - Mount the needle into a micromanipulator (see Table of Materials) connected to a magnetic stand and a pressure injector (see Table of Materials). Position under a stereomicroscope and set the injector to a pulse duration of approximately 50 ms and an injection pressure of approximately 40 psi.
- Cut the tip of the microinjection needle with clean fine tip forceps to generate an opening of approximately 5 μm in diameter.
- Calibrate the volume to be injected by injecting a bolus into a drop of mineral oil positioned over the grid lines of a hemocytometer. Adjust the pulse duration and/or the injection pressure until the diameter of the bolus covers 2.5 of the smallest grid lines (this corresponds to a volume of approximately 1 nL).
- Carefully inject 1 nL of the liposome injection mix into the hindbrain ventricle of each larvae.
NOTE: When injecting into the hindbrain ventricle, take care not to penetrate too far ventrally (beyond the hindbrain) as this can disrupt the underlying vasculature leading to hemorrhages. When injecting into the sinus venosus, care should be taken to ensure the tip of the needle is just within the pericardium and not penetrating the yolk. - Following injection, immediately transfer the injected larvae back into E3 media by adding E3 media to the injection plate and gently agitating the larvae out of the methyl cellulose using a plastic transfer pipette.
- Incubate the larvae at 28.5 °C (protected from light) until analysis by live confocal imaging.
5. Confocal Imaging and Image Analysis to Confirm Macrophage Targeting of Liposomes
- Heat a water bath to 50 °C.
- Anaesthetize liposome-injected larvae by transferring to a new Perti dish containing E3 media supplemented with 0.003% PTU and 200 µg/mL Tricaine.
- Prepare live imaging mounting medium by dissolving 1% low melting point agarose in E3 media, supplemented with 0.003% PTU, in a microwave. Place in the water bath and once the solution has cooled to 50 °C, add 200 µg/mL of Tricaine.
- Keep the mounting medium in the 50 °C water bath to prevent premature polymerization of the agarose.
- To image the dorsal surface of the hindbrain on a confocal microscope equipped with a water immersion lens (Figure 2E), embed the larvae as follows: Fill a 35 mm culture dish with the mounting medium (to a depth of approximately 5 mm) and let it polymerize. Excavate a small trench in the agarose that is of sufficient size to accommodate the yolk sac.
- Fill a plastic transfer pipette with molten mounting medium, expel a small quantity, and use it to collect the anaesthetized larvae. Immediately transfer the larvae into the culture dish and orientate the yolk sacs, ventral side down, into the excavated holes, using a microcapillary loader. Periodically maintain them in this position until the agarose polymerizes. Overlay with E3 media supplemented with 200 µg/mL Tricaine.
NOTE: When transferring and positioning larvae, take care not to cause epithelial damage and local inflammation which can influence immunological studies. For positioning of larvae to live image the CHT region (Figure 2J), mount as described above, but position the larvae within the excavated hole laterally. - When imaging on an inverted confocal microscope, embed the larvae, without the agarose bed, directly on the bottom of a glass bottom dish. Arrange them dorsal surface down (or laterally) and following polymerization, cover the entire dish surface with an even layer of mounting medium. Once polymerized, add a thin layer of E3 media supplemented with 200 µg/mL of Tricaine.
- To live image the hindbrain ventricle on a confocal microscope, use the following settings: 512 x 512 pixels; 40 x 3 μm Z-stacks (extending from dorsal-most surface of the hindbrain); 20x objective; 2.5x zoom.
NOTE: When comparing signal intensities between samples (for example when imaging the uptake of Marina Blue-labeled liposomes injected within reporter lines marking either macrophages [Tg(mpeg1:EGFP)33] or neutrophils [Tg(lyz:EGFP)34]), it is essential that laser settings are kept the same (e.g., pinhole diameter, laser voltage, offset and gain) to help ensure any differences are not an artifact of altered image acquisition settings. - Use 3D image analysis software to quantify macrophage or neutrophil uptake of Marina Blue-labeled liposomes. To quantify the number of cells containing liposomes (Figure 2F) within a Z-stack, scroll through the individual Z sections and count the number of individual cells containing Marina Blue-labeled liposomes. To quantify the fluorescence intensity of liposomes within cells (Figure 2G), draw a region of interest around each cell (in the X, Y and Z dimensions) to quantify the total or mean fluorescence intensity of intracellular Marina Blue.
Representative Results
The thin film hydration approach described here for the preparation of fluorescent liposomes enclosing drugs is a simple and cost-effective method. With the protocol used in this study, the liposomes are expected to be unilamellar23,24. The size, zeta potential, drug loading and entrapment efficiency of the liposomes produced are summarized in Table 1. The particle size of the liposomes (before and after drug loading) are similar (Table 1). The surface charge (zeta potential) of drug-loaded liposomes is slightly more neutral when compared with control liposomes, however, they are all negatively charged meaning this will not significantly change their biodistribution pattern.
Microinjection of Marina Blue-labeled liposomes into the hindbrain ventricle results in rapid uptake by resident macrophages that can be readily observed by confocal microscopy by 3 h post injection, when injected into the macrophage lineage-marking transgenic reporter line Tg(mpeg1:EGFP)33 (Figure 2C,E-G). This is in contrast to neutrophils (as marked within the neutrophil-specific Tg(lyz:EGFP)34 reporter line) that are rarely observed containing intracellular liposomes (Figure 2E-G). Within individual liposome-laden macrophages, the liposomes accumulate within phagolysosomal compartments (Figure 2H), which is necessary for liposome degradation and the subsequent release of their drug contents into the cytoplasm35. Selecting different microinjection sites for liposome delivery can impact which tissue-resident macrophages are targeted. As examples, delivery into the hindbrain ventricle efficiently targets hindbrain-resident macrophages (Figure 2D,E) while microinjection into the sinus venosus can deliver the liposomes to CHT-resident macrophages via the circulation (Figure 2I,J).
Consideration of the microinjection site for liposome delivery is important when using this technique to interrogate the macrophage response to an immunological challenge as it helps ensure the particular macrophages under investigation are receiving the drug. We have routinely used this protocol for the delivery of drug-loaded liposomes into the hindbrain ventricle to assess the impact of these drugs on the macrophage response to monosodium urate (MSU) crystals, similarly injected into the hindbrain compartment (Figure 3A). As the causative agent of acute gouty inflammation, MSU crystals activate tissue-resident macrophages to produce pro-inflammatory mediators including Interleukin-1β (IL-1β) through a process dependent upon mitochondrial reactive oxygen species (mROS)10,36,37,38. These activated macrophages then drive neutrophil infiltration, a hallmark of acute gouty inflammation. Microinjection of liposomes loaded with a mROS-inhibiting drug into the hindbrain ventricle can significantly suppress MSU crystal-driven mROS production within liposome-laden hindbrain-resident macrophages (Figure 3B-D). Using the protocol described here, we achieved an entrapment efficiency of 49.12 ± 0.17 %, which resulted in a formulation with a drug concentration of 103.05 ± 0.36 μM. Of note, injecting a 1 nL volume at this concentration resulted in no observable toxicity during our experiments, as evidenced by gross morphological changes or cardiac arrest. Further validation of the suppressive effects of this drug on macrophage activation state can be performed by investigating il1b expression (the zebrafish ortholog of IL-1β) by whole mount in situ hybridization (Figure 4A,B) and the temporal recruitment of neutrophils (Figure 4C,D).
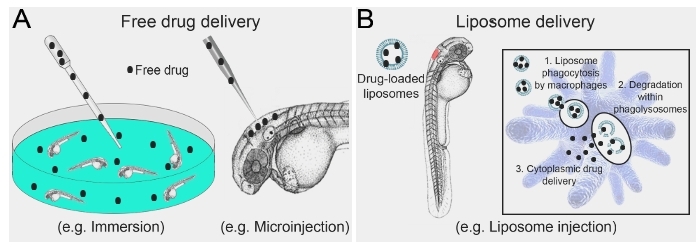
Figure 1: Schematic illustrating conventional free drug delivery versus liposome-mediated drug delivery to larval zebrafish. (A) Strategies routinely used for drug delivery to larval zebrafish are largely limited to immersion in, or microinjection of, free drug. (B) Microinjection of drug-loaded liposomes allows for direct targeting to macrophages, where liposome degradation within phagolysosomal compartments results in cytoplasmic drug delivery. Please click here to view a larger version of this figure.
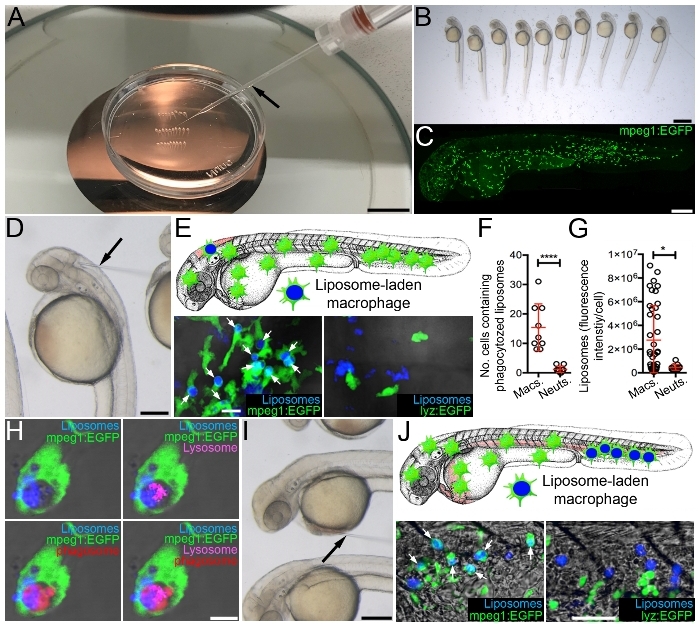
Figure 2: Targeting drugs to macrophages using fluorescent liposomes. (A) Microinjection set-up showing microinjection needle (black arrow) and larvae arrayed in a 35 mm tissue culture dish within 3% methylcellulose. (B) Magnified view of larvae arrayed as in A. (C) Live confocal image of 2 dpf Tg(mpeg1:EGFP) larvae, anterior to left. (D) Magnified view of arrayed larvae, as in A, demonstrating microinjection into the hindbrain ventricle (black arrow marks microinjection needle). (E) Schematic illustrating targeting of hindbrain-resident macrophages and live confocal images (dorsal views, anterior to left) of the hindbrain region of Tg(mpeg1:EGFP) and Tg(lyz:EGFP) larvae, 3 h following hindbrain microinjection of Marina Blue-labeled liposomes (white arrows mark liposome-laden macrophages). (F and G) Quantification of liposome uptake by macrophages and neutrophils (as detected in E), measured as the number of cells containing (F) and the fluorescence intensity/cell (G) of Marina Blue-labeled liposomes. (H) Live confocal image of liposome-laden macrophage within the hindbrain marked with a red fluorescent marker of phagosomes and a far red fluorescent marker of lysosomes within Tg(mpeg1:EGFP) larvae, 3 h following hindbrain microinjection of Marina Blue-labeled liposomes. (I) Magnified view of arrayed larvae, as in A, demonstrating microinjection into the sinus venosus (black arrow marks microinjection needle). (J) Schematic illustrating targeting of CHT-resident macrophages and live confocal images (lateral views, anterior to left) of the CHT region of Tg(mpeg1:EGFP) and Tg(lyz:EGFP) larvae, 3 h following microinjection of Marina Blue-labeled liposomes into the sinus venosus (white arrows mark liposome-laden macrophages). Error bars display mean ± SD. *p<0.05; ****p < 0.0001, Student's t-test. Scale bars = 100 mm (A), 100 μm (B), 250 μm (C, D, and I), 10 μm (E), 5 μm (H), 50 μm (J). This figure has been modified from previous publication24. Please click here to view a larger version of this figure.
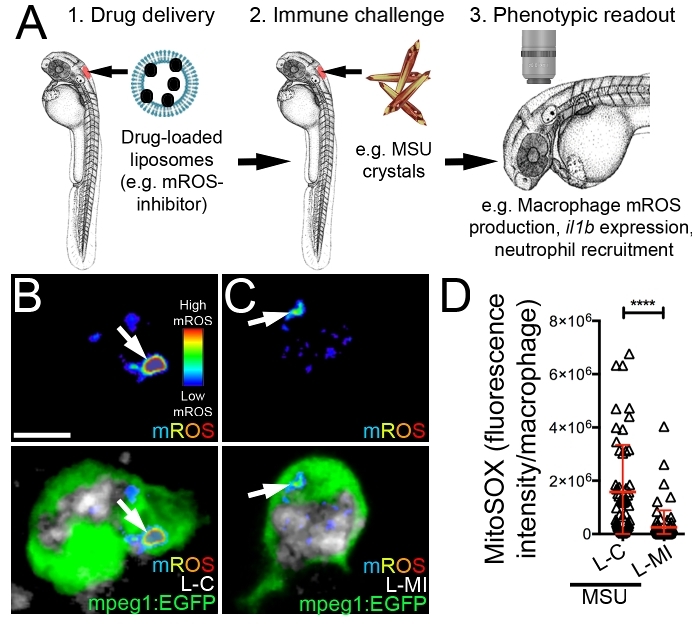
Figure 3: Microinjection of liposomes loaded with a mROS-inhibiting drug suppresses mROS production within activated macrophages. (A) Schematic illustrating the targeting of a mROS-inhibiting drug to macrophages and assessing it's impact on macrophage activation following MSU crystal stimulation. (B and C) Live confocal images of liposome-laden macrophages (control liposomes/L-C (B) or mROS-inhibiting liposomes/L-MI (C)) within the hindbrain of Tg(mpeg1:EGFP) larvae, also marked with a fluorescent mROS-specific probe (see Table of Materials, where the fluorescent signal is displayed as a heatmap with warm colors representing higher levels of mROS), 3 h following hindbrain microinjection of Marina Blue-labeled liposomes and MSU crystals. Marina Blue fluorescence is pseudo-colored in grayscale. (D) Quantification of fluorescence intensity of mROS-specific probe within macrophages, as detected in B and C. Error bars display mean ± SD. ****p < 0.0001, Student's t-test. Scale bar = 10 μm (B). This figure has been modified from previous publication24. Please click here to view a larger version of this figure.
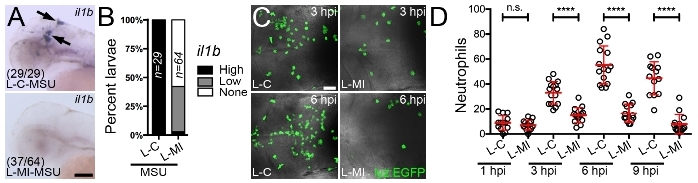
Figure 4: Microinjection of liposomes loaded with a mROS inhibitor suppresses il1b expression within activated macrophages and macrophage-driven neutrophil recruitment. (A) Expression of il1b (marked by black arrows), as detected by whole mount in situ hybridization, within the hindbrain region 3 h following hindbrain microinjection of control (L-C) or mROS-inhibiting liposomes (L-MI) and MSU crystals, anterior to left. Numbers represent proportion of larvae with displayed phenotypes. (B) Quantification of il1b expression, as detected in A, shown as percent larvae demonstrating high, low or no expression. (C) Confocal images of neutrophils within the hindbrain region of Tg(lyz:EGFP) larvae (dorsal views, anterior to left), as detected by immunofluorescence 3 (3 hpi) and 6 (6 hpi) h following hindbrain microinjection of L-C or L-MI and MSU crystals. (D) Temporal quantification of neutrophils, as detected in C, 1 (1 hpi), 3 (3 hpi), 6 (6 hpi) and 9 (9 hpi) h following hindbrain microinjection of L-C or L-MI and MSU crystals. Error bars display mean ± SD. ****p < 0.0001, n.s. not significant, one-way ANOVA, Dunnett's post hoc test. Scale bars = 100 μm (A), 50 μm (C). This figure has been modified from previous publication24. Please click here to view a larger version of this figure.
| Physicochemical property of liposomes (L) | Control liposomes | mROS-inhibiting liposomes |
| Size (μm) | 1.2 ± 0.07 | 1.1 ± 0.04 |
| Zeta potential (mV) | -25.9 ± 0.57 | -13.0 ± 0.11 |
| Entrapment efficiency (EE, %) | N/A | 49.12 ± 0.17 |
| Drug loading (DL, %) | N/A | 0.37 ± <0.01 |
Table 1: Physicochemical characteristics of PBS (control) or mROS-inhibiting liposomes (data are means ± standard deviation, n = 3). All liposomes were labeled with Marina Blue.
Discussion
Here, we have provided a detailed protocol to formulate drug-loaded liposomes to specifically target macrophages in larval zebrafish. This method can be used to dissect the role of macrophages in certain disease models by ensuring direct targeted delivery of drugs specifically to macrophages. Moreover, it can be used when general toxicity of drugs limits their use when delivered by more conventional routes, like immersion. The protocol described here provides an alternative to other nanoparticulate systems that have been used to target innate immune cells in larval zebrafish. These include targeting the antimalarial drug rifampicin to Mycobacterium marinum-infected macrophages to promote bacterial clearance using sub-micron size polymeric nanoparticles39. In another example, encapsulation of (R)-roscovitine, an inducer of neutrophil apoptosis, within polymerosomes has been shown to target this drug to neutrophils, thereby promoting inflammation resolution40. In this protocol, we have used liposomes as a vehicle for drug delivery due to their non-toxic, biocompatible and biodegradable properties. In addition, they are non-immunogenic, which is of particular importance when used to investigate immunological responses, such as those described here. A range of cargos can also be carried including hydrophilic, hydrophobic, amphipathic and lipophilic drugs.
When performing this protocol, there are a number of critical steps where special care must be taken. These include steps where larvae are physically handled and care must be taken to minimize any unintentional damage to the larvae. This includes when manually dechorionating the larvae (especially avoiding contact with the delicate epithelial lining of the yolk), arraying larvae onto the injection plate, their removal from the plate following injections and their mounting for live confocal imaging. An unavoidable component of this protocol is the need to delivery the liposomes (and any immune challenges) through microinjection which can cause local inflammation and therefore may influence the experimental outcome. Microinjection into larval zebrafish does require a certain degree of training to minimize tissue damage. In our work, generating a microinjection needle tip diameter of approximately 5 μm generates very minor surface epithelial damage (as evidenced by very low expression of the inflammatory marker matrix metallopeptidase 9 within surface epithelial cells in the immediate vicinity of the microinjection wound10) when injecting into the hindbrain ventricle of 2 dpf larvae. This diameter tip is also small enough to avoid leakage of injected contents, out of the hindbrain ventricle, when the microinjection needle is removed. It is important to note here that injection volumes greater than 2 nL will often result in some of the injected contents overflowing through the injection hole as a result of exceeding the volume of the ventricle. A tip diameter of 5 μm is also of sufficient size to inject subsequent immune challenges, such as MSU crystals10. Beveling the microinjection needle tip to 45° with a microgrinder so as to generate a sharp tip may provide better penetration of the outer periderm and inner basal epithelial layers covering the hindbrain ventricle41. It is important to note that control injections like those used here (i.e., control liposomes) are essential when assessing the effects of drug-loaded liposomes on macrophage function to control for the injection process itself. Selecting a concentration for a given drug when formulating into liposomes is also an important step as established efficacious drug concentrations, when delivered by immersion, might differ from those necessary when using liposome-mediated drug delivery. In addition, when using this protocol, one should be careful not to alter the physicochemical characteristics of the liposomes post modification with poloxamer as this may affect their biodistribution pattern.
An area for modification and future development of this protocol will be to investigate incorporating different surface features to the liposomes such as ligands or receptors to further enhance macrophage targeting and uptake, or to target different immune cells, such as neutrophils. This advanced approach will require further studies to uncover surface features that are unique to the different zebrafish immune cell compartments, which are currently poorly understood. Further areas for modification of this protocol include the incorporation of other fluorescent probes into the liposomes to expand their versatility (e.g., when injected into other transgenic reporter lines) and altering their physical properties such size and charge42,43. It will also be important to examine whether different routes of liposome administration at different larval stages can expand their versatility to target additional macrophage populations, for example intestinal epithelial cells. In the protocol detailed here, all injections were performed on 2 dpf larvae. During the third day of zebrafish development a single continuous lumen from the mouth to anus forms, following the opening of the mouth44. By the forth day the anus opens resulting in a completely open-ended tube lined with a polarized epithelium44. It will be interesting to see if immersion of older larvae in liposomes can facilitate the targeting of encapsulated drugs to intestinal epithelial cells.
Divulgaciones
The authors have nothing to disclose.
Acknowledgements
This work was supported by grants awarded to C.J.H. (Health research Council of New Zealand and Marsden Fund, Royal Society of New Zealand) and Z.W. (Faculty Research Development Fund from the University of Auckland). The authors thank Alhad Mahagaonkar for expert management of the zebrafish facility, the Biomedical Imaging Research Unit, School of Medical Sciences, University of Auckland for assistance with confocal imaging and Graham Lieschke for gifting the Tg(mpeg1:EGFP) reporter line.
Materials
| 1,2-dipalmitoyl-sn-glycero-3-phosphocholine (DPPC) | Avanti Polar Lipids, Inc. | 850355P | |
| 1,2-diseteroyl-sn-glycero-3-phosphocholine (DSPE) | Avanti Polar Lipids, Inc. | 850367P | |
| 1.0 µm Whatman Nuclepore Track-Etched polycarbonate membranes | GE Healthcare Life Sciences | 110610 | |
| 25 mL round-bottom flask | Sigma-Aldrich | Z278262 | |
| 35 mm culture dish | Thermo Scientific | 150460 | |
| Acetonitrile | Sigma-Aldrich | 34998 | |
| Agilent 1260 Infinity Diode Array Detector | Agilent Technologies | G4212B | |
| Agilent 1260 Infinity Quaternary Pump | Agilent Technologies | G1311B | |
| Agilent 1290 Infinity Series Thermostat | Agilent Technologies | G1330B | |
| Avanti mini-extruder Avanti Polar Lipids Inc. | Avanti Polar Lipids Inc. | ||
| borosilicate microinjection needles | Warner Instruments | 203-776-0664 | |
| CaCl2 | Sigma-Aldrich | C4901-100G | |
| cholesterol | Sigma-Aldrich | C8667 | |
| Dumont No.5 fine tip forceps | Fine Science Tools | 11251-10 | |
| Eppendorf Microloader pipette tip | Eppendorf | 5242956003 | |
| Eppendorf SmartBlock 1.5 mL, thermoblock for 24 reaction vessels | Eppendorf | 4053-6038 | |
| eyelash manipulator | Ted Pella Inc. | 113 | |
| hemocytometer | Hawksley | BS.748 | |
| HEPES | BDH Chemicals | 441474J | |
| HPLC system | Agilent Technologies | 1260 series HPLC system | |
| KCl | Sigma-Aldrich | P9541-1KG | |
| low melting point agarose | Invitrogen | 16520-100 | |
| LysoTracker Deep Red | Invitrogen | L12492 | 1 mM stock solution in DMSO, keep at -20 °C and protect from light. |
| LysoTracker Deep Red | Thermo Scientific | L12492 | |
| magnetic stand | Narishige | GJ-1 | |
| Marina Blue 1,2-dihexadecanoyl-sn-glycero-phosphoethanolamine (Marina Blue DHPE) | Invitrogen | M12652 | Keep at -20 °C and protect from light. |
| Methanol | Sigma-Aldrich | 34860 | |
| methyl cellulose | Sigma-Aldrich | M0387-500G | |
| methylene blue | Alfa Aesar | 42771 | |
| MgSO4 | Sigma-Aldrich | 230391-500G | |
| micromanipulator | Narishige | M-152 | |
| mineral oil | Sigma-Aldrich | M-3516 | |
| Mitochondria-targeting antioxidant MitoTEMPO | Sigma-Aldrich | SML0737 | |
| MitoSOX Red Mitochondrial Superoxide Indicator | Thermo Scientific | M36008 | |
| MitoTEMPO | Sigma-Aldrich | SML0737 | Keep at -20 °C and protect from light. |
| N-Phenylthiourea (PTU) | Sigma-Aldrich | P7629-10G | Take care when handling, toxic. |
| NaCl | BDH Chemicals | 27810.295 | |
| PBS (pH 7.4) | Gibco | 10010-023 | |
| Petri dish (100 mm x 20 mm) | Corning Inc. | 430167 | |
| Phenomenex C18 Gemini-NZ 3 mm 250 mm x 4.6 mm column | Phenomenex | 00G-4439-E0 | |
| pHrodo Red Escherichia coli BioParticles Conjugate | Thermo Scientific | P35361 | |
| pHrodo Red Escherichia coli BioParticles Conjugate | Invitrogen | P35361 | Keep at -20 °C and protect from light. Make 1 mg/mL stock solution by dissolving 2 mg lyophilized product in 2 mL of PBS supplemented with 20 mM HEPES, pH 7.4. |
| plastic transfer pipette | Medi'Ray | RL200C | |
| poloxamer 188 | BASF Corporation | ||
| pressure injector | Applied Scientific Instruments | MPPI-2 | |
| rotary evaporator | Büchi, Flawil, Switzerland | Büchi R-215 Rotavapor | |
| Scanning confocal microscope | Olympus | Olympus FV1000 FluoView | |
| Sorvall WX+ Ultracentrifuge | Thermo Scientific | 75000090 | |
| stereomicroscope | Leica | MZ12 | |
| Tricaine | Sigma-Aldrich | A5040-25G | Make 4 mg/mL stock solution (in deionzed H2O) and keep at -20 °C. |
| triton-X100 | Sigma-Aldrich | X100-100ML | |
| Ultrasonic bath | Thermo Scientific | FB-11205 | |
| Volocity Image Analysis Software | PerkinElmer | version 6.3 | |
| water bath | |||
| Zetasizer Nano | Malvern Instruments Ltd | Zetasizer Nano ZS ZEN 3600 |
Referencias
- Chow, A., Brown, B. D., Merad, M. Studying the mononuclear phagocyte system in the molecular age. Nature Reviews Immunology. 11 (11), 788-798 (2011).
- Li, Q., Barres, B. A. Microglia and macrophages in brain homeostasis and disease. Nature Reviews Immunology. 18 (4), 225-242 (2018).
- Krenkel, O., Tacke, F. Liver macrophages in tissue homeostasis and disease. Nature Reviews Immunology. 17 (5), 306-321 (2017).
- Alderton, G. K. Tumour immunology: turning macrophages on, off and on again. Nature Reviews Immunology. 14 (3), 136-137 (2014).
- Moore, K. J., Sheedy, F. J., Fisher, E. A. Macrophages in atherosclerosis: a dynamic balance. Nature Reviews Immunology. 13 (10), 709-721 (2013).
- Lawrence, T., Natoli, G. Transcriptional regulation of macrophage polarization: enabling diversity with identity. Nature Reviews Immunology. 11 (11), 750-761 (2011).
- Chawla, A., Nguyen, K. D., Goh, Y. P. Macrophage-mediated inflammation in metabolic disease. Nature Reviews Immunology. 11 (11), 738-749 (2011).
- Renshaw, S. A., Trede, N. S. A model 450 million years in the making: zebrafish and vertebrate immunity. Disease models and mechanisms. 5 (1), 38-47 (2012).
- Hall, C. J., et al. Immunoresponsive gene 1 augments bactericidal activity of macrophage-lineage cells by regulating beta-oxidation-dependent mitochondrial ROS production. Cell Metabolism. 18 (2), 265-278 (2013).
- Hall, C. J., et al. Blocking fatty acid-fueled mROS production within macrophages alleviates acute gouty inflammation. Journal of Clinical Investigation. 125 (5), 1752-1771 (2018).
- Cambier, C. J., et al. Mycobacteria manipulate macrophage recruitment through coordinated use of membrane lipids. Nature. 505 (7482), 218-222 (2014).
- Davis, J. M., et al. Real-time visualization of mycobacterium-macrophage interactions leading to initiation of granuloma formation in zebrafish embryos. Immunity. 17 (6), 693-702 (2002).
- Madigan, C. A., et al. A Macrophage Response to Mycobacterium leprae Phenolic Glycolipid Initiates Nerve Damage in Leprosy. Cell. 170 (5), 973-985 (2017).
- Tobin, D. M., et al. The lta4h locus modulates susceptibility to mycobacterial infection in zebrafish and humans. Cell. 140 (5), 717-730 (2010).
- Volkman, H. E., et al. Tuberculous granuloma induction via interaction of a bacterial secreted protein with host epithelium. Science. 327 (5964), 466-469 (2010).
- Bowman, T. V., Zon, L. I. Swimming into the future of drug discovery: in vivo chemical screens in zebrafish. ACS Chemical Biology. 5 (2), 159-161 (2010).
- Kaufman, C. K., White, R. M., Zon, L. Chemical genetic screening in the zebrafish embryo. Nature Protocols. 4 (10), 1422-1432 (2009).
- Zon, L. I., Peterson, R. T. In vivo drug discovery in the zebrafish. Nature Reviews Drug Discovery. 4 (1), 35-44 (2005).
- Malam, Y., Loizidou, M., Seifalian, A. M. Liposomes and nanoparticles: nanosized vehicles for drug delivery in cancer. Trends in Pharmacological Sciences. 30 (11), 592-599 (2009).
- Torchilin, V. P. Recent advances with liposomes as pharmaceutical carriers. Nature Reviews Drug Discovery. 4 (2), 145-160 (2005).
- Immordino, M. L., Dosio, F., Cattel, L. Stealth liposomes: review of the basic science, rationale, and clinical applications, existing and potential. International Journal of Nanomedicine. 1 (3), 297-315 (2006).
- Astin, J. W., et al. Innate immune cells and bacterial infection in zebrafish. Methods in Cell Biology. 138, 31-60 (2017).
- Zhang, W., et al. Post-insertion of poloxamer 188 strengthened liposomal membrane and reduced drug irritancy and in vivo precipitation, superior to PEGylation. Journal of Controlled Release. 203, 161-169 (2015).
- Wu, Z., et al. Liposome-Mediated Drug Delivery in Larval Zebrafish to Manipulate Macrophage Function. Zebrafish. 16 (2), 171-181 (2019).
- Cader, M. Z., et al. C13orf31 (FAMIN) is a central regulator of immunometabolic function. Nature Immunology. 17 (9), 1046-1056 (2016).
- Chono, S., Tanino, T., Seki, T., Morimoto, K. Influence of particle size on drug delivery to rat alveolar macrophages following pulmonary administration of ciprofloxacin incorporated into liposomes. Journal of Drug Targeting. 14 (8), 557-566 (2006).
- Chono, S., Tanino, T., Seki, T., Morimoto, K. Uptake characteristics of liposomes by rat alveolar macrophages: influence of particle size and surface mannose modification. Journal of Pharmact and Pharmacology. 59 (1), 75-80 (2007).
- Chono, S., Tauchi, Y., Morimoto, K. Influence of particle size on the distributions of liposomes to atherosclerotic lesions in mice. Drug Development and Industrial Pharmacy. 32 (1), 125-135 (2006).
- Rosen, J. N., Sweeney, M. F., Mably, J. D. Microinjection of zebrafish embryos to analyze gene function. Journal of Visualized Experiments. (25), (2009).
- Hall, C., Flores, M. V., Crosier, K., Crosier, P. Live cell imaging of zebrafish leukocytes. Methods in Molecular Biology. 546, 255-271 (2009).
- Kapellos, T. S., et al. A novel real time imaging platform to quantify macrophage phagocytosis. Biochemical Pharmacology. 116, 107-119 (2016).
- Shen, K., Sidik, H., Talbot, W. S. The Rag-Ragulator Complex Regulates Lysosome Function and Phagocytic Flux in Microglia. Cell Reports. 14 (3), 547-559 (2016).
- Ellett, F., Pase, L., Hayman, J. W., Andrianopoulos, A., Lieschke, G. J. mpeg1 promoter transgenes direct macrophage-lineage expression in zebrafish. Blood. 117 (4), 49-56 (2011).
- Hall, C., Flores, M. V., Storm, T., Crosier, K., Crosier, P. The zebrafish lysozyme C promoter drives myeloid-specific expression in transgenic fish. BMC Developmental Biology. 7, 42 (2007).
- Ahsan, F., Rivas, I. P., Khan, M. A., Torres Suarez, A. I. Targeting to macrophages: role of physicochemical properties of particulate carriers–liposomes and microspheres–on the phagocytosis by macrophages. Journal of Controlled Release. 79 (1-3), 29-40 (2002).
- Martin, W. J., Walton, M., Harper, J. Resident macrophages initiating and driving inflammation in a monosodium urate monohydrate crystal-induced murine peritoneal model of acute gout. Arthritis and Rheumatology. 60 (1), 281-289 (2009).
- Faires, J. S., McCarty, D. J. Acute arthritis in man and dog after intrasynovial injection of sodium urate crystals. Lancet. 280, 682-685 (1962).
- Martin, W. J., Harper, J. L. Innate inflammation and resolution in acute gout. Immunology and Cell Biology. 88 (1), 15-19 (2010).
- Fenaroli, F., et al. Nanoparticles as drug delivery system against tuberculosis in zebrafish embryos: direct visualization and treatment. ACS Nano. 8 (7), 7014-7026 (2014).
- Robertson, J. D., Ward, J. R., Avila-Olias, M., Battaglia, G., Renshaw, S. A. Targeting Neutrophilic Inflammation Using Polymersome-Mediated Cellular Delivery. Journal of Immunology. 198 (9), 3596-3604 (2017).
- Le Guellec, D., Morvan-Dubois, G., Sire, J. Y. Skin development in bony fish with particular emphasis on collagen deposition in the dermis of the zebrafish (Danio rerio). International Journal of Developmental Biology. 48 (2-3), 217-231 (2004).
- Kelly, C., Jefferies, C., Cryan, S. A. Targeted liposomal drug delivery to monocytes and macrophages. Journal of Drug Delivery. 2011, 727241 (2011).
- Fidler, I. J., et al. Design of liposomes to improve delivery of macrophage-augmenting agents to alveolar macrophages. Investigación sobre el cáncer. 40 (12), 4460-4466 (1980).
- Ng, A. N., et al. Formation of the digestive system in zebrafish: III. Intestinal epithelium morphogenesis. Developmenal Biology. 286 (1), 114-135 (2005).

