Isolation of Murine Spermatogenic Cells using a Violet-Excited Cell-Permeable DNA Binding Dye
Summary
Here we present a simple and efficient method to isolate live meiotic and post-meiotic germ cells from adult mouse testes. Using a low-cytotoxicity, violet-excited DNA binding dye and fluorescence-activated cell sorting, one can isolate highly enriched spermatogenic cell populations for many downstream applications.
Abstract
Isolation of meiotic spermatocytes is essential to investigate molecular mechanisms underlying meiosis and spermatogenesis. Although there are established cell isolation protocols using Hoechst 33342 staining in combination with fluorescence-activated cell sorting, it requires cell sorters equipped with an ultraviolet laser. Here we describe a cell isolation protocol using the DyeCycle Violet (DCV) stain, a low cytotoxicity DNA binding dye structurally similar to Hoechst 33342. DCV can be excited by both ultraviolet and violet lasers, which improves the flexibility of equipment choice, including a cell sorter not equipped with an ultraviolet laser. Using this protocol, one can isolate three live-cell subpopulations in meiotic prophase I, including leptotene/zygotene, pachytene, and diplotene spermatocytes, as well as post-meiotic round spermatids. We also describe a protocol to prepare single-cell suspension from mouse testes. Overall, the procedure requires a short time to complete (4-5 hours depending on the number of needed cells), which facilitates many downstream applications.
Introduction
Spermatogenesis is a complex process wherein a small population of spermatogonial stem cells sustain continuous production of a large number of sperm throughout adult life1,2. During spermatogenesis, dynamic chromatin remodeling takes place when spermatogenic cells undergo meiosis to produce haploid spermatids3,4,5. Isolation of meiotic spermatocytes is essential for molecular investigation, and several different approaches to isolate meiotic spermatocytes have been established, including sedimentation-based separation6,7 and fluorescence-activated cell sorting (FACS)8,9,10,11,12,13,14,15,16,17. However, these methods have technical limitations. While sedimentation-based separation yields a large number of cells5,6,7, it is labor intensive. The established FACS-based method uses Hoechst 33342 (Ho342) to separate meiotic spermatocytes based on DNA content and light scattering properties and requires FACS cell sorters equipped with an ultraviolet (UV) laser8,9,10,11. Alternative FACS-based methods require transgenic mouse lines that express florescent proteins, synchronization of spermatogenesis12, or cell fixation and antibody labeling that is not compatible with isolation of live cells13. While there is another alternative method using a cell-permeable DNA binding dye, DyeCycle Green stain14,15,16,17, this method is recommended for the isolation of spermatogenic cells from juvenile testis. Therefore, there is a critical need to develop a simple and robust isolation method for live meiotic spermatocytes that can be applied to any mouse strain of any age and that can be performed using any FACS cell sorter.
Here we describe such a long-sought cell isolation protocol using the DyeCycle Violet (DCV) stain. DCV is a low cytotoxicity, cell-permeable DNA binding dye structurally similar to Ho342 but with an excitation spectrum shifted toward the violet range18. In addition, DCV has a broader emission spectrum compared to DCG. Thus, it can be excited by both UV and violet lasers, which improves the flexibility of equipment, allowing the use of an FACS cell sorter not equipped with a UV laser. The DCV protocol presented here uses two-dimensional separation with DCV blue and DCV red, mimicking the advantage of the Ho342 protocol. With this advantage, our DCV protocol allows us to isolate highly enriched germ cells from the adult testis. We provide a detailed gating protocol to isolate live spermatogenic cells from adult mouse testes of one mouse (from two testes). We also describe an efficient and quick protocol to prepare single-cell suspension from mouse testes that can be used for this cell isolation. The procedure requires a short time to complete (preparation of single cell suspension – 1 hour, dye staining – 30 min, and cell sorting – 2-3 hours: total – 4-5 hours depending on the number of needed cells; Figure 1). Following cell isolation, a wide range of downstream applications including RNA-seq, ATAC-seq, ChIP-seq, and cell culture can be completed.
Protocol
This protocol follows the guidelines of the Institutional Animal Care and Use Committee (protocol no. IACUC2018-0040) at Cincinnati Children’s Hospital Medical Center.
1. Equipment and reagent setup for the preparation of testicular cell suspension
- Prepare each enzyme stock in 1x Hanks’ Balanced Salt Solution (HBSS) and store at -20 °C. (Table1).
NOTE: Prepare any time before the experiment. - One day before the experiment: Coat collecting tubes (1.5 mL tubes) with Fetal bovine serum (FBS) at 4 °C overnight.
- On the day of the experiment: Set the water bath to 37 °C.
- Pre-warm Dulbecco's Modified Eagle Medium (DMEM) at 37 °C and prepare 2 mL of 1x dissociation buffer for each sample right before use (Table 1) in 15 mL centrifuge tube.
NOTE: 2 mL dissociation buffer is prepared for two testes. For the dissociation buffer recipe, please refer to Table 1.
2. Animal dissection and preparation of testicular cell suspension
- Sacrifice an 8-week-old male mouse by leaving in a carbon dioxide chamber for at least 10 min.
- Remove both testes and place on a 60 mm Petri dish containing 2 mL of ice-cold phosphate-buffered saline (PBS).
- Remove the tunica albuginea from the testes. Slightly disperse the seminiferous tubules by gently separating with forceps.
- Transfer the seminiferous tubules to a 100 mm Petri dish with a new drop of ice-cold PBS and untangle seminiferous tubules gently with forceps. Repeat this wash 3 times to remove interstitial cells as much as possible.
- Incubate untangled seminiferous tubules in a 15 mL tube containing 2 mL of dissociation buffer at 37 °C for 20 min.
- Gently pipette the tubules 20 times using a 1000 µL micropipette.
- Incubate for 6 min. Repeat gentle pipetting 20 times.
- Incubate for 3 min. Repeat gentle pipetting 10 times until no visible chunks remain.
- Add 10 mL of FACS buffer to the suspension. Centrifuge at 300 x g for 5 min at room temperature and discard the supernatant.
- Repeat step 2.9 twice to remove the spermatozoa and as much debris as possible.
3. Cell staining
- Resuspend the cell pellet with 3 mL of FACS buffer (Table 1) and filter the cell suspension through a 70 µm nylon cell strainer into a 50 mL tube.
NOTE: The expected cell yield is approximately 100 million cells per two testicles (from an 8-week-old B6 wild-type mouse). - Count the cell number and split 10% of the cells into a new tube as the unstained negative control and leave on ice.
- Add 6 µL of DCV stain (the original concentration is 5 mM) to the remaining cell suspension and mix well. The final concentration is 10 µM, and the capacity is approximately 100 million cells.
- Incubate at 37 °C for 30 min in the dark. Gently shake the cell suspension every 10 min.
- After incubation, without a wash step, directly add 5 µL of DNase I (the stock concentration is 10 mg/mL) to the cell suspension and filter the cells into a 5 mL FACS tube through the 35 µm nylon mesh cap. Keep the samples on ice until sorting.
4. Flow cytometry and experimental gates
- Prepare the FACS cell sorter. Ensure that FACS cell sorter is equipped with Excitation optics: Violet (405-nm) laser; Detection optics: filter combination of 450/50 bandpass [same filter set of 4′,6-diamidino-2-phenylindole (DAPI) for DCV-blue detection] and 665/30 bandpass [same filter set of Allophycocyanin (APC) for DCV-red detection]. Here we use Sony SH800S cell sorter as an example.
- Create a new experiment and set up the following working plots displaying parameters to be optimized: Click “New Density” on the “Worksheet Tools” menu bar to create a forward scatter – area (FSC-A) vs. back scatter – area (BSC-A) density plot on a linear scale. Click “New Histogram” to create a DCV-blue histogram plot on logarithmic scale.
- Briefly vortex the unstained negative control (from step 3.2) and load the sample.
- Click “Start” and “Record” to begin processing the unstained sample. While the sample is running, click “Detector & Threshold Settings” to optimize FSC and BSC voltages by adjusting both the photomultiplier tube (PMT) voltages up or down to place the unstained cells on the scale of the FSC/BSC plot.
- Adjust the PMT voltage up and down on “FL1: DAPI” while the sample is running to locate the position of the DCV-negative population in the first decade of the DCV-blue histogram logarithmic plot (Figure 2A). After completing the PMT voltage adjustment, click “Stop” to unload the unstained sample.
- Briefly vortex the sample (from step 3.5) and load the sample.
- Click “Next Tube” to create a new worksheet for the DCV-stained sample; and click “Start” and “Record” to acquire the DCV-stained sample, record ≥ 1 x 106 events. Add the following working plots: Click “New Density” on the “Worksheet Tools” menu bar to create an FSC-H (height) vs. FSC-W (width) density plot on a linear scale; Click “New Density” to create a DCV-blue vs. DCV-red density plot on a linear scale. After recording ≥ 1 x 106 events, click “Stop” and unload the sample.
- On the FSC-A vs. BSC-A density plot, click “Polygon” on the “Plot Tools” menu bar to draw a big gate “Cells” to include most cells and exclude small debris (Figure 2B). Apply the gate “Cells” to FSC-H vs. FSC-W density plot. Click “Rectangle” to draw a “Single Cells” gate to exclude non-single cells (Figure 2C).
- Apply “Single Cells” gate to DCV-blue vs. DCV-red density plot and adjust the scale to capture an extended profile as shown in Figure 2D. Click “Polygon” on the “Plot Tools” menu bar to draw a “DCV” gate to exclude the unstained cells and side population (Figure 2D).
- Apply “DCV” gate to DCV-blue histogram plot on a linear scale. The three major peaks refer to different DNA content: 1C, 2C, and 4C (Figure 2E).
- Click “New Density” to create a DCV-blue vs. DCV-red density plot on a linear scale and apply “DCV” gate to perform back gating from Figure 2E to locate the 1C and 4C populations (Figure 2F). Click “Ellipse” to draw a gate on the 1C population, which is within a condensed area (gate 1C). Click “Polygon” to draw a gate on the 4C population which is a continuous curve (gate 4C).
- Click “New Density” to create a DCV-blue vs. DCV-red density plot on a linear scale and apply “4C” gate; adjust the scale to zoom in and click “Polygon” to draw a “4C_1” gate for more precise selection (Figure 2G).
- Click “New Density” to create a new FSC-A vs. BSC-A density plot on a linear scale and apply “4C_1” gate; there will be three enriched populations separated by size corresponding to leptotene (L) /zygotene (Z), pachytene (P) and diplotene (D) spermatocytes. Click “Polygon” or “Ellipse” to draw 3 gates: “L/Z”, “P”, and “D” based on the growing size (Figure 2H).
- Click “New Dot Plot” to create a DCV-blue vs. DCV-red color dot plot on a linear scale to apply gate and ensure the three populations are in a continuous order within the “4C_1” gate (Figure 2I).
- Similarly, for the 1C population, click “New Density” to create a new FSC-A vs. BSC-A density plot on a linear scale and apply “1C” gate, select the unified size of cells as pure round spermatid population, and click “Ellipse” to draw an “RS” gate (Figure 2J).
5. Sort male germ cell subpopulations
- Prepare 1.5 mL tubes containing 500 μL 50% FBS for cell collection and load the collection tube into the collector and click “Load Collection”.
- Click “Next Tube”, “Start”, “Record” and “Sort Start”. For using a two-way system that allows two populations of interest to be sorted at the same time into the collection device, follow pairwise combinations: leptotene (L) /zygotene (Z) and pachytene (P) spermatocytes based on L/Z and P back-gates; Round spermatids (RS) and diplotene (D) spermatocytes based on RS and D back-gates.
- While the sample is running, adjust the flow rate to ~3000 events/s to get the most efficient sorting.
6. Purity analysis of sorted cells
- Collect ≥ 10,000 cells/each population. Perform cell immunostaining to confirm the substage.
- Centrifuge at 300 x g for 5 min at 4 °C and carefully discard the supernatant, keeping around 110 µL of the liquid in the bottom of the tube.
- Take a 10 µL drop of cell suspension to observe under microscope and evaluate the overview cell morphology and number.
- Apply 100 µL of cell suspension to each of the sample chamber slides (see Table of Materials), and load the chambers to the Cytospin.
- Spin the samples at 30 x g for 5 min at room temperature. Draw a circle around the cell with a hydrophobic pen. Dry slides on lab bench for a few minutes at room temperature.
- Drop 50 µL of PBS in the circle of the slide and tap off.
- Add primary antibody solution (Dilute primary antibodies with 5% Donkey Serum in PBS with 0.02% Polysorbate 20) in the circle of the slide and incubate at 4 °C overnight.
NOTE: To judge the substage of meiotic spermatocytes, SYCP3 was detected, which is a marker of meiotic chromosome axes, and γH2AX, which is a marker of DNA damage response. (For the antibody working concentration, please refer to the Table of Materials). - Tap off the primary antibody solution from the slides.
- Drop 50 µL of PBS in the circle of the slide and tap off. Repeat one time.
- Add secondary antibody solution (dilute secondary antibodies in PBS with 0.02% Polysorbate 20) in the circle of the slide and incubate at room temperature for 1 h in dark.
- Drop 50 µL of PBS in the circle of the slide and tap off. Repeat one time.
- Add 50 µL of DAPI (stock concentration is 0.1 μg/mL) stain for 5 min and tap off.
- Add 1-2 drops of mounting media on the slides. Carefully cover the mounting media with a cover glass and gently press the cover glass to remove extra mounting media and air bubble. The slides are ready for microscopy evaluation.
Representative Results
A representative result of this sorting protocol is shown in Figure 3. The total sorting time of two testes (one mouse) is usually around 3 hours, which is dependent on the concentration of cell suspension and the sorting speed. After sorting, the purity of spermatocytes is confirmed by immunostaining of SYCP3 and γH2AX (Figure 3A). The representative purity of sorted L/Z, P, D spermatocyte fractions are around 80.4%, 90.6%, and 87.6%, respectively (Figure 3C). We have determined the substages based on the criteria we published previously19. Briefly, in the leptotene and zygotene stage, synapsis between homologous chromosomes is incomplete, which is indicated by thin threads of SYCP3 staining. Broad γH2AX domains are observed throughout the nuclear chromatin due to programmed DNA double-strand breaks. In the pachytene stage, homologous chromosomes have completely synapsed, and γH2AX specifically accumulates on the sex chromosomes. In the diplotene stage, homologous chromosomes progressively undergo desynapsis. The purity of RS is confirmed by nucleus staining with DCV (Figure 3B). RS can be precisely judged with DNA staining: a unique DAPI-intense chromocenter surrounded by euchromatin; or combine with specific markers, such as Sp56 that is expressed within the developing acrosomal granule of spermatid and histone variant H1T that is highly expressed in nucleus after the mid-pachytene stage (Figure 3B).
RS purity is around 90.1% after sorting (Figure 3C). The sample size of purity analysis is over 1,000 cells for each experiment; the purity of L/P and D populations is averaged from 6 independent experiments; the purity of P and RS populations is averaged from 3 independent experiments. The viability of these isolated cells is usually over 95% (Figure S1). The total yield of each fraction from a single adult mouse is estimated and listed in Fig 3C, which provides sufficient cells for various downstream analyses. Recently, we have used this protocol to isolate wild-type pachytene spermatocytes for ChIP-seq analysis20,21.
| Reagent | Ingredient | Stock concentration | Volume |
| (HBSS base) | |||
| Dissociation Buffer | DMEM | – | 2 ml |
| (DMEM base) | FBS | – | 40 μl |
| Hyaluronidase | 100 mg/ml | 30 μl | |
| DNase I | 10 mg/ml | 50 μl | |
| Collagenase Type I | 100 mg/ml | 40 μl | |
| Recombinant Collagenase | 14000 unit/ml | 100 μl | |
| FACS buffer | PBS | – | 980 ml |
| (PBS base) | FBS | – | 20 ml |
Table 1: Reagent Recipe. The dissociation buffer must be prepared right before use. Prewarm DMEM before starting dissection. The enzyme stocks can be prepared any time before the experiment and stored at -20 °C. FACS buffer needs to be vacuum-filtered and stored at 4 °C; prewarm to room temperature before use.
Figure 1: Workflow of murine spermatogenic cells isolation on DCV-based sorting. This image illustrates the general procedure, from tissue dissociation to FACS sorting, to harvesting of isolated spermatogenic cells within one day. Please click here to view a larger version of this figure.
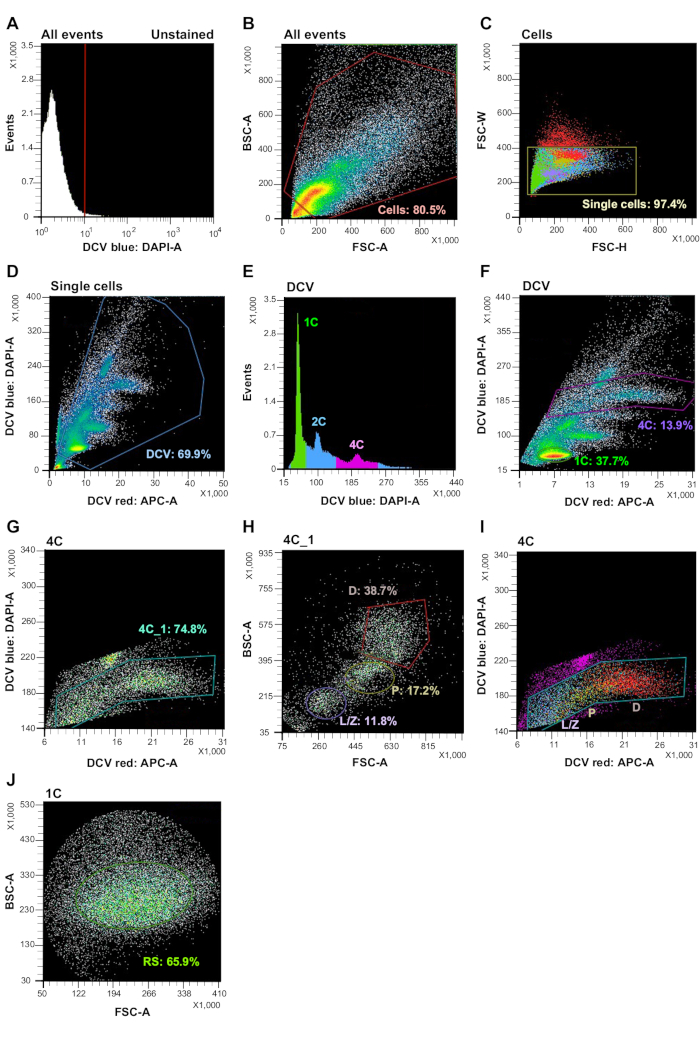
Figure 2: FACS analysis of adult murine testicular cells based on DCV fluorescence and light scattering. (A) Acquired unstained cells in the first decade of a DCV-blue histogram plot (left side of the red bar). (B)(C) Debris and non-single cell excluded by light scattering. (D) Unstained cell and side population exclusion based on DCV fluorescence. (E) DNA content determination based on DCV-blue fluorescence. Left peak (green) and right peak (pink) correspond to 1C and 4C populations. (F) Gating on 1C and 4C testicular populations based on DCV-blue/DCV-red fluorescence. (G) Precise gating on 4C testicular populations. (H) Back-gating of Gate 4C from the DCV plot on an FSC/BSC plot. Based on regions of minimal overlap on the FSC/BSC plot, the L/Z, P, and D gates are created to enrich their respective spermatocyte populations. (I) Color dot plot showing the L/Z, P, and D populations are in continuous order within Gate 4C. (J) Back-gating of Gate 1C from the DCV plot on an FSC/BSC plot. RS gate was created to enrich round spermatid population with uniform size, resulting in greater purity of populations during sorting. Please click here to view a larger version of this figure.
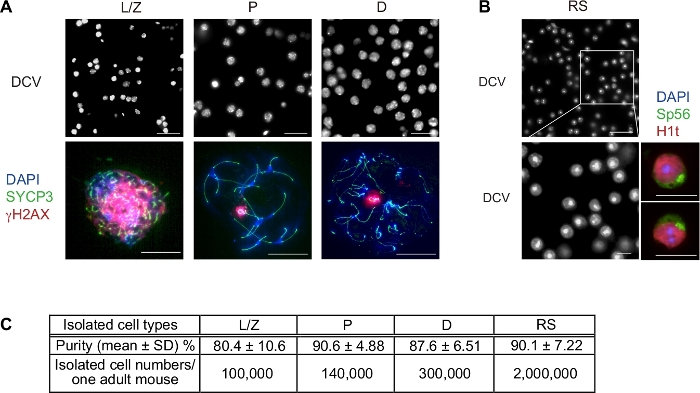
Figure 3: Representative result images and statistics of spermatogenic cells obtained from sorting. (A) Immunofluorescence characterization of sorted spermatocytes. Upper panel: DCV staining showing nucleus pattern of the live spermatocytes right after sorting; L/Z (leptotene/zygotene), P (pachytene), and D (diplotene). Lower panel: Confirmation of meiotic substages for each population by immunostaining for SYCP3 (green) and γH2AX (red). (B) Representative DCV image showing nucleus pattern of RS. Scale bars: 50 μm (upper panels), and 10 μm (lower magnified panels). Right Panels: Immunofluorescence confirmation of round spermatids stained with Sp56 and H1T. (C) The purity of L/Z, P, and D were confirmed by immunostaining, sample size was over 1,000 cells for each independent experiment, in total 6 independent experiments. The RS purity was confirmed by nucleus staining with a total of 3 independent experiments. The total cell number of the testicular cell suspension from one 8-week-old WT B6 mouse was around 100 million cells before sorting. Please click here to view a larger version of this figure.
Figure S1: The viability of isolated pachytene spermatocytes. A representative image shows the cell viability of isolated pachytene spermatocytes (Red: PI; Blue: DCV). PI could not be combined with DCV during sorting. However, under microscope, the DCV-red signal was quite low; therefore, PI-positive dead cells were easily distinguished from other live cells. The viability is usually over 95%. Scale bars: 10 μm. Please click here to download this figure.
Figure S2: Incomplete dissociation or debris disturbs gating. The A population (red circle) contains debris and polymer of spermatids (indicated by arrow). The bigger A population will cross with B population (yellow circle) and eventually contaminate the 4C population. Scale bars: 200 μm. Please click here to download this figure.
Discussion
Here we present a practical and simple protocol to isolate subpopulations of spermatocytes and spermatids from a single adult male mouse. To ensure the reproducibility of this protocol, there are some critical steps that need attention. Before enzyme digestion, wash step aims to remove interstitial cells; after digestion, this step helps to remove spermatozoa and debris. Washing/centrifuging 3 times is important. In our dissociation buffer recipe, the combination of several different enzymes facilitates the dissociation of testes into the single-cell suspension without excessive cell damage. Gently pipetting to avoid causing air bubbles also helps to protect cell integrity. Please check the cell suspension under a microscope after dissociation to make sure the suspension achieves single-cell level. Incomplete dissociation or debris contamination from excessive digestion will affect the purity of sorted cells; as shown in Figure S2, the upright population contains debris and tetramer of spermatids. DCV staining requires incubation in the dark and no washing afterwards.
To troubleshoot potential difficulties on gating and back-gating of spermatocyte subpopulations, as an option for optimization, we recommend using a synchronized wild type mouse to help locate a specific stage of spermatocytes22,23,24. It is also worth noting that some knockout mouse strains with spermatogenesis arrest phenotypes may have uncommon DCV profiles because they are missing some subpopulations. Proper wildtype control is strongly recommended in this case. In addition, this protocol can be potentially applied to adult mice of any age. However, the age of the experimental mouse could be a confounding factor due to the variable proportion of germ cells.
Over the years, several protocols to purify germ cells have been developed. As one of the most popular methods, STA-PUT velocity sedimentation separates germ cells by the BSA gradient and provides a good yield of intact germ cells6,7. However, STA-PUT not only requires special devices that may not be readily available to many laboratories but is also time-consuming and labor-intensive to conduct in a cold room at 4 °C. Unlike STA-PUT, which is suitable for large-scale separation, this FACS-based method could provide high purity and precise fraction for a small-scale experiment. A large-scale sorting using our protocol is possible but will prolong the sorting time significantly and may compromise cell viability. Therefore, STA-PUT is still a practical option when a large number of cells is needed5,25,26.
In comparison with the previous FACS method based on Ho342 dye staining8,9,10,11, our protocol utilizes DCV, which has a broader excitation spectrum and can be applied to most current FACS sorters equipped with a UV or 405 nm violet laser18. Although there is another protocol using DCG14,15,16,17, the difference between our protocol and the DCG protocol is that our DCV protocol uses two-dimensional separation with DCV blue and DCV red, mimicking the advantage of the Ho342 protocol. With this advantage, our DCV protocol allows us to isolate highly enriched germ cells from adult testis. The DCG protocol does not employ two-dimensional separation and is recommended for isolation of germ cells from juvenile mice. The two-dimensional separation can have better resolution to separate the substages of spermatocytes. However, our method is still incapable of isolating leptotene and zygotene spermatocytes separately, as well as “2C” cell types including spermatogonia, preleptotene spermatocytes, and secondary spermatocytes.
Since the wide emission spectrum of DCV stain causes leaking to other channels, most of the cell viability dyes like PI and 7AAD cannot be combined due to false positive signals. Other cell viability dyes with emission in far-red or near-infrared channels might be worth trying in the future. But in our experience, sorted cells usually show ≥ 95% viability after 2 hours of FACS sorting (Figure S1), which is sufficient for downstream analysis.
Sorted cells obtained from our procedure can be used for various downstream experiments, including next-generation sequencing analysis (RNA-seq, ATAC-seq, and ChIP-seq). Cells obtained here can also be used for short-term culture27. In conclusion, we provide a simple but efficient protocol including a one-hour single-cell suspension preparation procedure and the detailed gating strategy for FACS based on DCV dye staining, which is suitable for small-scale spermatogenic cell isolation and can be quickly adopted by many investigators, even flow cytometry beginners.
Divulgaciones
The authors have nothing to disclose.
Acknowledgements
We thank members of the Namekawa, Yoshida, and Maezawa laboratories for their help; Katie Gerhardt for editing the manuscript; Mary Ann Handel for sharing the H1T antibody, the Cincinnati Children’s Hospital Medical Center (CCHMC) Research Flow Cytometry Core for sharing the FACS equipment supported by NIH S10OD023410; Grant-in-Aid for Scientific Research (KAKENHI; 17K07424) to T.N.; Lalor Foundation Postdoctoral Fellowship to A.S.; AMED-CREST (JP17gm1110005h0001) to S.Y.; the Research Project Grant by the Azabu University Research Services Division, Ministry of Education, Culture, Sports, Science and Technology (MEXT)-Supported Program for the Private University Research Branding Project (2016–2019), Grant-in-Aid for Research Activity Start-up (19K21196), the Takeda Science Foundation (2019), and the Uehara Memorial Foundation Research Incentive Grant (2018) to S.M.; National Institute of Health R01 GM122776 to S.H.N.
Materials
| 1.5 ml tube | Watson | 131-7155C | |
| 100 mm Petri dish | Corning, Falcon | 351029 | |
| 15 mL Centrifuge tube | Watson | 1332-015S | |
| 5 ml polystyrene tube with cell strainer snap cap (35 µm nylon mesh) | Corning, Falcon | 352235 | |
| 50 mL Centrifuge tube | Watson | 1342-050S | |
| 60 mm Petri dish | Corning, Falcon | 351007 | |
| 70 µm nylon mesh | Corning, Falcon | 352350 | |
| Cell sorter | Sony | SH800S | |
| Centrifuge | |||
| Collagenase, recombinant, Animal-derived-free | FUJIFILM Wako Pure Chemical Corporation | 036-23141 | |
| Collagenase, Type 1 | Worthington | LS004196 | |
| Cover glass | Fisher | 12-544-G | |
| Cytospin 3 | Shandon | ||
| DAPI (4',6-Diamidino-2-Phenylindole, Dihydrochloride) | Fisher | D1306 | working concentration: 0.1 μg/mL |
| Dnase I | Sigma | D5025-150KU | |
| Donkey serum | Sigma | S30-M | |
| Dulbecco’s phosphate-buffered saline (DPBS) | Gibco | 14190144 | |
| Dulbecco's Modified Eagle Medium (DMEM) | Gibco | 11885076 | |
| Fetal bovine serum (FBS) | Gibco | 16000044 | |
| Hostone H1T antibody | gift from Mary Ann Handel | 1/2000 diluted | |
| Hank’s balanced salt solution (HBSS) | Gibco | 14175095 | |
| Hyaluronidase from bovine testes | Sigma | H3506-1G | |
| Phosphate buffered saline (PBS) | Sigma | P5493-4L | |
| Pipettemen | |||
| ProLong Gold Antifade Mountant | Fisher | P36930 | |
| rH2AX antibody | Millipore | 05-635 | working concentration: 2 μg/mL |
| Sperm Fertilization Protein 56 (Sp56) antibody | QED Bioscience | 55101 | working concentration: 0.5 μg/mL |
| Sterilized forceps and scissors | |||
| Superfrost /Plus Microscope Slides | Fisher | 12-550-15 | |
| SYCP3 antibody | Abcam | ab205846 | working concentration: 5 μg/mL |
| TWEEN 20 (Polysorbate 20) | Sigma | P9416 | |
| Vybrant DyeCycle Violet Stain (DCV) | Invitrogen | V35003 | |
| Water bath |
Referencias
- Griswold, M. D. Spermatogenesis: The Commitment to Meiosis. Physiological Reviews. 96 (1), 1-17 (2016).
- Yoshida, S. Mouse Spermatogenesis Reflects the Unity and Diversity of Tissue Stem Cell Niche Systems. Cold Spring Harbor Perspectives in Biology. , 036186 (2020).
- Rathke, C., Baarends, W. M., Awe, S., Renkawitz-Pohl, R. Chromatin dynamics during spermiogenesis. Biochimica et Biophysica Acta. 1839 (3), 155-168 (2014).
- Maezawa, S., et al. SCML2 promotes heterochromatin organization in late spermatogenesis. Journal of Cell Science. 131 (17), 217125 (2018).
- Maezawa, S., Yukawa, M., Alavattam, K. G., Barski, A., Namekawa, S. H. Dynamic reorganization of open chromatin underlies diverse transcriptomes during spermatogenesis. Nucleic Acids Research. 46 (2), 593-608 (2018).
- Bellve, A. R. Purification, culture, and fractionation of spermatogenic cells. Methods in Enzymology. 225, 84-113 (1993).
- Bryant, J. M., Meyer-Ficca, M. L., Dang, V. M., Berger, S. L., Meyer, R. G. Separation of spermatogenic cell types using STA-PUT velocity sedimentation. Journal of Visualized Experiments. (80), e50648 (2013).
- Gaysinskaya, V., Bortvin, A. Flow cytometry of murine spermatocytes. Current Protocols in Cytometry. 72, (2015).
- Bastos, H., et al. Flow cytometric characterization of viable meiotic and postmeiotic cells by Hoechst 33342 in mouse spermatogenesis. Cytometry Part A. 65 (1), 40-49 (2005).
- Getun, I. V., Torres, B., Bois, P. R. Flow cytometry purification of mouse meiotic cells. Journal of Visualized Experimments. (50), e2602 (2011).
- Gaysinskaya, V., Soh, I. Y., van der Heijden, G. W., Bortvin, A. Optimized flow cytometry isolation of murine spermatocytes. Cytometry Part A. 85 (6), 556-565 (2014).
- Romer, K. A., de Rooiji, D. G., Kojima, M. L., Page, D. C. Isolating mitotic and meiotic germ cells from male mice by developmental synchronization, staging, and sorting. Biología del desarrollo. 443 (1), 19-34 (2018).
- Lam, K. G., Brick, K., Cheng, G., Pratto, F., Camerini-Otero, R. D. Cell-type-specific genomics reveals histone modification dynamics in mammalian meiosis. Nature Communications. 10 (1), 3821 (2019).
- Geisinger, A., Rodriguez-Casuriaga, R. Flow Cytometry for the Isolation and Characterization of Rodent Meiocytes. Methods in Molecular Biology. 1471, 217-230 (2017).
- da Cruz, I., et al. Transcriptome analysis of highly purified mouse spermatogenic cell populations: gene expression signatures switch from meiotic-to postmeiotic-related processes at pachytene stage. BMC Genomics. 17, 294 (2016).
- Rodriguez-Casuriaga, R., et al. Rapid preparation of rodent testicular cell suspensions and spermatogenic stages purification by flow cytometry using a novel blue-laser-excitable vital dye. MethodsX. 1, 239-243 (2014).
- Trovero, M. F., et al. Revealing stage-specific expression patterns of long noncoding RNAs along mouse spermatogenesis. RNA Biology. 17 (3), 350-365 (2020).
- Telford, W. G., Bradford, J., Godfrey, W., Robey, R. W., Bates, S. E. Side population analysis using a violet-excited cell-permeable DNA binding dye. Stem Cells. 25 (4), 1029-1036 (2007).
- Alavattam, K. G., Abe, H., Sakashita, A., Namekawa, S. H. Chromosome Spread Analyses of Meiotic Sex Chromosome Inactivation. Methods in Molecular Biology. 1861, 113-129 (2018).
- Maezawa, S., et al. Super-enhancer switching drives a burst in gene expression at the mitosis-to-meiosis transition. Nature Structural & Molecular Biology. , (2020).
- Sakashita, A., et al. Endogenous retroviruses drive species-specific germline transcriptomes in mammals. Nature Structural & Molecular Biology. , (2020).
- Agrimson, K. S., et al. Characterizing the Spermatogonial Response to Retinoic Acid During the Onset of Spermatogenesis and Following Synchronization in the Neonatal Mouse Testis. Biology of Reproduction. 95 (4), 81 (2016).
- Hogarth, C. A., et al. Turning a spermatogenic wave into a tsunami: synchronizing murine spermatogenesis using WIN 18,446. Biology of Reproduction. 88 (2), 40 (2013).
- Patel, L., et al. Dynamic reorganization of the genome shapes the recombination landscape in meiotic prophase. Nature Structural & Molecular Biology. 26 (3), 164-174 (2019).
- Alavattam, K. G., et al. Attenuated chromatin compartmentalization in meiosis and its maturation in sperm development. Nature Structural & Molecular Biology. 26 (3), 175-184 (2019).
- Adams, S. R., et al. RNF8 and SCML2 cooperate to regulate ubiquitination and H3K27 acetylation for escape gene activation on the sex chromosomes. PLoS Genetics. 14 (2), 1007233 (2018).
- Wiltshire, T., Park, C., Caldwell, K. A., Handel, M. A. Induced premature G2/M-phase transition in pachytene spermatocytes includes events unique to meiosis. Biología del desarrollo. 169 (2), 557-567 (1995).

