Inducing Hairy Roots by Agrobacterium rhizogenes-Mediated Transformation in Tartary Buckwheat (Fagopyrum tataricum)
Summary
We describe a method of inducing hairy roots by Agrobacterium rhizogenes-mediated transformation in Tartary buckwheat (Fagopyrum tataricum). This can be used to investigate gene functions and production of secondary metabolites in Tartary buckwheat, be adopted for any genetic transformation, or used for other medicinal plants after improvement.
Abstract
Tartary buckwheat (TB) [Fagopyrum tataricum (L.) Gaertn] possesses various biological and pharmacological activities because it contains abundant secondary metabolites such as flavonoids, especially rutin. Agrobacterium rhizogenes have been gradually used worldwide to induce hairy roots in medicinal plants to investigate gene functions and increase the yield of secondary metabolites. In this study, we have described a detailed method to generate A. rhizogenes-mediated hairy roots in TB. Cotyledons and hypocotyledonary axis at 7–10 days were selected as explants and infected with A. rhizogenes carrying a binary vector, which induced adventitious hairy roots that appeared after 1 week. The generated hairy root transformation was identified based on morphology, resistance selection (kanamycin), and reporter gene expression (green fluorescent protein). Subsequently, the transformed hairy roots were self-propagated as required. Meanwhile, a myeloblastosis (MYB) transcription factor, FtMYB116, was transformed into the TB genome using the A. rhizogenes-mediated hairy roots to verify the role of FtMYB116 in synthesizing flavonoids. The results showed that the expression of flavonoid-related genes and the yield of flavonoid compounds (rutin and quercetin) were significantly (p < 0.01) promoted by FtMYB116, indicating that A. rhizogenes-mediated hairy roots can be used as an effective alternative tool to investigate gene functions and the production of secondary metabolites. The detailed step-by-step protocol described in this study for generating hairy roots can be adopted for any genetic transformation or other medicinal plants after adjustment.
Introduction
Tartary buckwheat (TB) (Fagopyrum tataricum (L.) Gaertn) is a type of dicotyledon belonging to the genus Fagopyrum and the family Polygonaceae1. As a type of Chinese medicine homologous food, TB has been receiving considerable interest owing to its distinctive chemical composition and diverse bioactivities against diseases. TB is primarily rich in carbohydrates, proteins, vitamins, and carotenoids as well as in polyphenols such as phenolic acids and flavonoids1. Various biological and pharmacological activities of flavonoids, including antioxidative, antihypertensive2, and anti-inflammatory as well as anticancer and antidiabetic properties, have been demonstrated3.
Agrobacterium rhizogenes is a soil bacterium that contributes to the development of hairy root disease in several higher plants, especially dicotyledons, by infecting wound sites4,5. This process is initiated by the transfer of the T-DNA in the root-inducing (Ri) plasmid5,6 and is commonly accompanied by the integration and expression of an exogenous gene from the Ri plasmid and the subsequent steps of generating the hairy root phenotype7. A. rhizogenes-mediated transgenic hairy roots, as a powerful tool in the field of plant biotechnology, have been most widely used owing to their stable and high productivity and easy obtainment in a short period. Moreover, hairy roots induced by A. rhizogenes are efficiently distinguished by their plagiotropic root development and highly branching growth in a hormone-free medium8. They can be used in several fields of research, including artificial seed production, root nodule research, and in studying the interactions with other organisms such as mycorrhizal fungi, nematodes, and root pathogens7,9. In addition, hairy root transformation cultures have been extensively used as an experimental system to investigate the biochemical pathways and chemical signaling and to produce plant secondary metabolites that are used as pharmaceuticals, cosmetics, and food additives8,10. The valuable secondary metabolites, including indole alkaloids, aconites, tropane alkaloids, terpenoids, and flavonoids, synthesized in wild-type hairy roots have been investigated for several decades in numerous species, such as ginsenoside in Panax ginseng11, coumarine in Ammi majus12, and phenolic compounds in TB2,13.
Hairy roots have been produced using A. rhizogenes in 79 plant species from 27 families14. For instance, A. rhizogenes-mediated hairy root transformation has been reported in soybean15,16, Salvia17, Plumbago indica18, Lotus japonicus19, and chicory (Cichorium intybus L.)20. TB hairy root transformation has also been investigated2. Few detailed protocols are available regarding the development of hairy roots mediated by A. rhizogenes either carrying a binary vector or not. For instance, Sandra et al.21 introduced a method of producing transgenic potato hairy roots sustained in wild-type shoots. The fully developed hairy roots could be visualized 5-6 weeks after the injection of A. rhizogenes carrying the gus reporter gene into the stem internodes of potato plants. Another study had also reported a transgenic hairy root system induced by A. rhizogenes harboring the gusA reporter gene in jute (Corchorus capsularis L.)22. Furthermore, Supaart et al.23 obtained transgenic tobacco hairy roots using A. rhizogenes transformed with the expression vector pBI121 carrying the gene of Δ1-tetrahydrocannabinolic acid (THCA) synthase to produce THCA.
However, a step-by-step process for an effective generation of hairy root transformation, especially in TB, has been relatively less demonstrated. In this study, we have described a detailed protocol using A. rhizogenes carrying the reporter gene (GFP), a selective marker (Kan), and a gene of interest (b4, an identified from our group but unpublished gene from basic helix-loop-helix (bHLH) family) to generate hairy root genetic transformation in TB. The experiment lasted for 5-6 weeks, from the inoculation of seeds to generation of hairy roots, involving the explant preparation, infection, coculturing, subculturing, and subsequent propagation. Furthermore, A. rhizogenes containing a binary plasmid carrying the TB transgene of myeloblastosis transcription factor 116 (FtMYB116) was used to determine whether FtMYB116 can promote accumulation of flavonoids, particularly rutin, in TB at the gene and metabolic level through the TB hairy root transformation. FtMYB116, which is a light-induced transcriptional factor, regulates the synthesis of rutin under different light conditions5. Chalcone synthase (CHS), flavanone-3-hydroxylase (F3H), flavonoid-3'-hydroxylase (F3'H), and flavonol synthase (FLS)24 are key enzymes involved in the metabolic pathway of rutin biosynthesis. Therefore, this study demonstrates the overexpression of FtMYB116 in TB hairy roots and the expression of key enzyme genes as well as the content of rutin and other flavonoids such as quercetin.
Protocol
The TB used in this study was named as BT18, which originated from the breed of "JinQiao No.2" cultivated by the Research Center of Small Miscellaneous Grain of Shanxi Academy of Agricultural Science. The primary steps of this protocol are illustrated in Figure 1.
NOTE: Operate explants-related manipulation rapidly, and when possible, keep the Petri dishes closed to avoid wilting and contamination. Unless otherwise stated, all the explant incubations were conducted under the condition of a 14-h light and a 10-h dark photoperiod at 25 °C. Unless otherwise stated, all explants- or bacteria-related operations were performed under aseptic conditions in a laminar flow hood. All the media ingredients for A. rhizogenes and in vitro plant cultures are provided in Table 1. After adjusting the pH, all media were autoclaved at 120 °C for 20 min. Solidified media were prepared by filling 25 mL of medium into a Petri dish of 9-cm diameter and allowing it to solidify.
CAUTION: Deposit all the genetically modified bacteria and plants into the appropriate waste container. Operate all hazardous chemicals in a fume cupboard and deposit them in the hazardous waste container.
1. Preparation of TB explants
- Preparation of TB seeds
- Select plump and undamaged TB seeds (Figure 1A1) that have been preserved at a temperature less than−20 °C for no more than 2 years.
- Soak the seeds in water at 28 °C for approximately 20 min so that the seed coat can be easily peeled off (Figure 1A2). If necessary, use scissors to cut a slot on the seeds to facilitate the peeling.
- Sterilization of TB seeds
- Place 100–200 peeled seeds into a 100 mL sterilized conical flask.
- Disinfect the seeds using 75% ethanol for 30 s.
- Replace the ethanol with 5% sodium hypochlorite and disinfect for 15 min.
NOTE: Disinfection using mercury bichloride at a concentration of 1 g/L for 8 min may be used as an alternative sterilizer to replace sodium hypochlorite in any case of inadequate sterilization.
CAUTION: Mercury bichloride is hazardous and not an environment-friendly material. Operate it in the fuming cupboard and deposit it into the hazardous waste container, if mercury bichloride is used in any case. - Pour out the sodium hypochlorite.
- Wash the seeds using sterile deionized water 5 times.
- Blot the seeds dry with a sterile bibulous paper.
- Preparation of TB seedlings
- Prepare 50 mL Murashige and Skoog (MS) basal medium (1962) supplemented with 30 g/L sucrose and 7 g/L agar powder (MSSA) (Table 1) in a 300 mL plant tissue culture bottle.
- Adjust the pH to 5.8 before autoclaving.
- Distribute 10 seeds evenly per bottle of MSSA medium.
- Germinate the seeds in a culture room at 25 °C ± 1 °C under the light condition for 7–10 days.
- Preparation of sterile explants
- Select robust seedlings of TB (Figure 1C), when the 2 pieces of cotyledons are unfolded.
- Cut off the seedlings from the roots (Figure 1C red-dash arrowheads), avoiding contact with the medium.
- Place them in a sterile Petri dish.
- Cut the hypocotyls into 0.8–1 cm segments, and shear the cotyledons into approximately 0.5 cm pieces.
- Preculture these explants on MSSA medium under the light condition for 24 h.
- Transfer them into a 100 mL sterilized conical flask, which are now ready for infection.
2. Preparation of A. rhizogenes for transformation
NOTE: The A. rhizogenes strain ACCC10060 was kindly provided by the Institute of Medicinal Plant Development and preserved at −80 °C. A. rhizogenes was transformed with the binary vector pK7GWIWG2D (II) that harbors a T-DNA carrying the b4 gene accompanying a GFP as an indicator gene and the Kan resistance gene as a selectable marker. The gene b4 is a member of the transcription factor bHLH family, which has not yet been published. To evaluate the potential of TB hairy roots, A. rhizogenes was transformed with the binary vector pK7WG2D containing the MYB116 gene to investigate its effect on the production of secondary metabolites such as flavonoids at the level of gene expression and by metabolic analyses. Activated A. rhizogenes should be well prepared at the same time with the explants.
- Activation of A. rhizogenes
- Thaw A. rhizogenes on ice
- Dip the bacteria and line them evenly onto yeast mannitol medium (YEB) supplemented with 15 g/L agar powder, 50 mg/L rifampicin, and 50 mg/L spectinomycin (YEBARS, pH 7.0).
- Incubate the bacteria at 28 °C for 12–16 h.
- Pick a monoclonal colony and culture it in another Petri dish in the same above-described manner.
- Select monoclonal colonies and culture them in a 100 mL sterilized conical flask containing 20 mL of YEB medium supplemented with 50 mg/L rifampicin and 50 mg/L spectinomycin (YEBRS, pH 7.0) at 28 °C and 200 rpm for 16–18 h until the OD600 value reaches 2.0.
- Incubate 2%–4% of the abovementioned culture in another 100-mL conical flask containing 20 mL of YEBRS medium at 28 °C and 200 rpm for 4-5 h until the OD600 value reaches approximately 0.5 (Figure 1D).
3. Infection and screening of TB explants
NOTE: The objective of this protocol is to obtain genetically transformed hairy roots. The wild-type roots were used as the negative control to assess the transgenic expression. In this protocol, A. rhizogenes was transformed with binary vector either pK7WG2D carrying the gene of FtMYB116 or pK7GWIWG2D (II) carrying the gene of b4 in advance.
- Resuspension of A. rhizogenes
- Transfer the culture obtained in step 2.1.6 into a 50-mL sterilized centrifugal tube.
- Spin at 4,000 x g for 10 min at 20 °C.
- Remove the supernatant and resuspend the bacterial pellet with MS medium supplemented with 30 g/L sucrose and 300 μM acetosyringone (AS) (MSSAS, pH 5.8) to OD600 ≈ 0.2.
- Infection of explants
- Infuse the bacterial suspension obtained in step 3.1.3 into a conical flask containing the explants prepared in step 1.4.6 for 10 min (Figure 1E).
- Take out the explants, and blot them dry using a sterile bibulous paper.
4. Coculture of explants with A. rhizogenes
- Place a sterile 9 cm diameter filter paper on the MS medium, which is solidified using 7 g/L agar powder supplemented with 30 g/L sucrose and 100 μM AS (MSSAAS medium, pH 5.2).
- Overlay the explants on the filter paper at 25 °C for 3 days in the dark (Figure 1F).
5. Induction and selective culture
- Place approximately 20 infected explants on the MSSA medium supplemented with 500 mg/L cefotaxime and 50 mg/L kanamycin (Kan) (MSSACK, pH 5.8) (Figure 1G).
- Incubate them vertically under the light condition at 25 °C ± 1 °C. The hairy roots occur approximately 1 week after incubation (Figure 1H black-dash arrowheads indicate occurrence of hairy roots).
NOTE: Replace MSSACK medium every 15 days if necessary.
6. Subculturing TB hairy roots
NOTE: This procedure aims to harvest vigorous hairy roots. Regularly observe the growth of hairy roots during propagation, and remove the contaminated and inactivated ones in a timely manner. If necessary, repeat the following steps to propagate more hairy roots. It takes approximately 10–14 days from subculturing to harvest.
- Select the hairy roots showing white appearance and rapid growth.
- Cut them into pieces of 2-3 cm.
- Clearly number them on a clean bench.
- Subculture them in a 100 mL sterilized conical flask containing 5 mL of MS medium supplemented with 30 g/L sucrose and 50 mg/L Kan (MSSK, pH 5.8) at a rotary speed of 80 rpm at 25 °C in the dark until they overspread to the bottom of the flask (Figure 1I).
7. Identification of transformed hairy roots and conservation
NOTE: Transformed hairy roots can be identified based on the aspects of morphology and gene level. Identification can also be conducted according to the hairy root genome and resistance, which are not covered in this protocol. This procedure primarily focuses on reporter gene and target gene identification.
- Remove tawny and contaminated hairy roots and select those with white appearance.
- Evaluate if there is green fluorescence under a blue/light dual ultraviolet transilluminator.
- Select the hairy roots exhibiting a strong fluorescence signal in the numbered tubes or wrapped using a marked tinfoil after drying them out with an absorbent paper.
- Lyophilize them in liquid nitrogen, followed by storing all the harvest at −80 °C for further investigation.
- Gene identification
- Triturate 0.1 g of the hairy roots into fine powder in liquid nitrogen.
- Prepare the genomic DNA of independent transgenic lines of TB using the modified cetyltrimethylammonium bromide (CTAB) method25 according to the instruction of the manufacturer of the plant genomic DNA kit.
- Perform polymerase chain reaction (PCR) using 100 ng of genomic DNA template and primers listed in Table 2.
- Perform the amplification cycle as follows: predenaturation at 94 °C for 5 min, denaturation at 94 °C for 30 s, primer annealing at 55 °C for 30 s, and primer extension at 72 °C for 30 s. After 36 cycles and a final extension step at 72 °C for 10 min, analyze the amplification products on 1% agarose gels.
- Stain the gels with nucleic acid staining and visualize them under UV light.
Representative Results
Agrobacterium rhizogenes-mediated TB hairy root transformation
This study describes the step-by-step protocol that was established to obtain genetically transformed hairy roots using A. rhizogenes. It took approximately 5-6 weeks from the inoculation of TB seeds to the harvesting of the identified hairy roots, and some key steps are depicted in Figure 1 (A-H). Briefly, sterilized shelled seeds were inoculated (Figure 1B) to achieve faster sterile germination. A. rhizogenes (Figure 1D) and sterile explants should be activated and prepared in advance, respectively. This is followed by some key steps, including infection of explants with activated A. rhizogenes (Figure 1E), coculture (Figure 1F), and selective culture (Figure 1G). The infected explants should be placed evenly on the solidified MS medium and space must be maintained between them to readily separate the different transgenic lines. Hairy roots appear with a fluffy white color in a plagiotropic manner in the wound sites of the explants (Figure 1H). The hairy roots form a highly branched and an interlocked matrix and can be propagated as required (Figure 1I). The harvested hairy roots can be used to investigate the gene function or the gene– or protein–protein interaction. Alternatively, the TB hairy roots can be massively propagated to yield secondary metabolites such as rutin in designated bioreactors.
The method to induce transgenic hairy roots in TB has been substantiated using a binary vector (pK7GWIWG2D (II)) carrying the genes GFP and b4 (a member of the transcriptional factor bHLH family, not yet published). The reporter gene GFP was used to easily distinguish the transgenic hairy roots from the nontransgenic ones by visualizing the signal under a blue/light dual ultraviolet transilluminator (Figure 2) or by identifying the target gene (Figure 3). The transformed hairy roots exhibited green fluorescence when illuminated under blue or ultraviolet light (represented using black arrowheads in Figure 2A), whereas the untransformed hairy roots did not exhibit the green fluorescence (Figure 2B). The hairy roots with a high GFP signal were propagated for a fortnight, as illustrated in Figure 2C.
To further identify whether the binary vector has been successfully transformed into the TB genome, gene identifications were conducted. Briefly, plant genomic DNA of the TB hairy roots was prepared for PCR analysis based on the modified CTAB method25. PCR was performed by amplifying the genes (Kan, GFP, and b4), which was present in Figure 3, respectively. The primers are listed in Table 2. The presence of the 3 genes in all the transgenic lines (Figure 3, lanes 5–11) indicated that the binary vector has been successfully transformed into the TB genome. Kan and GFP were absent in the wild-type roots (Figure 3, lane 3) and experimental negative control (Figure 3, lane 4), whereas b4 was detected in the wild-type roots. These 3 genes were undoubtedly presented in the positive control (Figure 3, lane 2) but were apparently absent in the negative control (Figure 3, lane 4).
Evaluation of the light-induced transcription factor FtMYB116 in TB using the aforementioned hairy root system
FtMYB116 was expressed by employing the abovementioned protocol of hairy root induction. This was accomplished by preinserting the gene FtMYB116 into the binary vector pK7WG2D and then infecting with A. rhizogenes to achieve gene overexpression. Briefly, hairy roots of 0.1 g were triturated into fine powder by using liquid nitrogen. Total RNA was extracted by following the instructions of manufacturer of plant RNA isolation kit26. Then reverse-transcription PCR and real time PCR were performed to amplify FtMYB116 and rutin synthesizing pathway related genes. Subsequently the regulatory effects of FtMYB116 on rutin synthesis-related gene expression and the yield of rutin were verified.
Figure 4A shows the relative expression of FtMYB116 in the transgenic lines of TB hairy roots. Compared with the control group, the relative expression of FtMYB116 exhibited a considerable increase in all 3 independent transgenic lines. Figure 4B and Figure 4C illustrate the promotion of the biosynthesis of rutin and quercetin at the metabolic level through FtMYB116 overexpression. The contents of rutin and quercetin in the transgenic were significantly (p < 0.01) increased compared with those in the wild-type, reaching 40 and 0.5 mg/g FW, respectively, which were 8 times those in the wild-type. The relative gene expressions of CHS, F3H, F3'H, and FLS in all 3 transgenic lines were remarkably higher than those in the control group (Figure 4D). Together, these results confirmed that the strategy described in this study could be successfully used to generate hairy root transformation in TB and investigate the gene expression and metabolic yield of secondary metabolites.
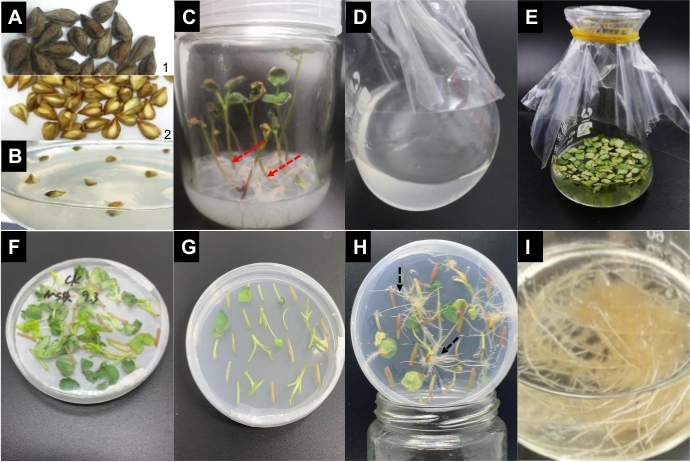
Figure 1: Processes to induce A. rhizogenes-mediated transgenic hairy roots in TB. Representative images of critical stages are displayed: (A1) and (A2) represent before and after peeling off the seed coats; (B) represents each 10 seeds inoculated in a tissue bottle containing MSSA medium; (C) denotes the seedlings of TB at 7–10 days after inoculation, and the red-dash arrowheads show the cutting points; (D) and (E) indicate the preparation of A. rhizogenes (OD600 = 0.5) and the infection of explants, respectively; (F) and (G) symbolize coculturing with activated A. rhizogenes on MSSAAS medium and selective culturing on MSSACK medium, respectively; hairy roots emerge from (H), as shown by the black-dash arrowheads; and (I) shows the propagation of hairy root formation; the black-dash arrowheads indicate the induced hairy roots. Please click here to view a larger version of this figure.
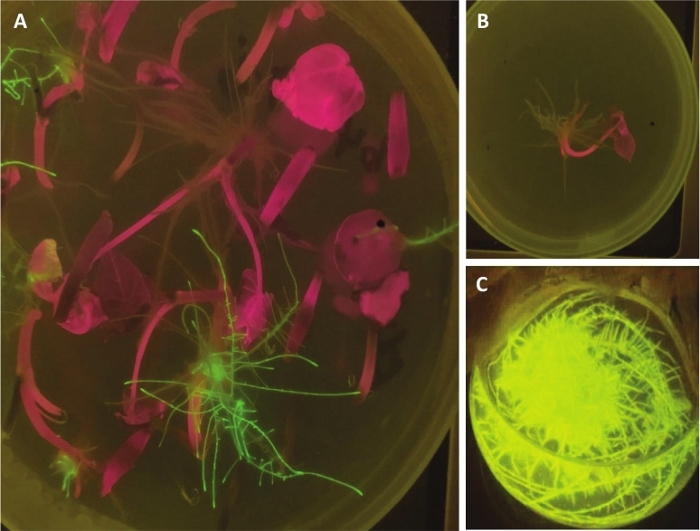
Figure 2: Transformation of the binary vector carrying the GFP reporter gene. (A) denotes the induced hairy roots after selective culture examined under the blue/light dual ultraviolet transilluminator. (B) and (C) represent wild-type root and propagation of transformed hairy roots, respectively. Please click here to view a larger version of this figure.
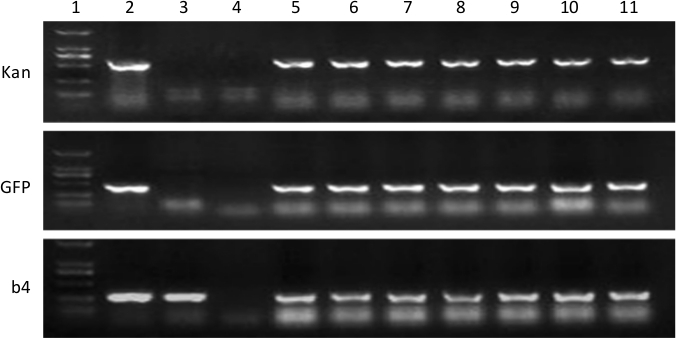
Figure 3: PCR amplification of genes (Kan, GFP, and b4) from genomic DNA isolated from wild-type root and hairy roots of TB in 7 independent transgenic lines. (A): Kan, (B): GFP, (C): b4. Lane 1: molecular size markers (white arrowhead indicates 750 bp), lane 2: plasmid (binary vector pK7GWIWG2D (II) carrying Kan, GFP, and b4 genes) as the positive control, lane 3: wild-type root, lane 4: purified H2O as the negative control, and lanes 5–11: the 7 independent transgenic lines. Please click here to view a larger version of this figure.
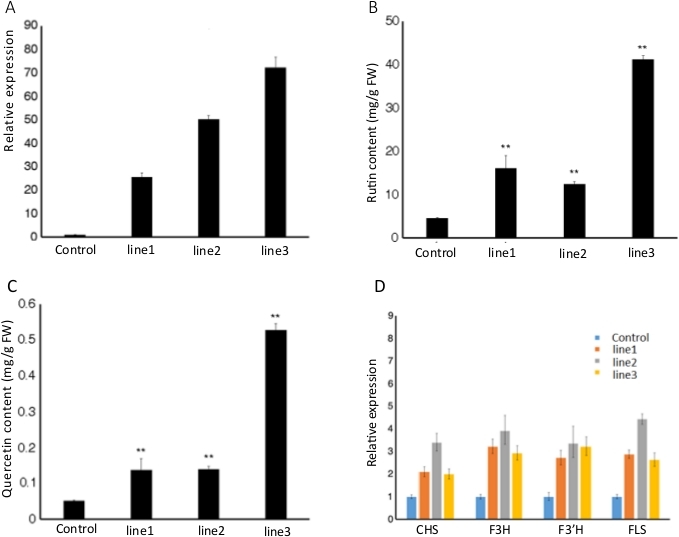
Figure 4: Relative expression of FtMYB116 in the transgenic lines of TB hairy roots. (A) and the promotive effect of the overexpression of FtMYB116 on the biosynthesis of (B) rutin and (C) quercetin (This figure has been modified from Dong et al.5). Experiments were performed in triplicate and conducted 3 times. "**" indicates a significant difference at p < 0.01 using Student's t-test. (D) Expression of genes related to flavonoid synthesis pathways in transgenic lines. The relative expression level was normalized to that of the actin control. Data are presented as mean ± standard deviation (n = 3). Please click here to view a larger version of this figure.
| Media | Medium ingredients | ||
| MSSA | Murashige and Skoog (MS) medium containing sucrose in 30 g/L, and agar powder in 7 g/L, pH 5.8 | ||
| YEBARS | Yeast Mannitol Medium (YEB) containing agar powder at 15 g/L, rifampicin at 50 mg/L, and spectinomycin at 50 mg/L, pH 7.0 | ||
| YEBRS | YEB containing rifampicin at 50 mg/L, and spectinomycin at 50 mg/L, pH 7.0 | ||
| MSSAS | MS medium containing sucrose at 30 g/L, and acetosyringone (AS) at 300 μM, pH 5.8 | ||
| MSSAA | MS medium containing sucrose at 30 g/L, agar powder at 7g/L, and AS at 100μM, pH 5.2 | ||
| MSSACK | MS medium containing sucrose at 30 g/L, agar powder at 7 g/L, cefotaxime at 500 mg/L, and kanamycin (kan) at 50 mg/L, pH 5.8 | ||
| MSSK | MS medium containing sucrose at 30 g/L, and kan at 50 mg/L, pH 5.8 | ||
Table 1: Media and their ingredients.
| Primer | Sequence (5'-3') | |
| GFP-F | CCACAAGTTCAGCGTGTCCG | |
| GFP-R | AAGTTCACCTTGATGCCGTTC | |
| b4-F | AAATCTTTTCCCTGTGG | |
| b4-R | ATGCCATCATTGCCAAG | |
| Kan-F | ATTCGGCTATGACTGGGCAC | |
| Kan-R | TGAATCCAGAAAAGCGGCCA | |
Table 2: Primer sequence.
Discussion
TB has been used in several studies related to secondary metabolites at genetic and metabolic levels1,2,5,27,28. Hairy root culture, as a unique source for metabolite production, plays a pivotal role in metabolic engineering29 and can be used to alter metabolic pathways by inserting the related genes. Kim et al.2 initially introduced the establishment of TB hairy root cultures by A. rhizogenes-mediated transformation to achieve the production of phenolic compounds. The content of rutin that they obtained in the TB hairy roots was more than 10 times higher than that in the wild-type roots. In the present study, the introduction of FtMYB116 led to a higher expression of rutin-related genes and surged the production of rutin in the TB hairy roots. This technique has been confirmed to be apt for phenotypic characterization and expression of phenylpropanoid-related genes such as FtF3H and FtFLS in TB hairy roots5,30,31. Zhang et al.32 used TB hairy roots to investigate the production of rutin by overexpressing a series of FtMYB transcriptional factors. Zhou et al.33 observed a decrease in the content of rutin owing to the overexpression of FtMYB11 in TB hairy roots. These results together with our findings indicate the feasible effects of hairy root transformation on the interaction between FtMYB transcriptional factors and rutin biosynthesis-related genes.
Although there are limited data regarding a step-by-step protocol for the induction of TB hairy roots, we describe herein the step-by-step protocol for the first time to obtain transgenic TB hairy roots in an efficient and stable manner using A. rhizogenes carrying a binary vector. During these experimental processes, numerous factors have to be carefully considered to obtain the optimal induced hairy roots. First, the selection of explants is a determining factor. TB cultivars are known to affect the morphology of hairy roots and the production of phenolic compounds. Thwe et al.30 illustrated that gene expression in the phenylpropanoid biosynthetic pathway and the contents of phenolic compounds varied among TB cultivars. They also found hairy roots in one cultivar, which was deep reddish-purple owing to its anthocyanin content13. In our study, 2 just unfolded cotyledons and hypocotyls were selected as the explants. This is because young and tender leaves favor a high hairy root induction rate2,30, whereas highly differentiated and old plant cells adversely affect the hairy root induction. Second, the strain of A. rhizogenes has a significant impact on hairy root induction. Different bacterial strains exhibit different transforming abilities in terms of morphologies and induction efficiency of hairy roots, which can be illuminated by the different plasmids harbored by the strains34. Aye et al.35 compared the effects of several A. rhizogenes strains (R1000, R1200, 15834, LBA9402, and A4) on TB hairy root induction and phenylpropanoid biosynthesis and found that the most promising strain for hairy root production in TB was R1000. This finding has been supported by Kim et al.2 Nevertheless, the strain ACCC10060 that was excluded in the study of Aye et al. but used in our study exhibited satisfactory infection efficiency. The fluffy white appearance of hairy roots obtained using our protocol is in agreement with the hairy roots generated in Salvia miltiorrhiza36, wherein the same strain ACCC10060 carrying the binary vector pK7GWIWG2D (II) was used to silence the target gene. Third, degerming including pretreatment of materials and a concentration of cefotaxime in selective culture also play vital roles in hairy root induction. Incomplete disinfection in any step could lead to the failure of hairy root transformation. In addition, the bacterial concentration has a significant influence on the production of transformed roots. High concentrations may reduce the plant cells by competitive inhibition, whereas low concentrations may cause low availability4.
Furthermore, culture conditions such as the growth medium, appropriate preculturing and coculturing time, and other biotic or abiotic factors play an important role in hairy root inducton. Huang et al.37 recommended 1/2 MS medium containing sucrose at a concentration of 30 g/L for cocultivation to achieve maximum TB hairy roots. This can be explained by the high salt medium that is suitable for hairy root formation, whereas a low salt medium favors excessive bacterial multiplication34. AS is a type of phenolic compound that can facilitate A. rhizogenes-mediated transformation in a number of plant species by the transcription of the vir region of Agrobacterium34,38, and vir could be effectively induced in a medium with a pH of < 5.739,40. Therefore, we recommend a coculture medium with pH 5.2 supplemented with 100 μM of AS. Huang et al.37 reported that TB hairy roots turned to brown after day 24 from white and pale yellow. Therefore, they subcultured hairy roots every 24 days; however, we recommend subculturing every fortnight to avoid browning of hairy roots. In addition, environmental conditions such as light, hormones, temperature, and UV radiation appear to affect the expression of flavonoid biosynthesis-related genes by highly stimulating or depressing signal transduction41,42. The previous study has demonstrated the significance of far-red light in monitoring rutin-related gene expression in TB hairy roots5.
The A. rhizogenes-mediated transformation has the advantage that any exogenous gene of interest inserted in a binary vector can be transferred to the transformed hairy root clone34 to achieve overexpression, loss-of-function via RNA silencing43, or discovery of new metabolic genes by transcriptome analyses5. Hairy roots have great potential to produce secondary metabolites, recombinant proteins, and evenantibodies44. This is primarily owing to their easy and rapid growth in hormone-free medium, being less expensive, no requirement for regeneration into complete plants21, and the relatively high yield of secondary metabolites compared to that from the starting plant material31. These roots can also be separated from the original explant to establish long-term, stable, and characterized root clones maintaining their biosynthetic capacity and phenotypes. Altogether, based on these findings, this protocol provides a rapid, distinct, and efficient method to produce transformed hairy roots to investigate the production of secondary metabolites and puts forward a reference for hairy root induction in other plants. However, the potential to explore hairy root cultures to generate massive yields of bioactive compounds depends on the appropriate bioreactor system in which certain parameters such as the supply of oxygen must be concerned4,8. This protocol is limited to the production of secondary metabolites derived in hairy roots and to investigate the visualized phenotype of functional genes such as the variance of color and the contents of secondary metabolites; however, the phenotypic changes in the entire plant regardless of the obtainment of regenerated plants from the hairy roots could not be evaluated in this study.
Divulgaciones
The authors have nothing to disclose.
Acknowledgements
This work was supported by the Fundamental Research Funds for the Central public welfare research institutes ZXKT17002.
Materials
| 2*Taq PCR MasterMix | Aidlab, China | PC0901 | |
| Agar powder | Solarbio Life Science, Beijing, China | A8190 | |
| Applied Biosystems 2720 thermo cycler | ThermoFisher Scientific, US | A37834 | |
| AS | Solarbio Life Science, Beijing, China | A8110 | Diluted in DMSO, 100 mM |
| binary vectors | ThermoFisher Scientific (invitrogen), US | / | pK7WG2D/pK7GWIWG2D (II) |
| Cefotaxime,sodium | Solarbio Life Science, Beijing, China | C8240 | Diluted in Water, 200 mg/mL |
| CF15RXII high-speed micro | Hitachi, Japan | No. 90560201 | |
| Diposable Petri-dish | Guanghua medical instrument factory, Yangzhou, China | / | |
| DYY-6C electrophoresis apparatus | Bjliuyi, Beijing China | ECS002301 | |
| EASYspin Plus Plant RNA Kit | Aidlab, China | RN38 | |
| ELGA purelab untra bioscience | ELGA LabWater, UK | 82665JK1819 | |
| Epoch Microplate Spectrophotometer | biotek, US | / | |
| Gateway BP/LR reaction enzyme | ThermoFisher Scientific (invitrogen), US | 11789100/11791110 | |
| HYG-C multiple-function shaker | Suzhou Peiying Experimental Equipment Co., Ltd. China | / | |
| Kan | Solarbio Life Science, Beijing, China | K8020 | Diluted in Water, 100 mg/mL |
| MLS-3750 Autoclave sterilizer | Sanyo, Japan | / | |
| MS salts with vitamins | Solarbio Life Science, Beijing, China | M8521 | |
| NaCl | Solarbio Life Science, Beijing, China | S8210 | |
| Other chemicals unstated | Beijing Chemical Works, China | ethanol, mercury bichloride, etc. | |
| PHS-3C pH meter | Shanghai INESA Scientific Instrument Co., Ltd, China | a008 | |
| Plant Genomic DNA Kit | TIANGEN BIOTECH (BEIJING) CO., LTD | DP305 | |
| Rifampin | Solarbio Life Science, Beijing, China | R8010 | Diluted in DMSO, 50 mg/mL |
| Spectinomycin | Solarbio Life Science, Beijing, China | S8040 | Diluted in Water, 100 mg/mL |
| Sucrose | Solarbio Life Science, Beijing, China | S8270 | |
| Trans2K DNA Marker | TransGen Biotech, Beijing, China | BM101-01 | |
| Tryptone | Solarbio Life Science, Beijing, China | LP0042 | |
| Whatman diameter 9 cm Filter paper | Hangzhou wohua Filter Paper Co., Ltd | / | |
| Yeast Extract powder | Solarbio Life Science, Beijing, China | LP0021 |
Referencias
- Fabjan, N., et al. Tartary Buckwheat ( Fagopyrum tataricum Gaertn .) as a Source of Dietary Rutin and Quercitrin. Agricultural and Food Chemistry. 51, 6452-6455 (2003).
- Kim, Y. K., et al. Production of Phenolic Compounds in Hairy Root Culture of Tartary Buckwheat (Fagopyrum tataricum Gaertn). Journal of Crop Science & Biotechnology. 12 (1), 53-57 (2009).
- Yao, Y., et al. D-chiro-inositol-enriched tartary buckwheat bran extract lowers the blood glucose level in KK-Ay mice. Journal of Agricultural and Food Chemistry. 56 (21), 10027-10031 (2008).
- Giri, A., Narasu, M. L. Transgenic hairy roots. Biotechnology Advances. 18 (1), 1-22 (2000).
- Zhang, D., et al. The light-induced transcription factor FtMYB116 promotes accumulation of rutin in Fagopyrum tataricum. Plant, Cell & Environment. 42, (2018).
- Chilton, M. -. D., et al. Agrobacterium thizogenes inserts T-DNA into the genomes of the host plant root cells. Nature. 295 (4), 129 (1982).
- Guillon, S., Trémouillaux-Guiller, J., Kumar Pati, P., Gantet, P. Hairy Roots: a Powerful Tool for Plant Biotechnological Advances. Bioactive Molecules and Medicinal Plants. , 271-283 (2008).
- Srivastava, S., Srivastava, A. K. Hairy root culture for mass-production of high-value secondary metabolites. Critical Reviews in Biotechnology. 27 (1), 29-43 (2007).
- Veena, V., Taylor, C. G. Agrobacterium rhizogenes: Recent developments and promising applications. In Vitro Cellular and Developmental Biology – Plant. 43 (5), 383-403 (2007).
- Ramachandra Rao, S., Ravishankar, G. A. Plant cell cultures: Chemical factories of secondary metabolites. Biotechnology Advances. 20 (2), 101-153 (2002).
- Palazón, J., et al. Growth and Ginsenoside Production in Hairy Root Cultures of Panax ginseng using a Novel Bioreactor. Planta Med. 69 (04), 344-349 (2003).
- Staniszewska, I., Królicka, A., Maliński, E., Łojkowska, E., Szafranek, J. Elicitation of secondary metabolites in in vitro cultures of Ammi majus L. Enzyme and Microbial Technology. 33 (5), 565-568 (2003).
- Uddin, M. R., Li, X., Won, O. J., Park, S. U., Pyon, J. Y. Herbicidal activity of phenolic compounds from hairy root cultures of Fagopyrum tataricum. Weed Research. 52, 25-33 (2011).
- Christey, M. C., Braun, R. H. Production of hairy root cultures and transgenic plants by Agrobacterium rhizogenes-mediated transformation. Methods in Molecular Biology. 286, 47-60 (2005).
- Olhoft, P. M., et al. A novel Agrobacterium rhizogenes-mediated transformation method of soybean [Glycine max (L.) Merrill] using primary-node explants from seedlings. In Vitro Cellular and Developmental Biology – Plant. 43 (6), 536-549 (2007).
- Kereszt, A., et al. Agrobacterium rhizogenes-mediated transformation of soybean to study root biology. Nature Protocols. 2 (4), (2007).
- Pistelli, L., et al. . Bio-Farms for Nutraceuticals: Functional Food and Safety Control by Biosensors. , (2010).
- Gangopadhyay, M., Sircar, D., Mitra, A., Bhattacharya, S. Hairy root culture of Plumbago indica as a potential source for plumbagin. Biologia Plantarum. 52 (3), 533-537 (2008).
- Okamoto, S., Yoro, E., T, S., K, M. Division Hairy Root Transformation in lotus Japonicus. Bio-Protocol. 3 (12), 14-17 (2013).
- Fathi, R., Mohebodini, M., Chamani, E. High-efficiency Agrobacterium rhizogenes-mediated genetic transformation in Cichorium intybus L. via removing macronutrients. Industrial Crops and Products. 128, 572-580 (2019).
- Fernández-piñán, S., et al. Transformation of Potato and the Promoter Activity of a Suberin Gene by GUS Staining. Journal Of Visualized Experiments. , e1 (2019).
- Chattopadhyay, T., Roy, S., Mitra, A., Maiti, M. K. Development of a transgenic hairy root system in jute (Corchorus capsularis L.) with gusA reporter gene through Agrobacterium rhizogenes mediated co-transformation. Plant Cell Reports. 30 (4), 485-493 (2011).
- Sirikantaramas, S., et al. The gene controlling marijuana psychoactivity. Molecular cloning and heterologous expression of Δ1-tetrahydrocannabinolic acid synthase from Cannabis sativa L. Journal of Biological Chemistry. 279 (38), 39767-39774 (2004).
- Zhou, M. L., et al. Characterization of Functional Genes in Buckwheat. Molecular Breeding and Nutritional Aspects of Buckwheat. , 327-331 (2016).
- Liang, C., et al. A Comparative Analysis of the Chloroplast Genomes of Four Salvia Medicinal Plants. Ingeniería. 5 (5), 907-915 (2019).
- Wang, J., Zhang, X., Yan, G., Zhou, Y., Zhang, K. Over-expression of the PaAP1 gene from sweet cherry (Prunus avium L.) causes early flowering in Arabidopsis thaliana. Journal of Plant Physiology. 170 (3), 315-320 (2013).
- Li, J., et al. Analysis of Flavonoid Metabolites in Buckwheat Leaves Using UPLC-ESI-MS/MS. Molecules. , (2019).
- Zhu, F. Chemical composition and health effects of Tartary buckwheat. Food Chemistry. 203, 231-245 (2016).
- Kaur, B., Malik, C. P. Hairy root culture -a unique source for metabolites production. Journal of Plant Science Research. 25 (2), 123-141 (2010).
- Thwe, A. A., et al. Metabolomic Analysis and Phenylpropanoid Biosynthesis in Hairy Root Culture of Tartary Buckwheat Cultivars. Plos One. 8 (6), (2013).
- Thwe, A. A., et al. Accumulation of Phenylpropanoids and Correlated Gene Expression in Hairy Roots of Tartary Buckwheat under Light and Dark Conditions. Applied Biochemistry and Biotechnology. 174 (7), 2537-2547 (2014).
- Zhang, K., et al. Jasmonate-responsive MYB factors spatially repress rutin biosynthesis in Fagopyrum tataricum. Journal of Experimental Botany. 69 (8), 1955-1966 (2018).
- Zhou, M., et al. FtSAD2 and FtJAZ1 regulate activity of the FtMYB11 transcription repressor of the phenylpropanoid pathway in Fagopyrum tataricum. New Phytologist. 216, (2017).
- Giri, A., Narasu, M. L. Transgenic hairy roots: Recent trends and applications. Biotechnology Advances. 18 (1), 1-22 (2000).
- Thwe, A., et al. Effect of different Agrobacterium rhizogenes strains on hairy root induction and phenylpropanoid biosynthesis in tartary buckwheat (Fagopyrum tataricum Gaertn). Frontiers in Microbiology. 7, 1-10 (2016).
- Cheng, Q., et al. RNA interference-mediated repression of SmCPS (copalyldiphosphate synthase) expression in hairy roots of Salvia miltiorrhiza causes a decrease of tanshinones and sheds light on the functional role of SmCPS. Biotechnology Letters. 36 (2), 363-369 (2014).
- Huang, X., et al. Efficient Rutin and Quercetin Biosynthesis through Flavonoids-Related Gene Expression in Fagopyrum tataricum Gaertn . Hairy Root Cultures with UV-B Irradiation. Frontiers In Plant Science. 7, 1-11 (2016).
- Godwin, I., Todd, G., Ford-lloyd, B., Newbury, H. J. The effects of acetosyringone and pH on Agrobacterium-mediated transformation vary according to plant species. Plant Cell Reports. 9, 671-675 (1991).
- Stachel, S. E., Messens, E., Van Montagiu, M., Zambryski, P. Identification of the signal molecules produced by wounded plant cells that activate T-DNA transfer in Agrobacterium tumefaciens. Nature. 318 (19), (1985).
- Bolton, G. W., Nester, E. W., Gordon, M. P. Plant Phenolic Compounds Induce Expression of the Agrobacterium tumefaciens loci needed for virulence. Science. 232 (10), 983-985 (1986).
- Ferri, M., et al. Chitosan treatment induces changes of protein expression profile and stilbene distribution in Vitis vinifera cell suspensions. Proteomics. 9 (3), 610-624 (2009).
- Bourgaud, F., Gravot, A., Milesi, S., Gontier, E. Production of plant secondary metabolites: a historical perspective. Plant Science. 161 (5), 839-851 (2001).
- Kumagai, H., Kouchi, H. Gene Silencing by Expression of Hairpin RNA in Lotus japonicus Roots and Root Nodules. Molecular Plant-Microbe Interactions. 16 (8), 663-668 (2003).
- Sunil Kumar, G. B., Ganapathi, T. R., Srinivas, L., Revathi, C. J., Bapat, V. A. Expression of hepatitis B surface antigen in potato hairy roots. Plant Science. 170 (5), 918-925 (2006).

