Establishing a Porcine Ex Vivo Cornea Model for Studying Drug Treatments against Bacterial Keratitis
Summary
This article describes a step-by-step protocol to set up an ex vivo porcine model of bacterial keratitis. Pseudomonas aeruginosa is used as a prototypic organism. This innovative model mimics in vivo infection as bacterial proliferation is dependent on the ability of the bacterium to damage corneal tissue.
Abstract
When developing novel antimicrobials, the success of animal trials is dependent on accurate extrapolation of antimicrobial efficacy from in vitro tests to animal infections in vivo. The existing in vitro tests typically overestimate antimicrobial efficacy as the presence of host tissue as a diffusion barrier is not accounted for. To overcome this bottleneck, we have developed an ex vivo porcine corneal model of bacterial keratitis using Pseudomonas aeruginosa as a prototypic organism. This article describes the preparation of the porcine cornea and protocol for establishment of the infection. Bespoke glass molds enable straightforward setup of the cornea for infection studies. The model mimics in vivo infection as bacterial proliferation is dependent on the ability of the bacterium to damage corneal tissue. Establishment of infection is verified as an increase in the number of colony forming units assessed via viable plate counts. The results demonstrate that infection can be established in a highly reproducible fashion in the ex vivo corneas using the method described here. The model can be extended in the future to mimic keratitis caused by microorganisms other than P. aeruginosa. The ultimate aim of the model is to investigate the effect of antimicrobial chemotherapy on the progress of bacterial infection in a scenario more representative of in vivo infections. In so doing, the model described here will reduce the use of animals for testing, improve success rates in clinical trials and ultimately enable rapid translation of novel antimicrobials to the clinic.
Introduction
Corneal infections are important causes of blindness and occur in epidemic proportions in low- and mid-income countries. The etiology of the disease varies from region to region but bacteria account for a large majority of these cases. Pseudomonas aeruginosa is an important pathogen that causes a rapidly progressive disease. In many cases, patients are left with stromal scarring, irregular astigmatism, require transplant or in the worst case scenario, lose an eye1,2.
Bacterial keratitis caused by P. aeruginosa is a difficult eye infection to treat particularly due to the increasing emergence of antimicrobial resistant strains of P. aeruginosa. Within the last decade, it has become apparent that testing and developing new treatments for corneal infections, in general, and those caused by Pseudomonas sp., in particular, are essential to combat the current trend in antibiotic resistance3.
For testing the efficacy of new treatments for corneal infections, conventional in vitro microbiological methods are a poor surrogate due to the difference in bacterial physiology during laboratory culture and during infections in vivo as well as due to the lack of the host interface4,5. In vivo animal models, however, are expensive, time-consuming, can only deliver a small number of replicates and raise concerns about animal welfare.
In this article, we demonstrate a simple and reproducible organotypic ex vivo porcine model of keratitis that can be used to test various treatments for acute and chronic infections. We have used P. aeruginosa for this experiment but the model also works well with other bacteria, and organisms such as fungi and yeast which cause keratitis.
Protocol
Albino laboratory rabbits were sacrificed in the laboratory for other planned experimental work under home office approved protocols. The eyes were not required for experimental use in those studies so they were used for this protocol.
1. Sterilization
- CRITICAL STEP: Disinfect all forceps and scissors by soaking for 1 h in 5% (v/v) solution of Distel in distilled water, clean with a brush, rinse with tap water and sterilize in an oven at 185 °C for a minimum of 2 h.
- Sterilize all other glassware and reagents by autoclaving at 121 °C for 15 minutes or prepare reagents according to the manufacturer’s instructions. Carry out the following procedures in a class II microbiology safety cabinet.
2. Sample collection
- Collection of porcine eyes
- Large white landrace sows, a cross with a Hampshire boar was used. The animals were stunned with an electric current and the eyes were enucleated 2 h later in the abattoir.
- CRITICAL STEP: Once enucleated, transfer the eyes to the lab in a sterile phosphate buffered saline (PBS) solution to prevent them from drying out and process them immediately upon arrival.
- Collection of rabbit eyes
- Excise the corneas and send to the lab in sterile PBS.
3. Preparation of the corneoscleral button
- Use sterile forceps to hold the tissue surrounding the eyeball and transfer it to a Petri dish. Remove the conjunctiva and muscle tissue around the eyeball on a Petri dish using scalpel blade no. 15 and forceps.
- Gently lift the eyeball while holding the optic nerve with forceps and transfer to a 0.5 L jar filled with sterile PBS.
- Once all eyes are cleared of surrounding tissue, move them using sterile forceps to another 0.5 L jar filled with 3% (v/v) povidone iodine in PBS and leave for 1 min.
- Transfer eyeballs to another 0.5 L jar with sterile PBS.
- Use forceps to hold the eye still on a Petri dish and make a cut near the cornea with a scalpel blade no 10A.
- CRITICAL STEP: Hold the edge of the cut and use scissors to excise the cornea leaving about 3 mm of sclera surrounding the cornea. Ensure the sharp end of scissors does not pierce the iris or the choroidal tissue and is in the supra-choroidal space.
- Hold the corneoscleral button with forceps and use another pair of pointed end forceps to gently separate the uveal tissue.
- Lift the corneoscleral button from remaining globe and briefly rinse it in 1.5% (v/v) povidone iodine solution in PBS in a 12 well plate.
- Place the corneoscleral button into another 12 well plate filled with sterile PBS.
- After processing all eyes (recommended maximum 40 eyes in one batch), place each corneoscleral button to an individual Petri dish (34 mm diameter) epithelial side up and pour in 3 mL of culture medium pre-warmed to 37 °C.
NOTE: The composition of the culture medium is as follows: Dulbecco’s modified Eagle’s medium (DMEM): Ham’s [1:1] supplemented with 5 μg∙mL-1 insulin and 10 ng∙mL-1 epidermal growth factor (EGF), 10% (v/v) foetal calf serum (FCS), 100 U∙mL-1 penicillin, 100 U∙mL-1 streptomycin and 2.5 μg∙mL-1 amphotericin B. As an optional step, the medium can be supplemented with 50 g∙L-1 dextran to prevent swelling of the excised cornea during the further incubation steps. - Incubate at 37 °C in a humidified tissue culture incubator.
4. Maintenance of the corneoscleral buttons
- After 24 hours, use aseptic technique to remove media and replace with 3 mL of fresh pre-warmed culture media containing antibiotics. Keep the corneoscleral buttons in media with antibiotics for 48 h to disinfect the corneas. Incubate at 37 °C in a humidified tissue culture incubator.
- CRITICAL STEP: After 48 hours, remove the media and rinse corneas with 2 mL of PBS. Then keep the corneoscleral buttons in antibiotic-free media for a minimum of two or ideally three days before experimental infection, to remove residual antibiotics from the tissue.
- Incubate at 37 °C in a humidified tissue culture incubator. Change media at least one more time within these three days. Discard corneas if any turbidity develops in the antibiotic-free medium.
5. Preparation of an inoculum
- Pour 10 mL of LB broth into a 50 mL conical flask with a foam stopper.
- Transfer a colony of P. aeruginosa strain PAO1 or strain PA14 from a fresh agar plate and incubate at 37 °C for 3-4 h until the bacteria are in mid-log phase.
- Transfer the culture of bacteria to a 50 mL tube and centrifuge at 3,000 x g for 5 min. Remove the supernatant and re-suspend the cell pellet in PBS.
- Repeat step 5.3 two more times to wash the cells. Re-suspend the cell pellet in PBS and adjust the optical density at 600 nm to approximately 0.6 using sterile PBS as a blank.
6. Infecting the corneoscleral button
- Remove media from the Petri dish and rinse corneas twice with 1 mL of sterile PBS.
- Gently squeeze forceps while holding the cornea in-between. Use a 10A scalpel to make four cuts – two vertical, two horizontal – in the central section of the corneoscleral button through the epithelial layer to the underlying stroma.
- Place a sterile glass mold in a 6-well plate with the wide part up and place the cornea in the middle of the glass mold, epithelium side facing down. Make the cut right in the center of the bottom part of the glass mold.
- CRITICAL STEP: Pour 1 mL of 1% (w/v) low melting point agar dissolved in DMEM to fill the glass mold with cornea completely.
- Allow the agar to set and then invert the glass mold so that the corneal epithelium is facing upwards.
- Pipette 15 μL of the bacterial culture with OD600nm = 0.6 (for P. aeruginosa this equates to approximately 1 x 107 colony forming units (CFU) in 15 μL) directly into a cut area and then add 85 μL of PBS to the top to keep the corneal epithelium moist. Dilute the remaining bacterial culture and plate on agar to count colony forming units for the inoculum.
- Add 1 mL of DMEM without antibiotics to the bottom of each well with the glass mold. Incubate the 6-well plate with the infected corneoscleral buttons in a humidified incubator at 37 °C with 5% CO2 for up to 24 h.
- Set up uninfected control cornea alongside every experiment. To set up uninfected control, replace the 15 μL of bacterial culture in step 6.6 with sterile PBS.
7. Homogenization of the cornea to harvest the bacteria
- Discard the DMEM medium from the bottom of the 6 well plate and add 1 mL of sterile PBS to rinse the bottom of the well.
- Remove PBS gently by pipetting without touching the central part of the corneoscleral button. Remove the glass ring using sterile forceps and place it in the 5% Distel.
- Gently rinse the top of the corneoscleral button with 1 mL of PBS twice [optional].
- Hold the edge of the corneoscleral button with fine tip forceps and detach it from the agar underneath.
- Transfer the cornea to a 50 mL tube filled with ice cold 1-2 mL of PBS.
- Use a fine tip homogenizer to sheer the top of the infected cornea. The tissue does not have to be completely liquidized. The homogenizer helps to detach bacteria from the corneal epithelium and the cut area.
- Vortex the cornea in PBS for a few seconds to mix the contents.
- Add 20 μL of the homogenate to 180 μL of PBS and perform serial dilutions in a 96 well plate.
- Serially dilute the suspension to 10-4 and 10-5 dilution and pipette 10 μL of the diluted homogenate with bacteria onto a blood agar plate. Incubate the plate for 8 hours and count the number of CFU. When testing the effect of antimicrobials, the appropriate dilution factor must be arrived at experimentally.
Representative Results
The design of the glass molds are an innovative and original idea, the use of which allowed us to set up the model in a consistent fashion with minimal/no issues with contamination. The molds were prepared by a glass blower at the University of Sheffield based on a design (Figure 1A). The experimental setup maintains the convex shape of the cornea and holds bacteria on the top of the epithelium where infection takes place (Figure 1B).
Porcine corneas usually swell after few days in medium. This is normal and we found that there was no significant difference between corneas with and without addition of dextran, which is usually added to prevent swelling of the cornea (Figure 1H). The corneas are typically wounded to help the bacteria penetrate the epithelium. Although there was no significant difference in the progress of infection between wounded (cut) and unwounded (uncut) corneas, we noticed more variations between replicates in uncut corneas (Figure 1C). Washing the corneas twice with PBS removes excess bacteria that did not attach to the epithelium. There was a significant difference in CFU between washed and unwashed porcine corneas infected with P. aeruginosa PAO1 for 24 hours (Figure 1D). There was no significant difference in CFU counts between porcine and rabbit corneas infected with PA14 and PAO1 (Figure 1E,1F). The results for both models were reproducible. After 24 hours, the cornea infected with either Pseudomonas strain always develop opacity and the cut area becomes more visible and open in comparison to the uninfected cornea (Figure 1G).
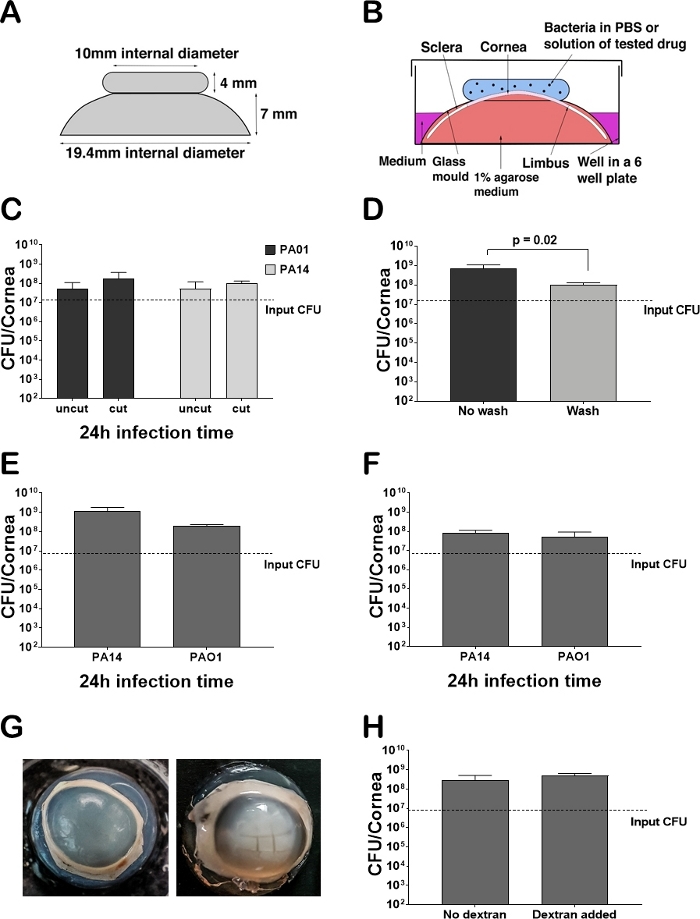
Figure 1: Ex vivo cornea infected with Pseudomonas aeruginosa. (A) Schematic picture of a glass mold used for maintaining the shape of the cornea and facilitating the introduction of bacteria and treatments. The thickness of the glass molds is 1.5 mm and is the same as the thickness of test tubes made from borosilicate glass. (B) Schematic picture of the experimental set up. (C) Testing the effect of wounding on the final CFU count after homogenization. Uncut (n = 16) and cut (n = 28) corneas were infected with P. aeruginosa PAO1 and P. aeruginosa PA14 for 24 hours. The corneas were washed with 1 mL of PBS before homogenization. Error bars indicate standard deviation. (D) Testing the effect of washing corneas with 2 x 1 mL of PBS (n = 6) and not washing (n = 6) on the final CFU count after infection with P. aeruginosa PAO1 for 24 hours. Error bars indicate standard deviation. (E) Final CFU count in porcine corneas infected with P. aeruginosa PAO1 and P. aeruginosa PA14 for 24 hours (n = 10). Corneas were washed and cut. Error bars indicate standard deviation. (F) Final CFU count in rabbit corneas infected with P. aeruginosa PAO1 and P. aeruginosa PA14 for 24 hours (n = 6). Corneas were washed and cut. Error bars indicate standard deviation. (G) Pictures of ex vivo porcine corneas infected with P. aeruginosa PAO1 for 24 hours. The control was wounded but no bacteria were added. The infected corneas were wounded and 107 CFU were added to the cut side. No CFU were recovered from the control cornea. (H) Final CFU recovered after 24 hours of infection with P. aeruginosa PAO1 from corneas treated with dextran (n = 2) and those without dextran (n = 9). Corneas were washed and cut. Error bars indicate standard deviation. Please click here to view a larger version of this figure.
Discussion
The main driver behind the development of this keratitis model using ex vivo porcine cornea is to provide researchers developing novel antimicrobials with a representative in vitro model to more accurately determine antimicrobial efficacy at the preclinical stages. This will provide researchers involved in developing new antimicrobials greater control over drug design and formulation at the pre-clinical stages, increase success at clinical trials, reduce use of animals by enabling targeted studies and result in faster translation of new antimicrobials to clinic.
A number of studies have investigated the effect of infections on ex vivo corneas from various animals such as: rabbit6, dog7, goat8 and pigs9,10,11. Most of these studies focus on ways of establishing6 and visualizing an infection9 but so far there have only been a few publications focusing on drug testing and accurate quantification of bacteria6,7,8,12.
The primary advantage of our model is the availability of the porcine corneas as part of the food chain. The use of ex vivo porcine corneas therefore aligns with the principle of 3Rs, which is to replace, refine and reduce the use of animals in research, whilst providing a representative model of the host interface. We have observed no issues with contamination of the corneal explants if the protocol is strictly followed. The glass molds are very easy, quick and straightforward to use without any requirement for specialized equipment. The narrow ring at the top makes the addition of a small quantity of a tested drug (100 µL) or bacteria convenient. The ring of the glass mold allows PBS with bacteria or a drug solution to be retained in the central part of the cornea and prevents the bacteria from getting underneath the cornea. The ring is easy to clean and sterilize, and allows the observation of the changes that occur on the top of the cornea during infection. Strains of fluorescently-tagged bacteria can be used to visualize infection or quantify the spread of infection in the tissue using fluorescent confocal microscopy. The whole corneas can be further processed for histology or electron microscopy imaging.
The critical steps are marked in the protocol. Extra attention must be paid to these steps when carrying out the protocol to ensure successful infection. The most critical steps within the protocol are ensuring that the corneas are treated with sufficient antibiotics to prevent infection during preparation and then that the antibiotics are sufficiently eliminated before the introduction of the infective organism, in this case P. aeruginosa. When setting up the experiments using this protocol, in some instances, turbidity developed during incubation in the antibiotic-free medium. This turbidity was indicative of growth of microorganisms in the antibiotic-free medium. This might be due to incomplete treatment of the cornea using the antibiotics or due to contamination during handling. These corneas were not taken forward for further experiments and were discarded. Development of turbidity when incubating corneas in antibiotic-free medium was avoided by employing frequent sterilization runs in the incubator, using disposable pipette tips with a filter and taking adequate care when sterilizing the tools used for excising the cornea from the porcine eyes. Another critical step is when the corneas are placed in the glass mold prior to infection. The glass mold enables one to maintain the convex shape of the cornea. The convexity of the cornea is a challenge for retention of either the infective dose or the therapeutic agent on the surface of the cornea. Therefore, it is essential to ensure the presence of adequate seal between the cornea and the glass mold. When there is adequate seal between the cornea and the glass mold, the ring structure above the mold creates a reservoir to retain either the infective dose or the therapeutic agent. An adequate seal is ensured by completely filling the wide section of the glass mold with DMEM agar up to the brim.
As is the case with any model, there are limitations associated with the ex vivo porcine cornea model described. The model described herein does not mimic the composition, flow and replenishment of the tear film across the cornea. The mechanical action provided by blinking is also not incorporated into the model. There is agreement in the literature that tear film composition and dynamics, and blinking are important defense mechanisms that remove foreign particles and microorganisms from the eye13. Indeed, the model also lacks an immune response that is triggered during infection in vivo. It is likely that the progression of infection in vivo in the presence of these defense mechanisms is different to that observed in the ex vivo model described here. Despite these limitations, the ex vivo porcine corneal model is relevant for testing the effectiveness of existing and emerging antimicrobials for two main reasons: 1) the physiology of the bacteria in the ex vivo model mimics the in vivo conditions as bacterial proliferation is dependent on their ability to damage the corneal tissue, and 2) the model incorporates the three dimensional tissue as a diffusion barrier for therapeutics much like in the in vivo situation. Therefore, the ex vivo model is advantageous over conventional techniques for antimicrobial susceptibility testing.
The ex vivo porcine cornea model described here can be also used for studying different strains of bacteria, fungi and yeast that cause keratitis. This ex vivo cornea model is reproducible and allows one to generate replicates within a short time unlike in vivo models. Instead of PBS, artificial tears or host immune defense cells can theoretically be added to mimic the live scenario. Corneas are obtained from the same breed of pigs and about 21-23 weeks old when slaughtered. Therefore, there is less variability between replicates compared to those obtained from human cadavers. The concept of using a porcine ex vivo cornea model for biomedical applications has gained more popularity within the last few years because of its biological similarity to the human eye which makes this model easier to compare14. There is increased interest in using porcine corneas for transplantation15,16 or as a model for dry eye17 or wound healing18.
Divulgaciones
The authors have nothing to disclose.
Acknowledgements
The authors would like to thank Elliot Abattoir in Chesterfield for providing porcine eyes. The glass rings were made based on our design by the glass blower Dan Jackson from the Department of Chemistry at the University of Sheffield. The authors would like to thank the Medical Research Council (MR/S004688/1) for funding. The authors would like to also thank Mrs Shanali Dikwella for technical help with cornea preparation. The authors would like to thank Mr Jonathan Emery for help with formatting pictures.
Materials
| 50 mL Falcon tube | SLS | 352070 | |
| Amphotericin B | Sigma | A2942 | |
| Cellstar 12 well plate | Greiner Bio-One | 665180 | |
| Dextran | Sigma | 31425-100mg-F | |
| Distel | Fisher Scientific | 12899357 | |
| DMEM + glutamax | SLS | D0819 | |
| Dual Oven Incubator | SLS | OVe1020 | Sterilising oven |
| Epidermal growth factor | SLS | E5036-200UG | |
| F12 HAM | Sigma | N4888 | |
| Foetal calf serum | Labtech International | CA-115/500 | |
| Forceps | Fisher Scientific | 15307805 | |
| Handheld homogeniser 220 | Fisher Scientific | 15575809 | Homogeniser |
| Heracell VIOS 160i | Thermo Scientific | 15373212 | Tissue culture incubator |
| Heraeus Megafuge 16R | VWR | 521-2242 | Centrifuge |
| Insulin, recombinant Human | SLS | 91077C-1G | |
| LB agar | Sigma | L2897 | |
| Multitron | Infors | Not appplicable | Bacterial incubator |
| PBS | SLS | P4417 | |
| Penicillin-Streptomycin | SLS | P0781 | |
| Petri dish | Fisher Scientific | 12664785 | |
| Petri dish 35x10mm CytoOne | Starlab | CC7672-3340 | |
| Povidone iodine | Weldricks pharmacy | 2122828 | |
| Safe 2020 | Fisher Scientific | 1284804 | Class II microbiology safety cabinet |
| Scalpel blade number 15 | Fisher Scientific | O305 | |
| Scalpel Swann Morton | Fisher Scientific | 11849002 |
Referencias
- Vazirani, J., Wurity, S., Ali, M. H. Multidrug-Resistant Pseudomonas aeruginosa Keratitis Risk Factors, Clinical Characteristics, and Outcomes. Ophthalmology. 122 (10), 2110-2114 (2015).
- Sharma, S. Keratitis. Bioscience Reports. 21 (4), 419-444 (2001).
- Sharma, G., et al. Pseudomonas aeruginosa biofilm: Potential therapeutic targets. Biologicals. 42 (1), 1-7 (2014).
- Ersoy, S. C., et al. Correcting a Fundamental Flaw in the Paradigm for Antimicrobial Susceptibility Testing. EBioMedicine. 20, 173-181 (2017).
- Kubicek-Sutherland, J. Z., et al. Host-dependent Induction of Transient Antibiotic Resistance: A Prelude to Treatment Failure. EBioMedicine. 2 (9), 1169-1178 (2015).
- Pinnock, A., et al. Ex vivo rabbit and human corneas as models for bacterial and fungal keratitis. Graefe’s Archive for Clinical and Experimental Ophthalmology. 255 (2), 333-342 (2017).
- Harman, R. M., Bussche, L., Ledbetter, E. C., Van de Walle, G. R. Establishment and Characterization of an Air-Liquid Canine Corneal Organ Culture Model To Study Acute Herpes Keratitis. Journal of Virology. 88 (23), 13669-13677 (2014).
- Madhu, S. N., Jha, K. K., Karthyayani, A. P., Gajjar, D. U. Ex vivo Caprine Model to Study Virulence Factors in Keratitis. Journal of Ophthalmic & Vision Research. 13 (4), 383-391 (2018).
- Vermeltfoort, P. B. J., van Kooten, T. G., Bruinsma, G. M., Hooymans, A. M. M., vander Mei, H. C., Busscher, H. J. Bacterial transmission from contact lenses to porcine corneas: An ex vivo study. Investigative Ophthalmology & Visual Science. 46 (6), 2042-2046 (2005).
- Duggal, N., et al. Zinc oxide tetrapods inhibit herpes simplex virus infection of cultured corneas. Molecular Vision. 23, 26-38 (2017).
- Brothers, K., et al. Bacterial Impediment of Corneal Cell Migration. Investigative Ophthalmology & Visual Science. 56 (7), (2015).
- Alekseev, O., Tran, A. H., Azizkhan-Clifford, J. Ex vivo Organotypic Corneal Model of Acute Epithelial Herpes Simplex Virus Type I Infection. Journal of Visualized Experiments. (69), (2012).
- Sack, R. A., Nunes, I., Beaton, A., Morris, C. Host-Defense Mechanism of the Ocular Surfaces. Bioscience Reports. 21 (4), 463-480 (2001).
- Kunzmann, B. C., et al. Establishment of a porcine corneal endothelial organ culture model for research purposes. Cell and Tissue Banking. 19 (3), 269-276 (2018).
- Oh, J. Y., et al. Processing Porcine Cornea for Biomedical Applications. Tissue Engineering Part C-Methods. 15 (4), 635-645 (2009).
- Shi, W. Y., et al. Protectively Decellularized Porcine Cornea versus Human Donor Cornea for Lamellar Transplantation. Advanced Functional Materials. 29, 1902491-1902503 (2019).
- Menduni, F., Davies, L. N., Madrid-Costa, D., Fratini, A., Wolffsohn, J. S. Characterisation of the porcine eyeball as an in-vitro model for dry eye. Contact Lens & Anterior Eye. 41 (1), 13-17 (2018).
- Castro, N., Gillespie, S. R., Bernstein, A. M. Ex vivo Corneal Organ Culture Model for Wound Healing Studies. Journal of Visualized Experiments. (144), (2019).

