Microalgae Cultivation and Biomass Quantification in a Bench-Scale Photobioreactor with Corrosive Flue Gases
Summary
Bench-scale, axenic cultivation facilitates microalgal characterization and productivity optimization before subsequent process scale-up. Photobioreactors provide the necessary control for reliable and reproducible microalgal experiments and can be adapted to safely cultivate microalgae with the corrosive gases (CO2, SO2, NO2) from municipal or industrial combustion emissions.
Abstract
Photobioreactors are illuminated cultivation systems for experiments on phototrophic microorganisms. These systems provide a sterile environment for microalgal cultivation with temperature, pH, and gas composition and flow rate control. At bench-scale, photobioreactors are advantageous to researchers studying microalgal properties, productivity, and growth optimization. At industrial scales, photobioreactors can maintain product purity and improve production efficiency. The video describes the preparation and use of a bench-scale photobioreactor for microalgal cultivation, including the safe use of corrosive gas inputs, and details relevant biomass measurements and biomass productivity calculations. Specifically, the video illustrates microalgal culture storage and preparation for inoculation, photobioreactor assembly and sterilization, biomass concentration measurements, and a logistic model for microalgal biomass productivity with rate calculations including maximum and overall biomass productivities. Additionally, since there is growing interest in experiments to cultivate microalgae using simulated or real waste gas emissions, the video will cover the photobioreactor equipment adaptations necessary to work with corrosive gases and discuss safe sampling in such scenarios.
Introduction
Photobioreactors are useful for controlled experiments and cultivation of purer microalgal products than can be achieved by open ponds. Microalgal cultivation in bench-scale photobioreactors supports the development of fundamental knowledge that may be used for process scale-up. Slight changes to environmental conditions can significantly alter microbiological experiments and confound the results1. A sterile process with temperature, pH, and gas sparging control is advantageous for studying microalgal properties and performance under varied conditions. Additionally, the control over input gas concentrations, temperature, shear force from mixing, and medium pH can support diverse species that are otherwise challenging to cultivate. Photobioreactors can be run as a batch process with continuous gas feed and sparging, or as a chemostat flow-through system with continuous gas feed and sparging plus influent and effluent wastewater nutrient inputs. Here, we demonstrate the batch process with continuous gas feed and sparging.
The use of photobioreactors addresses several microalgal cultivation and production challenges. The field generally struggles with concerns of contamination by other microorganisms, efficient substrate utilization (which is especially important in the case of CO2 mitigation or wastewater treatment)2, pH control, illumination variability, and biomass productivity3. Photobioreactors enable researchers to study a wide range of phototrophs in closely-controlled batch systems, where even slow growing species are protected from predators or competing microorganisms4. These batch systems are also better at facilitating greater CO2 utilization rates and biomass productivity because they are closed systems that are more likely to be in equilibrium with supplied gases. Photobioreactor technology also offers pH control, the lack of which has hindered high biomass productivity in past studies5. At bench scale, the level of control offered by photobioreactors is advantageous to researchers. At larger industrial scales, photobioreactors can be used to maintain commercial bioproduct purity and improve production efficiency for nutraceutical, cosmetic, food, or feed applications6.
Microalgae are of great interest for biosequestration of CO2 because they can rapidly fix CO2 as biomass carbon. However, most anthropogenic sources of CO2 are contaminated with other corrosive and toxic gases or contaminants (NOx, SOx, CO, Hg), depending on combustion process fuel source. Growing interest in sustainable CO2 sequestration has prompted development of photobioreactor technologies to treat CO2-rich emissions, such as those from coal-fired power plants (Table 1). Unfortunately, there is inherent risk of human and environmental exposure to the corrosive and toxic contaminants during research and scale-up processes. As such, describing the safe assembly and operation of bioreactors using corrosive gases is necessary and instructive.
This method is for the use of a 2 L bench-scale photobioreactor for the growth of microalgae under carefully controlled experimental conditions. The protocol describes microalgal storage, inoculum preparation, and photobioreactor setup and sterilization. Beyond basic operation, this work describes microalgal biomass measurements and biomass productivity calculations, and adaptation of the equipment for microalgal cultivation with corrosive gases. The protocol described below is appropriate for researchers seeking to exert greater experimental control, optimize microalgal growth conditions, or axenically culture a range of phototrophic microbes. This method does not describe appropriate materials for cultivation of microbes that produce or consume flammable gases (e.g. CH4, H2, etc.)7.
Protocol
1. Safe use and sampling of a photobioreactor sparged with corrosive gases
NOTE: This method does not describe appropriate procedures for safe sampling of microalgal cultures that produce or consume highly flammable gases.
- Manage toxic gas as a risk to human health.
NOTE: Per the University of Iowa’s Chemical Hygiene Plan, the authors worked with the University Fire Safety Coordinator and University Environmental Health & Safety Industrial Hygiene Officer to develop a safety protocol for working with the toxic gases. - Set up a toxic gas monitoring system with sensors for each of the toxic gases in use. Calibrate the sensors according to manufacturer instructions. Bump test (check for sensor and alarm functionality with calibration gases) frequently, according to manufacturer instructions. Locate the gas monitor just outside the fume hood.
- Prior to beginning any corrosive gas experiments, notify nearby personnel of the risk and alarm system. Also, notify the appropriate local emergency responders. Post signs on laboratory entrances specifying which hazardous gases are in use.
- Instruct all nearby personnel to evacuate if toxic gas is detected. Notify laboratory supervisors and emergency response personnel.
NOTE: In a power outage, the gas-regulating tower will stop gas flow when it loses power. However, if the room heating, ventilation, and air conditioning (HVAC) system or fume hood go down without a power outage, this will result in leaking toxic gas.
- Instruct all nearby personnel to evacuate if toxic gas is detected. Notify laboratory supervisors and emergency response personnel.
- Model the possible accumulated concentration of toxic gases in the room if the fume hood were to fail using the American Industrial Hygiene Association’s (AIHA) mathematical modeling spreadsheet IH MOD8 for each gas.
- Obtain the room supply/exhaust air rate, Q, in m3 min-1 from building HVAC maintenance personnel or HVAC technician. Calculate the volume, V, of the laboratory (L x W x H) in m3. Calculate the contaminant emission rate, G, of each type of toxic gas in mg min-1, using Equation 1 adapted from the ideal gas law:
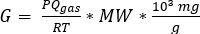 (1)
(1)
where P is the fraction of pressure exerted by the toxic gas at 1 atm (ppm gas/106 ppm), Qgas is the flow rate of the gas in L min-1, R is the universal gas constant (0.082057 L·atm·mol-1·K-1), T is temperature in K, and MW is the gas’ molecular weight in g mol-1. - Use the values for V, Q, and G for each gas (calculated in step 1.4.1) in the “Well-Mixed Room Model With option to cease generation and model room purge” algorithm in the IH MOD spreadsheet to calculate the accumulated room gas concentrations for each gas over a 1440 min (24 h) simulation period. Compare these values to the exposure limits (Table 2)9.
- Obtain the room supply/exhaust air rate, Q, in m3 min-1 from building HVAC maintenance personnel or HVAC technician. Calculate the volume, V, of the laboratory (L x W x H) in m3. Calculate the contaminant emission rate, G, of each type of toxic gas in mg min-1, using Equation 1 adapted from the ideal gas law:
2. Preparation of the microalgal inoculum
- Prepare the Scenedesmus obliquus inoculum, or other microalgal species selected for the photobioreactor, prior to the start of the experiment by transferring it from storage, whether cryopreserved or cultured on agar media.
- Add 30−50 mL of sterile microalgal growth medium (triple-nitrogen Bold’s basal medium [3N-BBM], Table 3) to a sterile (150−250 mL) shake flask capped with a foam stopper.
NOTE: Unless the flask is sparged, only one-fifth of the shake flask volume should be occupied by liquid media. - Use a biosafety cabinet to maintain sterility when transferring cells to a slant or shake flask. For cultures on agar, use a sterile loop to transfer microalgae from its agar plate or slant to the shake flask. For cryopreserved cultures, gradually thaw the cryopreserved sample and rinse away the cryoprotectant according to the chosen protocol10, then add the cells to the shake flask.
- Culture cells in 3N-BBM at 20−25 °C under 16 h:8 h light:dark and shaking at 115−130 rpm.
- Track microalgal growth over time using optical density (OD) measurements (as in sections 5 and 6). Allow the microalgae to reach its exponential growth phase (2−4 days) before transferring cells to the photobioreactor.
NOTE: Depending on the goal of the experiment, the cells may be rinsed of culture medium (this study) and/or concentrated with multiple centrifugation steps prior to inoculation of the bioreactor.
- Add 30−50 mL of sterile microalgal growth medium (triple-nitrogen Bold’s basal medium [3N-BBM], Table 3) to a sterile (150−250 mL) shake flask capped with a foam stopper.
3. Setup and operation of photobioreactor
- Use the photobioreactor (Figure 1) to control temperature, pH, stirring rate, gas flow rates, and input solution flow rates.
NOTE: The photobioreactor may be used to control the flow of up to four different input solutions, commonly acid, base, antifoam, and substrate.- Prepare 100 mL each of 1 N NaOH and 1 N HCl and add each to a 250 mL input solution bottle. Use secondary containment for these solutions.
- Store metered input solutions in autoclavable capped bottles equipped with dip tubes and a vent tube with a sterile inline air filter. Connect the dip tubes to the photobioreactor’s four input ports using autoclavable tubing and, during photobioreactor operation, submerse the dip tubes in the input solutions. Pass the 1.6 mm inside dimeter (ID) autoclavable tubing between the input solutions and their ports through separate peristaltic pumps which may be controlled either by manually selected flow rates or by feedback from pH and foam-level probes (in the case of acid, base, and antifoam solutions).
- Calibrate the photobioreactor pH meter prior to autoclaving.
- Connect the pH probe to the photobioreactor control tower by fitting the probe to the connecting line and twisting to lock. Use pH 4 and pH 7 buffers to calibrate the pH meter. Wait until values have stabilized before accepting the pH meter value.
- Disconnect the pH probe from the pH meter cord that connects the probe to the control tower.
- Surface sterilize with 70% ethanol or autoclave the probe with the reactor. To autoclave, cap the pH probe tightly with aluminum foil.
NOTE: If the probe is autoclaved, there is a risk of corrosion of the probe interior from steam damage. This capping method does not completely guarantee damage prevention. - Add 10 mM 4-(2-hydroxyethyl)piperazine-1-ethanesulfonic acid (HEPES) buffer to the culture medium to better control pH.
- Insert and screw closed the cold finger and exhaust condenser on the photobioreactor headplate.
- Insert the inoculation port and screw tightly in place. Add a length of autoclavable tubing to the section of inoculation port above the photobioreactor headplate. Prior to autoclaving the bioreactor, clamp the tubing closed with an autoclavable hose clamp.
- Attach tubing capped with sterile filters to any unused photobioreactor ports.
- Attach acid and base input solutions to the photobioreactor input ports via autoclavable tubing. Add 1.5 L of culture medium.
- Autoclave the reactor and associated input solutions for 30−45 min at 121 °C according to working volume.
NOTE: If the culture medium is adversely affected by autoclaving, add the media after autoclaving under sterile conditions in a laminar flow hood. The protocol can be paused here.
CAUTION: Reactor will be hot after removing from autoclave. - Attach the impeller motor to the impeller shaft and tighten the fitting.
- Arrange LED light panels symmetrically outside the bioreactor according to illumination requirements.
- Prior to autoclaving, measure and record the light intensity with a photometer. Place the illuminance sensor inside the photobioreactor vessel and face the sensor toward the light source.
- Connect up to two gas cylinders to supply the simulated coal-fired power plant emissions (Table 1) to the microalgae in the photobioreactor.
NOTE: The gas concentrations used in this study approximated those of the University of Iowa power plant.- Assemble the connections between the gas cylinder, gas regulating tower, and photobioreactor sparging ring. Attach appropriate regulators capable of 20 psi outlet pressure to the gas cylinders. Attach 6 mm ID pressure resistant tubing to the regulator outlet hose barb and secure with a hose clamp. Attach the other end of the pressure resistant tubing to the gas regulating tower gas inlet using a hose barb to 6 mm stem quick connect fitting secured with a hose clamp. Connect 3.2 mm ID tubing to the gas-regulating tower gas outlet using another 6 mm quick connect fitting and connect the other end of the outlet tubing to the sparging ring port at the photobioreactor headplate.
NOTE: For a second input gas, repeat step 3.9.1, but use a T-shaped hose barb to consolidate the two input gas lines to a single section of tube connected to the sparging ring port. - Set the outlet pressure to 20 psi on each gas regulator and use the bioreactor interface to set experimental gas flow rates.
NOTE: Calculate and report the volume of air under standard conditions per volume of liquid per minute (vvm); divide the volumetric gas flow rate by the culture volume. Report in units of per miniute.
- Assemble the connections between the gas cylinder, gas regulating tower, and photobioreactor sparging ring. Attach appropriate regulators capable of 20 psi outlet pressure to the gas cylinders. Attach 6 mm ID pressure resistant tubing to the regulator outlet hose barb and secure with a hose clamp. Attach the other end of the pressure resistant tubing to the gas regulating tower gas inlet using a hose barb to 6 mm stem quick connect fitting secured with a hose clamp. Connect 3.2 mm ID tubing to the gas-regulating tower gas outlet using another 6 mm quick connect fitting and connect the other end of the outlet tubing to the sparging ring port at the photobioreactor headplate.
- When sparging with more than one gas cylinder, confirm the supplied CO2 concentration to the photobioreactor with a CO2 sensor.
- Connect a software (e.g., GasLab) compatible CO2 sensor to the USB port of a computer. Download the most recent software that corresponds to the CO2 sensor model. Open the software and input the sensor model, measurement time interval, and duration of measurement data logging.
- Place the CO2 sensor and combined gas flow tube (prior to connecting the tube with the bioreactor) in a 100−250 mL, capped, vented vessel (external to the bioreactor).
NOTE: During the experiment, the headspace CO2 concentrations may be measured from one of the venting tubes on the photobioreactor headplate. - Start the CO2 concentration measurements on the user interface and wait for the measurements to equilibrate.
- Use the photobioreactor user interface to adjust the gas flow rates from each tank until the desired total flow rate (0.1 L min-1) and CO2 concentration (12%) is achieved.
- On the photobioreactor user interface, use the STIRR function to set the impeller rotation rate. Ensure that the mixing rate is rapid enough for the culture medium to assimilate the sparged gas bubbles.
NOTE: Certain microalgal species have weak cell structures and will be damaged or ruptured by high shear force.
4. Adapting the photobioreactor and experimental setup for toxic gas use
CAUTION: The corrosive gases in real or simulated flue gas are corrosive and toxic. These gases pose serious risk if inhaled.
NOTE: This method does not describe appropriate materials for safe cultivation of microbes that produce or consume highly flammable gases (i.e., methane, hydrogen, etc.).
- Replace brass, plastic, and standard tubing components with corrosion resistant materials.
- Use stainless steel to reliably resist corrosion from the strong acids formed by the reaction between NOx or SOx and water. Replace plastic quick connect fittings at the gas inlets and outlets on the gas-regulating tower with stainless steel quick connect fittings. Use stainless steel regulators for gas cylinders including the outlet hose barb rather than brass.
- Use polytetrafluoroethylene (PTFE) or natural ethyl vinyl acetate (EVA) tubing to resist corrosion from NOx and SOx gases (respectively) on the connections between the gas cylinder to the gas-regulating tower and the gas-regulating tower to the photobioreactor.
- After autoclaving, assemble the photobioreactor and gas cylinders within a walk-in fume hood. Place the photobioreactor on a table inside secondary containment and place gas cylinders in free standing cylinder collars or a cylinder rack.
- After initiating gas flow, use a bubble type liquid leak detector to check for gas leaks in all connections between the gas cylinders and bioreactor. Use a wash bottle filled with a 1:100 (v:v) dilution of dish soap:water to cover the connection with a small stream of soap solution.
NOTE: Leaks will be indicated by bubbling at the connections. - When initiating the microalgal experiments, begin sparging then adjust pH before inoculation (as in standard photobioreactor experiments).
NOTE: Buffering the culture medium during corrosive gas experiments is highly recommended since the input gases are strongly acidic. - Inoculate the photobioreactor by aspirating the prepared microalgal inoculum into a sterile syringe, fitting the syringe to the tubing attached to the inoculation port, opening the inoculation tubing clamp, and depressing the syringe.
- Check the gas monitor, gas cylinder pressures, and photobioreactor twice daily (and prior to sampling) for elevated levels of toxic gas or indication of leaks.
- Limit the fume hood sash opening to a width that allows the bioreactor and gas cylinder regulators to be reached. To avoid inhalation exposure risk, ensure that the opening does not expose personnel above the torso region.
- Turn the gas cylinder regulators to the closed position to cease gas flow to the reactor. Close the fume hood sash and allow 5 min for the hood to evacuate the corrosive gases before sampling the photobioreactor culture.
- Sample within the fume hood either by opening a headplate port and using a sterile serological pipet or drawing culture into a syringe through the inoculation/sampling port. Secure the photobioreactor ports prior to opening the gas cylinders and resuming the experiment.
5. Measuring microalgal biomass productivity
- Use a calibration curve to relate microalgal culture OD750 measurements to dried microalgal biomass concentrations.
- Prepare several (minimum: 4, minimum working volume: 500 mL) flasks with sterile microalgal medium and inoculate with the species of interest (e.g., S. obliquus in this study).
- Measure the culture OD750 until growth is exponential, and promptly sacrificially sample the flasks by filtering known volumes (minimum of 100 mL) of the contents with a 0.45 μm filter membrane of known mass. Use covered aluminum weigh boats or glass vessels to support the biomass and filters as they are dried.
- Mass the biomass and filters after drying for approximately 18−24 h in an oven between 80−100 °C. To verify complete drying, measure again after an additional 2−3 h to determine if the mass has stabilized.
- Subtract the filter mass from the combined biomass and filter mass to calculate the biomass mass.
- Plot the calibration curve as measured OD750 against the biomass concentration (mass of biomass divided by the volume of filtered culture) and fit the data with a linear regression.
6. Biomass productivity modeling and rate calculations
- Calculate experiment biomass concentrations from OD750 measurements using the calibration curve linear regression (determined in section 5).
- Fit batch microalgal growth data from lag to exponential to stationary phase with a logistic regression (Equation 2) in graphing and statistics software (Table of Materials):
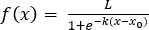 (2)
(2)
where L is the curve’s maximum biomass concentration value, k is the relative steepness of the exponential phase (time-1), xo is the time of the sigmoidal curve’s midpoint, and x is time.- Manually enter the logistic equation above. Select Fit a curve with nonlinear regression on the Analysis tab in the software. On the left of the Parameters: Nonlinear Regression box, choose Create new equation under the Nuevo dropdown box. Use the default explicit equation as equation type, name the new function, and define the new function as Y = L/(1+ exp(-k*(x-b))), where b represents xo.
- Calculate the overall biomass productivity of the microalgal batch by dividing the difference between the final and initial biomass concentrations by the difference between the final and initial times.
- Calculate the maximum biomass productivity of the microalgal batch from the derivative of Equation 2 (Equation 3) at the sigmoid midpoint, when x = xo.
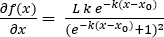 (3)
(3)
Representative Results
A calibration curve for the green microalgae, S. obliquus, harvested in the exponential phase, was established with OD750 and dried biomass concentrations (Figure 2). The linear regression had an R2 value of 0.9996.
An S. obliquus culture was started in a 250 mL Erlenmeyer flask from a culture stored on a refrigerated agar plate. The microalga was inoculated in 3N-BBM with 10 mM HEPES buffer and sparged with 2.2% CO2 in a 2 L photobioreactor with 1.5 L working volume (0.07 vvm) (Figure 1). The batch was tracked via OD750; the biomass concentrations were calculated from the calibration curve, and then modeled with a logistic curve (Figure 3). The photobioreactor maintained the culture at pH 6.8, 100 cm3 min-1 total gas flow rate, continuous 280 μmol m-2 s-1 illumination, and 27 °C. The logistic curve fit biomass concentration data from lag to exponential to stationary phase. From the logistic model, the maximum biomass concentration during the batch was 2070 ± 20 mg L-1, maximum biomass productivity occurred at 4.6 day, and the rate of specific biomass productivity was 1.0 d-1. The maximum biomass productivity, calculated from the derivative of the logistic curve at the time of maximum growth, was 532 ± 60 mg L-1 d-1.
The well-mixed room model was used to calculate the accumulated concentration of NO2, SO2, and CO in the case of fume hood failure for 24 h. These values were compared to the exposure limits (Table 2). For example, in the scenario where 0.05 L min-1 of 400 ppm NO2 is released during a fume hood failure period of 24 h, the well-mixed room model with inputs of calculated G = 0.0377 mg min-1, Q = 0.0001 m3 min-1, V = 100 m3, and maximum time for simulation = 1440 min predicts NO2 accumulation to 0.54 mg m-3 (0.29 ppm), which is above the acceptable chronic exposure limit (American Conference of Governmental Industrial Hygienists threshold limit value [ACGIH TLV]) and below the short-term exposure limit (STEL).
A promising preliminary trial with simulated flue gas achieved a greater maximum microalgal biomass productivity rate (690 ± 70 mg L-1 d-1) than that of 12% CO2 and ultra-zero air (510 ± 40 mg L-1 d-1) (Figure 4). Prior to the experiment, a gas monitor was calibrated with CO, NO2, and SO2. The simulated flue gas experiment was carried out without any risk to personnel or damage to equipment from corrosive gases.
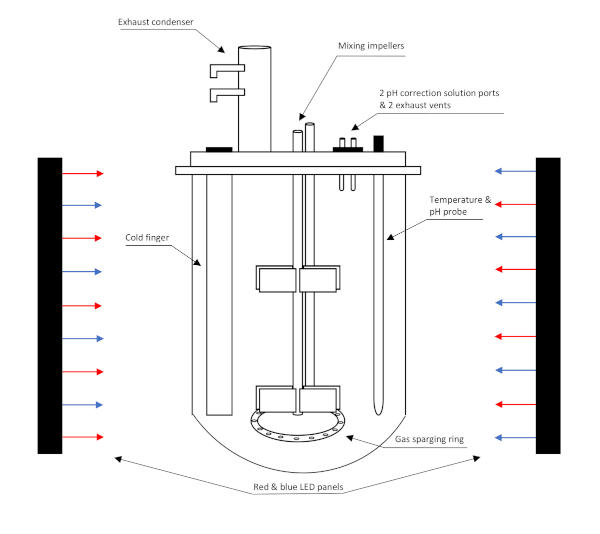
Figure 1: Bench-top photobioreactor illuminated by red and blue LED lights. The photobioreactor operates as a 2 L batch reactor with 1.5 L working volume. The photobioreactor is continuously fed with gases through the sparging ring and excess gas vents through ports in the headplate. Adapted with permission from Molitor et al.5. Please click here to view a larger version of this figure.
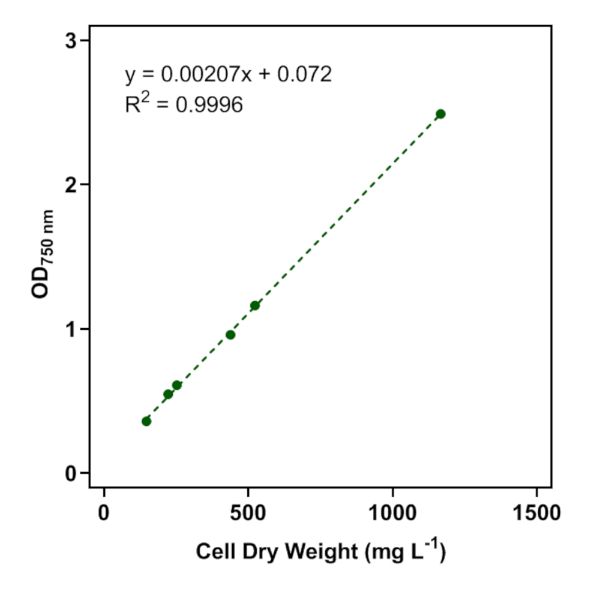
Figure 2: Calibration curve relating OD750 a S. obliquus cell dry weight. S. obliquus cell culture light absorption was measured at 750 nm, then cells were filtered and dried to obtain cell dry weight measurements. Reprinted with permission from Molitor et al.5. Please click here to view a larger version of this figure.
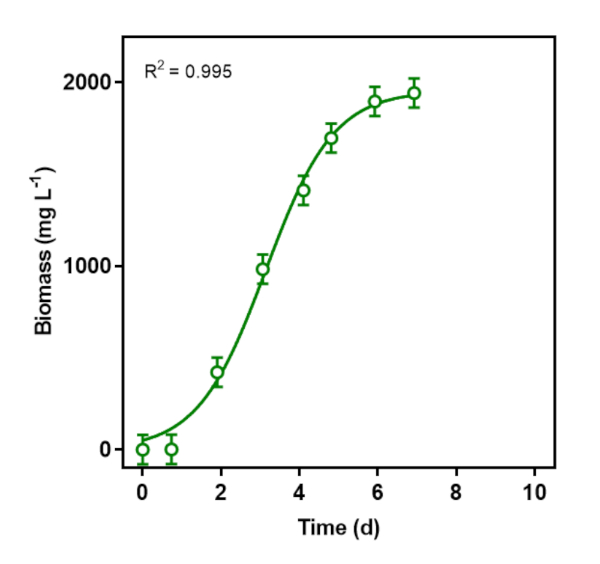
Figure 3: S. obliquus growth data at 2.2% CO2 input modeled with a logistic regression. The data points represent biomass values as calculated from optical density measurements. The data have been modeled with a logistic regression through a least squares fit; 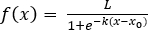 where L = 1955 mg L-1, k = 1.154 d-1, and x0 = 3.317 d. R2 = 0.995. Please click here to view a larger version of this figure.
where L = 1955 mg L-1, k = 1.154 d-1, and x0 = 3.317 d. R2 = 0.995. Please click here to view a larger version of this figure.
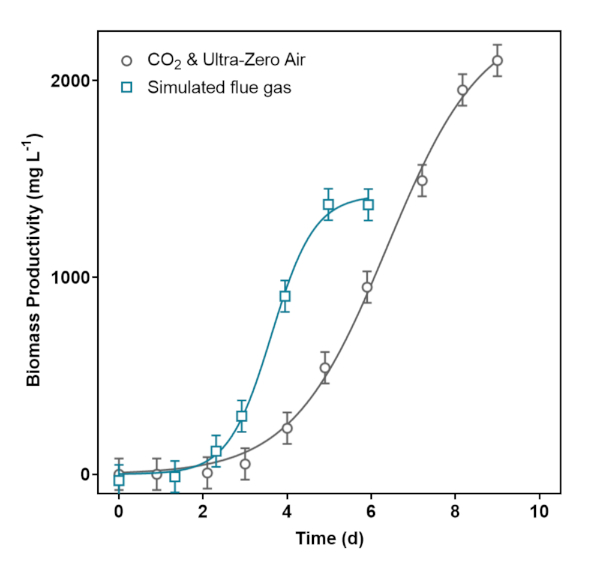
Figure 4: Modeled S. obliquus growth at 12% CO2, with and without additional simulated flue gas components. The biomass measurements from each batch of microalgae were modeled with logistic regressions. Please click here to view a larger version of this figure.
| Component | Percent |
| H2O | 12.6% |
| CO2 | 11.6% |
| O2 | 5.8% |
| CO | 0.048% |
| SO2 | 0.045% |
| NO2 | 0.022% |
| N2 | 69.9% |
Table 1: Composition of coal-fired power plant emissions. These quantities were averaged from the University of Iowa power plant emissions data collected at minute intervals over the span of 10 h.
| Toxic gas | TWA | CEILING | STEL | NIOSH IDLH | NIOSH REL | ACGIH TLV | CDC Description |
| CO | 35 ppm | 200 ppm | – | 1,200 ppm | 35 ppm | 25 ppm | Colorless, odorless |
| SO2 | 2 ppm | 100 ppm | 5 ppm | 100 ppm | 2 ppm | 2 ppm | Colorless gas with a characteristic, irritating, pungent odor |
| NO2 | 3 ppm | 5 ppm | 1 ppm | 13 ppm | 1 ppm | 0.2 ppm | Yellowish-brown liquid or reddish-brown gas (above 70 °F) with a pungent, acrid odor |
Table 2: Exposure limits and descriptions for toxic gases (CO, SO2, NO2) in flue gas. OSHA TWA: time weighted average (usually 8 h period), CEILING: value never to be reached, STEL: short-term exposure limit (TWA over 15 min), NIOSH IDLH: danger to life and health, NIOSH REL: 15 min exposure limit, ACGIH TLV: acceptable chronic exposure limit, no ill effects.
| Compound | mM |
| NaNO3 | 8.82 x 100 |
| MgSO4·7H2O | 3.04 x 10-1 |
| NaCl | 4.28 x 10-1 |
| K2HPO4 | 4.31 x 10-1 |
| KH2PO4 | 1.29 x 100 |
| CaCl2·2H2O | 1.70 x 10-1 |
| ZnSO4·7H2O | 3.07 x 10-2 |
| MnCl2·4H2O | 7.28 x 10-3 |
| MoO3 | 4.93 x 10-3 |
| CuSO4·5H2O | 6.29 x 10-3 |
| Co(NO3)2·6H2O | 1.68 x 10-3 |
| H3BO3 | 1.85 x 10-1 |
| EDTA | 1.71 x 10-1 |
| KOH | 5.52 x 10-1 |
| FeSO4·7H2O | 1.79 x 10-2 |
| H2SO4 (concentrated) | 1 x 10-3 μL |
Table 3: Composition of triple-nitrogen Bold’s basal medium (3N-BBM). The quantity of nitrogen has been tripled from the original Bold’s basal medium11.
Discussion
Batch, axenic photobioreactor experiments with regulated pH, temperature, gas flow rate, and gas concentration promote meaningful results by eliminating contamination by non-target algal strains and variability in culture conditions. Accurate pure culture growth kinetics can be obtained even in the presence of corrosive gases (CO2, SO2, NO2), which serve as nutrients, turning waste gases into a valuable product such as animal feed.
Prior to beginning any microalgal experiment, the chosen microalga culture should be taken from storage and readapted to liquid culture. Growing the microalgae into exponential phase improves the probability that experiments have equivalent initial conditions and that the microalgae do not stagnate in lag phase after inoculation.
Calibration curves relating optical density and biomass concentrations are especially important during studies of biomass productivity. High microalgal biomass productivity is one of the key goals of the microalgal industry and, as such, is often an indicator of research success12. Therefore, accurate calculations of biomass concentrations from optical density measurements must stem from species-specific, precise, and accurate calibration curve data. To avoid potential optical interferences, it is important that measurements for the calibration curve and during the experiment be made in equivalent background solutions. Additionally, the calibration curve should be made with measurements taken from microalgae in the growth phase(s) most representative of those in the experiments. Certain microalgal species can have dramatic differences in cell size during different growth phases which can alter the optical density and perceived biomass concentrations. It should be noted that biomass productivity is related to, but not equivalent to, growth rate. Specific growth rate depends on the number of cells (change in cell density over time/cell density) present, and specific biomass productivity depends on the bulk mass of cells (change in mg/L biomass over time per mg/L biomass)13 present.
When microalgal biomass concentrations are modeled with a logistic curve, experimental results can be meaningfully compared by interpolating biomass concentrations and accurately calculating biomass productivities. However, care should be taken when interpreting these experimental results; it is inappropriate to compare overall and maximum batch biomass productivity. While maximum biomass productivity values are useful to compare batch results, overall biomass productivity is misleading without further information on the experiment duration and microalgal growth phases. These rates change continuously during the lag, log growth, and stationary phases.
During experiments with corrosive gases which are characteristic of power plant or industrial combustion emissions, caution should be exercised for both human health and equipment longevity. Standard parts must be replaced with more robust materials, and consumables such as tubing should be inspected and replaced more frequently to resist corrosion, prevent leaks, and avoid human exposure. Extra safety measures and risk awareness are essential to safe and successful operation and sampling. The method is not appropriate for flammable gases because there is potential for gas accumulation within the headspace and the equipment is neither designed for such risks nor suitable for safe adaptation to such conditions.
Divulgaciones
The authors have nothing to disclose.
Acknowledgements
This material is based upon work supported by the National Science Foundation Graduate Research Fellowship under Grant No. 1546595. Any opinion, findings, and conclusions or recommendations expressed in this material are those of the authors and do not necessarily reflect the views of the National Science Foundation. The work was also supported by a University of Iowa Graduate and Professional Student Government research grant, and the University of Iowa Foundation, Allen S. Henry endowment. Research was conducted in the W. M. Keck Phytotechnologies Laboratory. The authors would like to thank the University of Iowa power plant staff, especially Mark Maxwell, for expertise and financial support for the simulated flue gases. The authors would also like to acknowledge Emily Moore for her assistance with sampling and analysis and Emily Greene for her assistance and participation in the protocol video.
Materials
| Biostat A bioreactor | Sartorius Stedim | 2-liter bioreactor for microbial fermentation; designed to be autoclaved; pH, temperature, gas flow rate control | |
| Bump test NO2 gas | Grainger | GAS34L-112-5 | Calibration gas for MultiRAE gas detector |
| Bump test O2, CO, LEL gas | Grainger | GAS44ES-301A | Calibration gas for MultiRAE gas detector |
| Bump test SO2 gas | Grainger | GAS34L-175-5 | Calibration gas for MultiRAE gas detector |
| Corrosion resistant tubing for NO2 gas | Swagelok | SS-XT4TA4TA4-6 | PTFE Core Hose Smooth Bore X Series—Fiber Braid and 304 SS Braid Reinforcement |
| Corrosion resistant tubing for SO2 gas | QC Supply | 120325 | Reinforced Braided Natural EVA Tubing – 1/4" ID |
| cozIR 100% CO2 meter | Gas Sensing Solutions Ltd. | CM-0121 at CO2meter.com | CO2 meter for concentrations up to 100% |
| cozIR 20% CO2 meter | Gas Sensing Solutions Ltd. | CM-0123 at CO2meter.com | CO2 meter for concentrations up to 20% |
| Durapore Membrane Filter, 0.45 μm | Millipore Sigma | HVLP04700 | Hydrophilic, plain white, 47 mm diameter, 0.45 μm pore size, PVFD membrane filters |
| Gas cylinder regulators | Praxair | PRS 40221331-660 | Single-stage stainless steel regulator configured for 0-15 psi outlet assembly diaphragm valve with 1/4" MNPT threads, Stainless steel to resist corrosion from NOx and SOx |
| Gas cylinders | Praxair | Ulta-zero air, high purity CO2, or custom gas composition | Dependent on study objectives |
| Gas monitoring and leak detection system | RAE Systems by Honeywell | MAB3000235E020 | Pumped model that detects O2, SO2, NO2, CO, and LEL |
| GasLab software | GasLab | v2.0.8.14 | Software for CO2 meter measurements and data logging |
| Hose barb | Grainger | Item # 3DTN3 | Used to adapt regulators to tubing, Stainless steel to resist corrosion from NOx and SOx |
| K30 1% CO2 meter | Senseair | CM-0024 at CO2meter.com | CO2 meter for concentrations less than 1% |
| LED grow panels | Roleadro | HY-MD-D169-S | Red & blue LED light panels |
| Memosens dissolved oxygen probe | Endress+ Hauser | COS22D-19M6/0 | Autoclavable (with precautions) dissolved oxygen probe for bioreactor |
| Memosens pH probe | Endress+ Hauser | CPS71D-7TB41 | Autoclavable (with precautions) pH probe for bioreactor |
| Oven, Isotemp 500 Series | Fisher Scientific | 13246516GAQ | Small oven for drying |
| Prism GraphPad software | GraphPad Software | Version 7.03 or 8.0.1 | Graphing software for data organization, data analysis, and publication-quality graphs |
| Stem to hose barb fitting | Swagelok | SS-4-HC-A-6MTA | Stainless Steel Hose Connector, 6 mm Tube Adapter, 1/4 in. Hose ID |
| Tubing, dilute acid/base transfer | Allied Electronics and Automation | 6678441 | Silicone TP Process Tubing; 1.6mm Bore Size; 3000mm Long; Food Grade |
| Tubing, gas transfer | Allied Electronics and Automation | 6678444 | Silicone TP Process Tubing; 3.2mm Bore Size; 3000mm Long; Food Grade |
Referencias
- Obom, K. M., Magno, A., Cummings, P. J. Operation of a Benchtop Bioreactor. Journal of Visualized Experiments. (79), e50582 (2013).
- Cheah, W. Y., Pau Loke, S., Chang, J. -. S., Ling, T., Juan, J. C. Biosequestration of atmospheric CO2 and flue gas-containing CO2 by microalgae. Bioresource Technology. 184, 190-201 (2014).
- Xu, L., Weathers, P. J., Xiong, X. -. R., Liu, C. -. Z. Microalgal bioreactors: Challenges and opportunities. Engineering in Life Sciences. 9 (3), 178-189 (2009).
- Tsang, Y. F. . Photobioreactors: Advancements, Applications and Research. , (2017).
- Molitor, H. R., Moore, E. J., Schnoor, J. L. Maximum CO2 Utilization by Nutritious Microalgae. ACS Sustainable Chemistry & Engineering. 7 (10), 9474-9479 (2019).
- Khan, M. I., Shin, J. H., Kim, J. D. The promising future of microalgae: current status, challenges, and optimization of a sustainable and renewable industry for biofuels, feed, and other products. Microbial Cell Factories. 17 (1), 36 (2018).
- Benemann, J. R. Hydrogen production by microalgae. Journal of Applied Phycology. 12 (3), 291-300 (2000).
- . IH MOD Available from: https://aiha.org/public-resources/consumer-resources/topics-of-interest/ih-apps-tools (2019)
- Centers for Disease Control and Prevention, Immediately Dangerous To Life or Health (IDLH) Values. The National Institute for Occupational Safety and Health Available from: https://www.cdc.gov/niosh/idlh/intridl4.html (2019)
- Nakanishi, K., Deuchi, K., Kuwano, K. Cryopreservation of four valuable strains of microalgae, including viability and characteristics during 15 of cryostorage. Journal of Applied Phycology. 24 (6), 1381-1385 (2012).
- Bischoff, H. W., Bold, H. C. . Some soil algae from Enchanted Rock and related algal species. , (1963).
- Mata, T. M., Martins, A. A., Caetano, N. S. Microalgae for biodiesel production and other applications: A review. Renewable and Sustainable Energy Reviews. 14 (1), 217-232 (2010).
- Wood, A. M., Everroad, R. C., Wingard, L. M., Andersen, R. A. Measuring growth rates in microalgal cultures. Algal Culturing Techniques. , 270-272 (2005).

