Generating Acute and Chronic Experimental Models of Motor Tic Expression in Rats
Summary
We present protocols for generating acute and chronic experimental models of tic expression in freely behaving rats. The models are based on striatal cannula implantation and subsequent GABAA antagonist application. The acute model uses transient injections whereas the chronic model utilizes prolonged infusions via a subcutaneous implanted mini-osmotic pump.
Abstract
Motor tics are sudden, rapid, recurrent movements that are the key symptoms of Tourette syndrome and other tic disorders. The pathophysiology of tic generation is associated with abnormal inhibition of the basal ganglia, particularly its primary input structure, the striatum. In animal models of both rodents and non-human primates, local application of GABAA antagonists, such as bicuculline and picrotoxin, into the motor parts of the striatum induces local disinhibition resulting in the expression of motor tics.
Here, we present acute and chronic models of motor tics in rats. In the acute model, bicuculline microinjections through a cannula implanted in the dorsal striatum elicit the expression of tics lasting for short time periods of up to an hour. The chronic model is an alternative enabling the extension of tic expression to periods of several days or even weeks, utilizing continuous infusion of bicuculline via a sub-cutaneous mini-osmotic pump.
The models enable the study of the behavioral and neural mechanisms of tic generation throughout the cortico-basal ganglia pathway. The models support the implantation of additional recording and stimulation devices in addition to the injection cannulas, thus allowing for a wide variety of usages such as electrical and optical stimulation and electrophysiological recordings. Each method has different advantages and shortcomings: the acute model enables the comparison of the kinematic properties of movement and the corresponding electrophysiological changes before, during and after tic expression and the effects of short-term modulators on tic expression. This acute model is simple to establish; however, it is limited to a short period of time. The chronic model, while more complex, makes feasible the study of tic dynamics and behavioral effects on tic expression over prolonged periods. Thus, the type of empirical query drives the choice between these two complementary models of tic expression.
Introduction
Tics are the defining symptom of Tourette syndrome (TS) and other tic disorders. Tics are described as sudden, rapid, recurrent movements (motor tics), or vocalizations (vocal tics)1. Tic expression typically fluctuates in its temporal (frequency)2 and spatial (intensity, body location)3 properties over multiple time scales (hours, days, months, and years). These changes are affected by different factors, such as environmental features4,5, behavioral states6,7, and voluntary and temporary suppression8.
Although the neuronal mechanism governing motor tics is still not fully understood, an increasing number of theoretical and experimental studies have provided new evidence as to its nature9. Currently, the pathophysiology of tic generation is thought to involve the cortico-basal ganglia (CBG) loop, and specifically is associated with abnormal inhibition of the striatum, the primary basal ganglia input nucleus10,11,12. Previous studies in rodents and primates have demonstrated that the striatum can be disinhibited by local application of different GABAA antagonists, such as bicuculline and picrotoxin13,14,15,16,17,18. This pharmacological intervention leads to transient motor tic expression in the contralateral side to the injection, thus establishing a robust acute model of tic disorders with face and construct validity. The acute model is simple to induce and makes it possible to study the effects of short-term modulation such as electrical and optical stimulation concurrent with electrophysiological and kinematic recordings before, during and after tic expression. However, the acute model is limited to the short time period following the injection. Based on the acute model, we recently proposed a chronic model of tic generation in rats that utilizes a prolonged, fixed-rate infusion of bicuculline to the striatum via a subcutaneous-implanted mini-osmotic pump19. This model extends the period of tic expression to multiple days/weeks. The constant release of bicuculline over a lengthy period of time allows for the examination of the effects of a variety of factors such as pharmacological treatments and behavioral states on tic expression.
Here, we present protocols for generating the acute and chronic models of tic expression in rats. As a function of the specific research question, the protocols enable the fine-tuning of the parameters including unilateral versus bilateral implantation, the site of the tics (according to the somatotopic organization of the striatum)18 and the angle of the implant-cannula (depending on the location of additional implanted devices). The method used in the chronic model is partially based on commercial products but with critical adjustments to fit the tic model. This article details the adjustments needed to custom tailor these tic models.
Protocol
All procedures were approved and supervised by the Institutional Animal Care and Use Committee and adhered to the National Institutes of Health Guide for the Care and Use of Laboratory Animals and the Bar-Ilan University Guidelines for the Use and Care of Laboratory Animals in Research. This protocol was approved by the National Committee for Experiments in Laboratory Animals at the Ministry of Health.
NOTE: This protocol utilizes female Long-Evans rats (acute and chronic models) and female Sprague Dawley rats (acute model) aged 3-10 months, 280-350 g. The implementation of these models in other strains, weights or ages should be tested carefully for different reaction.
1. Acute model
- Pre-surgery preparation
- Implant-cannula preparation
NOTE: The implant-cannula enables local bicuculline injections into the striatum.- Cut a stainless steel, 25 G (OD 0.02'', ID 0.015'') hypo-tube to obtain an implant-cannula (Figure 1, device #1). Use a rotary tool to achieve straight edges. The length of the cannula depends on the implantation target depth, the angle of cannula implantation, and the final cemented cap height. The implantation target depth needs to be 2 mm (0.079'') higher than the final injection target to prevent tissue damage.
NOTE: The highest object implanted determines the cap height. - Sand and smooth the implant-cannula edges, preventing additional mechanic friction to the brain. Insert a 30 G (0.01'') needle through it to remove any internal obstructions.
- Cut a stainless steel, 25 G (OD 0.02'', ID 0.015'') hypo-tube to obtain an implant-cannula (Figure 1, device #1). Use a rotary tool to achieve straight edges. The length of the cannula depends on the implantation target depth, the angle of cannula implantation, and the final cemented cap height. The implantation target depth needs to be 2 mm (0.079'') higher than the final injection target to prevent tissue damage.
- Dummy preparation
NOTE: The dummy is a removable internal wire placed inside the implanted cannula. The dummy seals the implanted cannula, thus preventing its obstruction.- Make a dummy by cutting a 0.013'' wire with a rotary tool. The dummy should be 3 mm (0.118'') longer than the implant-cannula length (Figure 1, device #2).
- Insert the dummy into the implant-cannula until it reaches the end. Bend the excess wire by pinching it against the cannula. The bent part should be flush with the implant-cannula to prevent the dummy from falling out of the implanted cannula, and to prevent the rat from removing it.
- Injector preparation
NOTE: The injector, composed of a flexible tube and an injection-cannula (Figure 1, device #3), enables direct bicuculline injection into the striatum.- Cut a 70 cm (27.559'') flexible polymer microbore tube (OD 0.06'', ID 0.02'') (Figure 1, device #3.1).
NOTE: The length of the flexible tube is defined by the distance between the experimental cage and the infusion pump machine location. It must be long enough to enable free movement of the rat during the injection period, but not too long, to avoid the rat getting tangled in it (see Figure 3A). - Cut a stainless steel, 30 G (OD 0.012'', ID 0.007'') hypo-tube to obtain injection-cannula (Figure 1, device #3.2). Use a rotary tool to achieve straight edges. It should measure 5 mm (0.197'') longer than the implant-cannula: 2 mm (0.079'') longer than the implanted cannula within the brain to reach the final injection target, and 3 mm (0.118'') to insert it into the flexible tube.
- Sand and smooth the tip of the injection-cannula, preventing additional mechanic friction to the brain. Insert a wire measuring 0.005'' diameter to verify that it is unobstructed.
- Insert 3 mm (0.118'') of the injection-cannula into the flexible tube and glue the joint between them, to obtain an injector. Use cyanoacrylate (CA) glue and CA accelerator.
- Attach a syringe with 25 G needle (0.018'') filled with sterile water to the injector and wash it through. This ensures that the flow orientation coming out of the injection-cannula is straight and effortless. Crucially, if the flow is not straight, use the tip of 30 G (OD 0.01'') needle to remove any obstructions and enlarge the injection-cannula hole, and re-verify the flow.
- Cut a 70 cm (27.559'') flexible polymer microbore tube (OD 0.06'', ID 0.02'') (Figure 1, device #3.1).
- Cannula-holder preparation
NOTE: The cannula-holder is connected to the stereotaxic arm and holds the implant cannula during the implantation. The cannula-holder consists of cannula-holder base and cannula-holder lead, which are glued together (Figure 1, device #4). During the implantation, the cannula-holder base is attached to the stereotaxic arm, and the cannula-holder lead is attached to the implant-cannula.- Cannula-holder base: Cut 10 cm (3.947'') of stainless steel, 22 G (OD 0.028'', ID 0.017'') hypo-tube (Figure 1, device #4.1).
- Cannula-holder lead: Cut 0.013'' wire to a length of 3 mm (0.118'') longer than the desired implant-cannula (Figure 1, device #4.2).
- Insert the cannula-holder lead into the cannula-holder base and glue the joint between them, using CA glue and CA accelerator. The lead should be 1 mm (0.039'') shorter than the implant-cannula, to avoid tissue damage during implantation.
- Bicuculline preparation: dissolve bicuculline methiodide in physiological saline or artificial cerebrospinal fluid (ACSF) to a final concentration of 1 μg/μL. Divide the dissolved bicuculline into 1 mL syringes, cover with aluminum foil, and freeze at -20 °C until needed. When necessary, thaw the syringe before use.
- Implant-cannula preparation
- Surgery
- Induce initial anesthesia by placing the rat in a designed chamber and deliver 4-5% isoflurane mixed with an oxygen at a rate of0.5-1 L/min. Then, inject the rat intramuscular (IM or IP) with Ketamine and Xylazine (100 and 10 mg/kg, respectively) mixture.
- Shave the rat's head using an electric clipper.
- Put lidocaine gel in the rat's ears. Put petroleum jelly on the rat's eyes to prevent corneal drying and trauma.
- Secure the rat in the stereotactic frame using ear bars and toothbar.
- Swab the rat's scalp with povidone iodine and then with alcohol wipe to sterilize the area. Infiltrate along the desired incision line with 0.5 – 1% lidocaine solution subcutaneously (SC). Using a scalpel blade, make an incision along the scalp.
- Pull the fascia toward the edges to open the surgical area.
- Clean the skull with sterile saline, using cotton swabs. In case of bleeding, use a cauterizer to cauterize the blood capillary. This step is crucial for cap stability over time.
- Clamp the fascia with four curved hemostats (two anterior, two posterior) to enlarge the surgical site.
- Measure the bregma and lambda coordinates. Level the dorsoventral (DV) coordinates of the two points, so that they are within a range of 100 µm.
- Using the stereotaxic apparatus, measure and mark the coordinates of the areas of interest and the anchor screws to be implanted. The straight-implantation cannula coordinates for tic induction in the forelimb area are: AP: +1 to +1.5, mL: ±2.5, DV: 3; hindlimb area: AP: -0.4 to -0.5, mL: ±3.5, DV:318,20.
NOTE: In case of the implantation of multiple devices that prevent implanting cannula straight, change the angle of cannula implantation and its coordinates accordingly (forelimb coordinates: AP: +2.7, mL: ±2.5, DV: 3, angle 15° from anterior to posterior). - Drill holes in the skull under the microscope. Use a dental drill machine with 1/4-1/2 bit size carbide round burs. To minimize risks of brain injury, adjust the drill speed according to drilling skills and avoid any mechanic pressure. Drill until the brain is visible, for about 1 mm. Absorb any blood with a cotton swab and wash with sterile saline.
NOTE: The anchor screws serve to stabilize the cap. Make sure the screws are located in both hemispheres and along the anterior-posterior axis. - Cannula implantation
- Screw the anchor screws into the holes. Use stainless steel #0 x 1/8 size screws.
NOTE: The number of anchor screws depends on the total number of implanted devices. Ground screws (e.g. for the electrical recordings or electrical stimulations) should reach the brain surface. - Attach the cannula-holder to the stereotaxic arm.
- Slide the implant-cannula onto the cannula-holder. Slowly position the implant-cannula above the hole until it reaches the brain.
- Measure the DV coordinates starting from brain surface. Lower the implant-cannula up to the implantation target. Absorb any blood coming out of the hole with a cotton swab, wash with sterile saline and then dry thoroughly.
- Glue the implanted cannula to the skull using gel glue. Wait until dry.
- Apply dental cement along the implanted cannula to attach it to the skull. Leave 2 mm (0.079'') extend from its upper end to enable dummy insertion. Wait until dry.
NOTE: Do not put cement on the cannula-holder. - Lift the cannula-holder, leaving the implanted cannula in place.
- Insert the dummy into the implanted cannula.
- Implant all other devices such as recording arrays, optic fibers, stimulation electrodes etc. Apply dental cement over the rest of the skull, covering all the implants.
- Inject 3 mL of room temperature Ringer’s solution and carprofen 5 mg/kg SC21.
- Monitor the rat until it regains consciousness (animal is upright, has control of its airway and is not in danger of aspiration). Return the rat to its home cage for full recovery.
- Screw the anchor screws into the holes. Use stainless steel #0 x 1/8 size screws.
- Microinjections
NOTE: During the injection, it is crucial to verify that the flow of the bicuculline is intact. This can be done by letting a small air bubble form in the injector and monitoring its movement. The remaining volume of the injector may be filled with saline, so that no bicuculline is wasted.- Attach the injector to a bicuculline syringe with a 25 G needle (OD 0.018''). Fill ~1/3-1/2 of the injector and remove the syringe, allowing for the formation of a small air bubble.
- Attach the injector to a sterile saline-filled syringe with a 25 G needle (OD 0.018''). Fill the injector until the bicuculline reaches the end and a small drop comes out of it.
- Remove the plunger of a 10 µL precision glass microsyringe.
- Cut and attach a short-flexible polymer tube (~3 cm, 1.181'') to the precision glass microsyringe.
- Connect the other end of the short-flexible tube to a 1 mL syringe, 25 G needle (OD 0.018'') filled with sterile water.
- Inject water through the short-flexible tube into the precision glass microsyringe until water comes out of it. Disconnect the short-flexible tube.
- Reinsert the plunger until it reaches the ~7 µL mark on the precision glass microsyringe.
- Insert the precision glass microsyringe into the destined slot in the infusion pump machine.
- Attach the injector to the precision glass microsyringe and configure the settings to a rate of 0.35 µL/min and a total volume of 0.35 µL.
- Put a paper wipe under the injector tip. Mark the air bubble location on the injector, start the infusion pump machine and verify that a bicuculline drop appears. After the injection, mark the air bubble location again.
NOTE: The difference between the two marks corresponds to the desired difference during the experimental injection. - Put the rat in the experimental cage and remove the dummy.
- Insert the injector into the implanted cannula through the end (see Figure 3A).
- Start the infusion pump machine. Verify that the air bubble is moving. Start the stopwatch to keep track of tic initiation and termination times.
- One minute following the injection, remove the injector and slowly reinsert the dummy.
NOTE: Inserting the dummy after the injection pushes the bicuculline into the injection target.
- Post injection
- Disconnect the injector from the precision glass microsyringe.
- Wash out the remaining solution from the injector, using an air-filled syringe. Clean the injector with sterile water and then drain it by injecting air through the injector.
- Disconnect the precision glass microsyringe from the infusion pump machine and clean it with sterile water.
2. Chronic model
- Pre-surgery preparation
- Cannula-guide preparation
NOTE: The cannula-guide is part of the infusion-tube and is used to attach the infusion-cannula to the cannula-holder during the implantation.- Cut 12 mm (0.472'') of stainless steel, 25 G (OD 0.02'', ID 0.015'') hypo-tube to obtain a cannula-guide (Figure 2, device #1). Use a rotary tool to achieve straight edges.
- Prepare a cannula-holder as described in step 1.1.4. Insert the cannula-holder into the cannula-guide to verify it is properly attached and remove it.
- Infusion-cannula preparation
NOTE: The infusion-cannula is also a part of the infusion tube. It is implanted into the final target of the striatum and allows focal infusion of bicuculline.- Cut stainless steel, 30 G (OD 0.012'', ID 0.007'') hypo-tube to obtain an infusion-cannula. Use a rotary tool to achieve straight edges. The total infusion-cannula length is the sum of the desired implantation depth plus a safety factor (~1-2 mm, 0.039''-0.079''), the infusion-cannula bent part (2 mm, 0.079''), the overlap with the cannula-guide (3 mm, 0.118''), and the horizontal part (4 mm, 0.157'') (Figure 2, device #2).
NOTE: Unlike the acute model, the implantation depth is equal to the final infusion target. - Insert a 0.005'' diameter wire into the infusion-cannula and bend them into an L shape in the intended location. The vertical part corresponds to the desired implantation depth plus 4-5 mm (0.157''-0.197''), and the horizontal part is 4 mm (0.157'') long.
NOTE: The insertion of the inner wire prevents obstruction of the cannula during bending.
- Cut stainless steel, 30 G (OD 0.012'', ID 0.007'') hypo-tube to obtain an infusion-cannula. Use a rotary tool to achieve straight edges. The total infusion-cannula length is the sum of the desired implantation depth plus a safety factor (~1-2 mm, 0.039''-0.079''), the infusion-cannula bent part (2 mm, 0.079''), the overlap with the cannula-guide (3 mm, 0.118''), and the horizontal part (4 mm, 0.157'') (Figure 2, device #2).
- Flexible catheter-tubing preparation
NOTE: It is also a component of the infusion-tube. It connects the infusion-cannula to the mini-osmotic pump via a tubing-adapter.- Cut 8 cm (3.149'') of polyethylene (PE)-10 tubing (ID 0.011'', OD 0.025'') (Figure 2, device #3).
NOTE: The length of the catheter is determined by the distance between the implantation target and pump location, allowing free movement of the rat’s head and neck (see Figure 3B).
- Cut 8 cm (3.149'') of polyethylene (PE)-10 tubing (ID 0.011'', OD 0.025'') (Figure 2, device #3).
- Assembly of the infusion-tube
NOTE: The infusion-tube conducts the bicuculline from the mini-osmotic pump to the brain. It consists of the cannula-guide, the infusion-cannula, the flexible catheter-tubing, the tubing-adapter and the flow-moderator (Figure 2).- Remove the inner wire from the infusion-cannula. Inspect the cannula under the microscope to make sure its edges are open and clean on both sides; if not, use a 30 G (OD 0.01'') needle to open it.
- Glue the cannula-guide to the vertical section of the infusion-cannula, near the bent part, on the 3 mm (0.118'') overlap, using CA glue and CA accelerator.
- Insert the horizontal part of the infusion-cannula into the flexible catheter-tubing. The overlap should be at least 2 mm (0.079'').
- Eject the translucent cap of the pump flow-moderator. This will reveal the short stainless steel cannula tube (Figure 2, device #5.1).
NOTE: The flow-moderator is a part of the mini-osmotic pump kit. It is composed of a translucent cap, a short cannula-part, a white flange and a long cannula-part. The long cannula-part is inserted into the mini-osmotic pump and the short cannula-part is connected to the catheter-tubing via tubing-adapter. - Immerse the tubing-adapter (Figure 2, device #4) in 70% alcohol. Wait several minutes to allow the material to swell.
- Attach the tubing-adapter to the short cannula-part of the flow-moderator, until it touches the white flange (Figure 2, device #5.2). The tubing-adapter will shrink in the air to form a tight sealed connection.
- Insert the flexible catheter-tubing into the open end of the tubing-adapter, until it touches the short cannula-part of the flow-moderator.
- Hold the long cannula-part (Figure 2, device #5.3) using a clip stand and glue all the connections. The connections are between the tubing-adapter and white flange, the tubing-adapter and the flexible catheter-tubing, and finally the flexible catheter-tubing and the horizontal part of the infusion-cannula. Wait several hours until the glue is completely dry (depending on the glue type).
NOTE: Use PE compatible adhesive to prevent the connections from coming loose. - Inject sterile water through the long cannula-part of the infusion tube, using a syringe with a 27 G (0.014'') blunt needle. Verify that the water flows smoothly through the infusion-cannula. Inject air through the infusion-tube to drain the water.
- Priming of the mini-osmotic pump
NOTE: The priming is a start-up procedure that enables the pump to start the infusion immediately after the implantation.- Fill a heating bath with water at body temperature (~37 °C). Fill a small beaker with sterile saline and place it in the heating bath.
- Wrap the mini osmotic pump with a paper wipe, and fix it vertically with the opening facing upwards, using a clip holder stand.
- Fill the pump with ACSF using a syringe with a 27 G (0.014'') blunt needle. While removing the syringe, continue to inject the ACSF to prevent air from entering. An ACSF bubble will appear in the aperture of the pump.
NOTE: The initial ACSF infusion enables the rat to fully recover from surgery before tics are induced. Optionally, the bicuculline-filled pump can be implanted during the primary surgery to avoid the following pump replacement, but it is not optimal19. - Attach a syringe, 27 G (0.014'') blunt needle to the long cannula-part of the infusion-tube and inject ACSF through it. While removing the syringe, continue to inject the ACSF, to prevent air from entering. An ACSF bubble will appear in the long cannula-part.
- Insert the long cannula-part into the pump, bubble to bubble. An ACSF bubble should appear at the tip of the infusion-cannula.
- Place the pump in the beaker. Prime the pump, attached to the infusion-tube, for at least 4-6 hours (at ~37 °C) preceding pump implantation. Make sure only the pump contacts the saline.
- Pump implantation surgery
- Anaesthetize the rat according to the anesthesia protocol. See step 1.2.1.
- Shave the rat's head and back, using an electric clipper, slightly posterior to the scapulae.
- Perform the basic steps in surgery, as described in steps 1.2.3-1.2.11. The incision should be along the scalp up to the occipital bone.
- Sterilize a large hemostat (~14 cm long, 5.512'') in autoclave. Insert the hemostat through the incision and create a subcutaneous pocket in the rat’s back by alternately opening and closing it under the skin through the midscapular line.
NOTE: The pocket should be large enough to contain the pump and allow it to move slightly.
- Mini-osmotic pump and infusion tube implantation
- Attach the cannula-holder to the stereotaxic arm and place it in the desired position for implantation.
- Remove the pump from the heating bath and place it on the rat's back covered with a paper wipe.
- Slide the cannula-guide of the infusion-tube on the cannula-holder.
- Hold the pump with a hemostat and gently insert it into the subcutaneous pocket.
- Implant the anchor screws.
NOTE: Implant the anchor screws after inserting the pump, to avoid blockage of the pocket opening, and before cannula implantation to avoid cannula displacement. - Implant the infusion-cannula in the target and glue it to the skull using gel glue. Wait until dry. The coordinates for forelimb tic induction are: AP: +1 to +1.5, mL: ±2.5, DV: 5.
- Apply dental cement along the infusion-cannula to fix it to the skull. Wait until dry.
- Lift the cannula-holder leaving the implanted cannula in place.
- Implant all other devices. Apply dental cement on the rest of the skull, covering all the implants. Leave enough flexible catheter-tubing in the subcutaneous pocket unfixed to enable free movement of the rat.
NOTE: Make sure there are no exposed areas between the skull and the pocket opening, and that the catheter is not bent. - Finalize the surgery as detailed in steps 1.2.12.10-1.2.12.11.
- Cannula-guide preparation
- Pump replacement surgery
NOTE: Each mini-osmotic pump type has its own predetermined delivery infusion period. Hence, the pump replacement surgery should be performed prior to the expiration date.- Pre-surgery preparation
- Repeat steps 2.1.5.1-2.1.5.2.
- Fill the pump with bicuculline using a syringe with a 27 G (0.014'') blunt needle. While removing the syringe, continue to inject bicuculline, to prevent air from entering.
- Insert the flow-moderator (attached to its translucent cap) inside the pump.
- Place the pump in the beaker. Prime the pump for at least 4-6 hours (at ~37 °C) preceding pump replacement.
- Surgery
- Anesthetize the rat (see step 1.2.1.1) and shave its back using an electric clipper.
- Swab the rat's back with povidone iodine and then with an alcohol wipe to sterilize the area. Infiltrate along the desired incision line with a 0.5-1% lidocaine solution (SC).
- Make an incision on the skin above the implanted pump. Wash the pocket with room temperature ACSF and dry with gauze pads. Use autoclaved disposable drapes to cover the area near the incision.
- Detach the ACSF-filled pump from the flow-moderator using a hemostat and discard.
- Remove the bicuculline-filled pump from the heating bath. Detach and discard the flow-moderator from the bicuculline-filled pump.
- Gently attach the bicuculline-filled pump to the implanted flow-moderator. Avoid touching the surrounding skin.
NOTE: Steps 2.2.2.4-2.2.2.6 should be performed quickly to prevent air bubbles. However, the pump should be inserted slowly to prevent rapid entry of bicuculline into the brain. - Press the two margins of the incision closely together, using forceps. Glue the incision line with a tissue adhesive. As an alternative, close the incision using sutures.
- Swab the area with povidone iodine and finalize the surgery as detailed in steps 1.2.12.10-1.2.12.11.
- Pre-surgery preparation
Representative Results
Protocols for generating the acute and chronic models for tic induction in rats were presented above. The protocols cover the full preparation for surgery and experiments (Figure 1 for the acute model, Figure 2 for the chronic model). The application of bicuculline into the motor areas of the striatum results in the expression of ongoing motor tics. Tics appear on the contralateral side to the application and are characterized by brief and repetitive muscle contractions. After bicuculline application to the anterior parts of the striatum, tics are typically expressed in the rat’s forelimb, head and/or jaw, whereas after posterior injections, tics are expressed in the hindlimb18. In the acute model (Figure 3A), tics start to appear several minutes after the bicuculline microinjection, last for dozens of minutes and eventually decay and cease18. In the chronic model (Figure 3B), tics typically start to appear on the first day following the bicuculline-filled pump implantation19. Tics fluctuate during the day and are most clearly observable during the quiet-waking state19. Tic expression remains ongoing over a period of multiple days and up to a few weeks, depending on the type of mini-osmotic pump.
Tic expression may be monitored and quantified by simultaneous recordings of video, kinematic sensors and neural activity15,19,22. Motor tics have a stereotypic kinematic signature that can be detected in the accelerometer and gyroscope signals (Figure 4), thus enabling the measurement of their frequency and intensity. Tic timing can also be assessed using the local field potential (LFP) signal throughout the CBG pathway, because of the appearance of large amplitude LFP transient spikes15 (Figure 4). The results presented here and additional implementations of the acute and chronic models are described in detail in our previous works15,18,19,22,23. The striatal disinhibition model in both rodents and non-human primates replicated key properties of tic expression in Tourette syndrome and other tic disorders concerning both motor15,18 and vocal24 tics and their expression following a different behavioral, environmental and pharmacological interventions22,25,26. However, existing findings form only the tip of the iceberg of the complex manifestation of tic disorders. We believe that the model will enable the study of a wide range of such factors, ranging from environmental effects such as sensory input, behavioral effects such as concurrent action performance and clinical effects such as the response to different treatments.
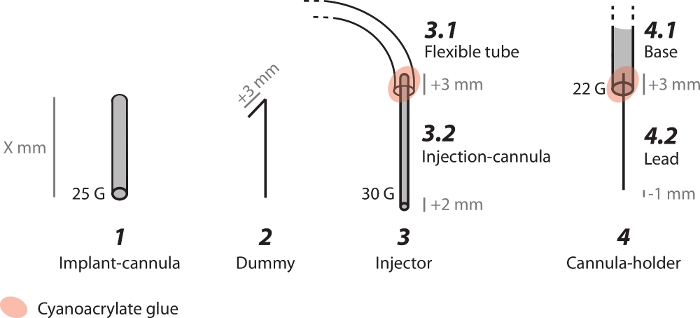
Figure 1: Schematic representation of the custom-made devices used in the acute model. (1) Implant-cannula which is chronically implanted in the striatum. (2) Dummy, a removable inner wire, is used to seal the implanted cannula. (3) Injector, composed of (3.1) flexible tube and (3.2) injection-cannula, is used for acute delivery of the bicuculline into the striatum. (4) Cannula-holder, composed of (4.1) base and (4.2) lead, is used to hold the implant-cannula during the implantation. Please click here to view a larger version of this figure.
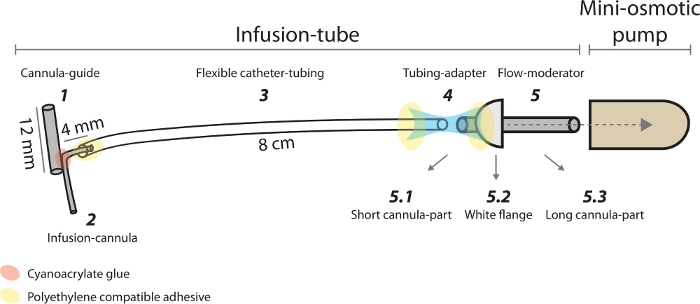
Figure 2: Schematic representation of the custom-made devices and the mini-osmotic pump used in the chronic model. (1) Cannula-guide is used to hold the infusion-cannula during the implantation. (2) Infusion-cannula is chronically implanted in the striatum. (3) Flexible catheter-tubing connects the infusion-cannula to the mini-osmotic pump. (4) Tubing-adapter connects the flexible catheter-tubing to the flow moderator. (5) Flow-moderator is composed of (5.1) short cannula-part, (5.2) white flange and (5.3) long cannula-part. Please click here to view a larger version of this figure.
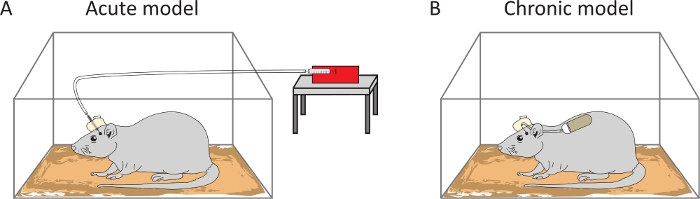
Figure 3: Schematic representation of the experimental setups. In the acute model, tics are induced following a bicuculline injection using a pump-infusion machine (A). In the chronic model, ongoing tics are achieved by prolonged infusion of bicuculline via mini-osmotic pump implantation (B). Please click here to view a larger version of this figure.
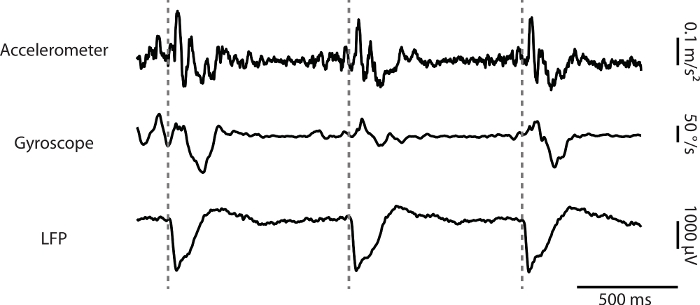
Figure 4: An example of synchronized signals from the kinematic and neurophysiological recordings. Accelerometer, gyroscope and the corresponding LFP from the primary motor cortex during tic expression. Dashed gray line: tic onset time as detected by the LFP signal. Please click here to view a larger version of this figure.
Discussion
In this manuscript, we detailed the protocols of the acute and chronic models for tic induction in a freely behaving rat. These protocols describe the preparation of all components, the surgery and the experimental process which can be adapted for customization to meet specific research needs. The primary principle underlying these models is the direct local application of bicuculline to the motor areas of the striatum, which is known to play a key role in the pathophysiology of tic disorders10,11,12. In both models, bicuculline is delivered to the target through custom-made implanted cannulas. The specific cannula implantation target depends on the desired body location of tic expression. The striatum is somatotopically organized27,28,29,30. Application of bicuculline to its anterior parts leads to tic expression in the forelimb, jaw, and head, whereas its application to the posterior parts results in hindlimb tics18. Moreover, application to the ventral striatum (nucleus accumbens – NAc) leads to hyperactivity31. The models enable the implantation of cannulas in both hemispheres and in both striatal targets for simultaneous injection to produce bilateral symptoms. This method is not only applicable to tic expression models, but also valid in other neuroscience models that require injection of neuroactive compounds.
In the acute model, we suggest implanting the cannula 2 mm (0.079'') above the injection target to prevent tissue damage to the target area. To minimize subsequent damage by the injection-cannula, we use a thin 30 G tube to reach the final target. Note that multiple injections to the same target will eventually lead to tissue necrosis from mechanical stress, which will cause decreased tic expression. One possible solution is to insert the injector to deeper targets during the subsequent injections, as long as they remain localized in the motor areas of the striatum. This tissue necrosis does not occur in the chronic model, since the bicuculline infusion is ongoing through a static directly implanted infusion-cannula into the striatal target. To minimize potential tissue damage from chronic infusion-cannula implantation, we also used a 30 G tube. However, to connect the infusion-cannula to the flow-moderator via flexible-catheter tubing, we needed to use a tubing-adapter, creating a potential failing point in the process. Thicker flexible-catheter tubing can be used to fit the flow-moderator, leading to a reasonable cost of a larger tissue damage from the larger infusion-cannula.
Ongoing research over the last 10 years has enabled us to define specific concentrations and delivery rates of bicuculline15,18,22,23, resulting in a reproducible behavioral phenomenon of observable tic expression. Deviation from these values towards higher volumes, concentrations or injection rates, may cause episodic seizures15,18,32 and unilateral rotations of the rats. Lower concentrations result in more subtle, less detectable tics, expressed over shorter periods of time. In the chronic model, no seizures were observed throughout the whole period; however, extensive tic expression and tendency to unilateral rotations were observed on the first day after the bicuculline-filled pump implantation, which stabilized during the second day. This, combined with post brain surgery recovery, interferes with the animal’s comfort level and wellbeing. To dissociate the recovery period from tic expression, we suggest implanting an ACSF-filled pump first19. This period of ACSF infusion can also be used to conduct control experiments prior to tic induction. Control experimental sessions may also be carried out in the acute model, utilizing ACSF injections18,33.
Both the acute and the chronic models can be used to study the kinematic characteristics and neural correlates of tic expression. Tics can be identified by frame-by-frame offline video analysis, which however is time-consuming and less accurate. More sensitive evaluation methods include electromyography (EMG) and kinematic sensors (accelerometer and gyroscopes) (Figure 4). For this purpose, the kinematic devices need to be located near the tic-expressing site on the body for accurate movement assessment. The neural correlates of tic expression may be captured by neurophysiological recordings throughout the CBG pathway (Figure 4). When considering the implantation of additional recording devices, their locations both inside and outside the brain need to be planned carefully to prevent interference with the injection.
The nature of the experimental query should dictate the choice of model of tic expression. The acute model is simple and easy to implement. Multiple transient injections can be conducted over a relatively long period of time, can be run simultaneously in several brain regions and enable combining control and experimental sessions. The chronic model is more complicated and requires daily monitoring of the rat's wellbeing. Yet, the constant and prolonged bicuculline application provides the opportunity to address the dynamics of tic expression and its modulation over time.
Divulgaciones
The authors have nothing to disclose.
Acknowledgements
This study was supported in part by an Israel Science Foundation (ISF) grant (297/18). The authors thank M. Bronfeld for establishing the acute rodent model and M. Israelashvili for her comments.
Materials
| Anchor screws | Micro Fasteners | SMPPS0002 | #0 x 1/8 – Pan Head Sheet Metal Screws |
| Bicuculline methiodide | Sigma Aldrich | 14343 | |
| Cyanoacrylate (CA) accelerator | Zap | PT29 | |
| Cyanoacrylate (CA) glue | BSI | IC-2000 | This glue was found to be stronger than others |
| Dental cement | Coltene | H00322 | Hygenic Perm Repair Material Reline Resin Self Cure |
| Glue gel | Loctite | Ultra Gel Control | |
| Hemostat | WPI | 501242 | Any hemostat sized approximately 14 cm would be sufficient |
| Hypo-tube, extra-thin wall 25G | Component supply company | HTX-25X | |
| Hypo-tube, regular wall 22G | Component supply company | HTX-22R | |
| Hypo-tube, regular wall 30G | Component supply company | HTX-30R | |
| Infusion pump machine | New Era Pump Systems | NE-1000 | |
| Mini-osmotic pump | ALZET | 2001 | 1.0µl per hour, 7 days |
| PE compatible adhesive | CEYS | Special difficult plastics (suitable for PE) | |
| PE-10 Catheter Tubing | ALZET | PE-10 | ID = 0.28mm, OD = 0.61mm |
| Precision glass microsyringe, 10µl | Hamilton | 80065 | 1701 RNR 10µl syr (22s/51/3) |
| Tissue adhesive | 3M | 1469Sb | Vetbond |
| Tubing-adapter | CMA | 3409500 | |
| Tygon micro bore tubing, 0.02 inch ID * 0.06 OD | Component supply company | TND80-020 | |
| Wire 0.005-inch | Component supply company | GWX-0050 | |
| Wire 0.013-inch | Component supply company | GWX-0130 |
Referencias
- American Psychiatric Association. DSM-5. American Psychiatric Association. , (2013).
- Peterson, B. S., Leckman, J. F. The temporal dynamics of tics in Gilles de la Tourette syndrome. Biol.Psychiatry. 44, 1337-1348 (1998).
- Ganos, C., et al. The somatotopy of tic inhibition: where and how much. Movement Disorders. , (2015).
- Barnea, M., et al. Subjective versus objective measures of tic severity in Tourette syndrome – The influence of environment. Psychiatry Research. 242, 204-209 (2016).
- Silva, R. R., Munoz, D. M., Barickman, J., Friedhoff, A. J. Environmental Factors and Related Fluctuation of Symptoms in Children and Adolescents with Tourette’s Disorder. Journal of Child Psychology and Psychiatry. 36 (2), 305-312 (1995).
- Rothenberger, A., et al. Sleep and Tourette syndrome. Advances in Neurology. 85, 245-259 (2001).
- Conelea, C. a., Woods, D. W., Brandt, B. C. The impact of a stress induction task on tic frequencies in youth with Tourette Syndrome. Behaviour Research and Therapy. 49 (8), 492-497 (2011).
- Ganos, C., Rothwell, J., Haggard, P. Voluntary inhibitory motor control over involuntary tic movements. Movement Disorders. 33 (6), 937-946 (2018).
- Yael, D., Vinner, E., Bar-Gad, I. Pathophysiology of tic disorders. Movement Disorders. 30 (9), 1171-1178 (2015).
- Kurvits, L., Martino, D., Ganos, C., Eddy, C. M. Clinical Features That Evoke the Concept of Disinhibition in Tourette Syndrome. Frontiers in Psychiatry. 11, 1-10 (2020).
- Mink, J. W. Basal ganglia dysfunction in Tourette’s syndrome: a new hypothesis. Pediatric Neurology. 25, 190-198 (2001).
- Bronfeld, M., Bar-Gad, I. Tic disorders: what happens in the basal ganglia. The Neuroscientist. 19 (1), 101-108 (2013).
- Tarsy, D., Pycock, C. J., Meldrum, B. S., Marsden, C. D. Focal contralateral myoclonus produced by inhibition of GABA action in the caudate nucleus of rats. Brain. 101 (1), 143-162 (1978).
- Crossman, A. R., Mitchell, I. J., Sambrook, M. A., Jackson, A. Chorea and Myoclonus in the Monkey Induced By Gamma-Aminobutyric Acid Antagonism in the Lentiform Complex. Brain. 111 (5), 1211-1233 (1988).
- McCairn, K. W., Bronfeld, M., Belelovsky, K., Bar-Gad, I. The neurophysiological correlates of motor tics following focal striatal disinhibition. Brain. 132 (8), 2125-2138 (2009).
- Worbe, Y., et al. Behavioral and movement disorders induced by local inhibitory dysfunction in primate striatum. Cerebral Cortex. 19 (8), 1844-1856 (2009).
- Pogorelov, V., Xu, M., Smith, H. R., Buchanan, G. F., Pittenger, C. Corticostriatal interactions in the generation of tic-like behaviors after local striatal disinhibition. Experimental Neurology. 265, 122-128 (2015).
- Bronfeld, M., Yael, D., Belelovsky, K., Bar-Gad, I. Motor tics evoked by striatal disinhibition in the rat. Frontiers in Systems Neuroscience. 7, 50 (2013).
- Vinner, E., Israelashvili, M., Bar-Gad, I. Prolonged striatal disinhibition as a chronic animal model of tic disorders. Journal of Neuroscience Methods. 292, 20-29 (2017).
- Paxinos, G., Watson, C. . The Rat Brain in Stereotaxic Coordinates. 6, (2007).
- Flecknell, P. Analgesia and Post-Operative Care. Laboratory Animal Anaesthesia. , (2016).
- Israelashvili, M., Bar-Gad, I. Corticostriatal divergent function in determining the temporal and spatial properties of motor tics. Journal of Neuroscience. 35 (50), 16340-16351 (2015).
- Bronfeld, M., Belelovsky, K., Bar-Gad, I. Spatial and temporal properties of tic-related neuronal activity in the cortico-basal ganglia loop. Journal of Neuroscience. 31 (24), 8713-8721 (2011).
- McCairn, K. W., et al. A Primary Role for Nucleus Accumbens and Related Limbic Network in Vocal Tics. Neuron. 89 (2), 300-307 (2016).
- Rizzo, F., et al. Aripiprazole Selectively Reduces Motor Tics in a Young Animal Model for Tourette’s Syndrome and Comorbid Attention Deficit and Hyperactivity Disorder. Frontiers in Neurology. 9, 1-11 (2018).
- Vinner, E., Matzner, A., Belelovsky, K., Bar-gad, I. Dissociation of tic expression from its neuronal encoding in the striatum during sleep. bioRxiv. , (2020).
- Webster, K. E. Cortico-striate interrelations in the albino rat. Journal of Anatomy. 95, 532-544 (1961).
- Ebrahimi, A., Pochet, R., Roger, M. Topographical organization of the projections from physiologically identified areas of the motor cortex to the striatum in the rat. Neuroscience Research. 14, 39-60 (1992).
- Brown, L. L., Sharp, F. R. Metabolic mapping of rat striatum: somatotopic organization of sensorimotor activity. Brain Research. 686, 207-222 (1995).
- Brown, L. L., Smith, D. M., Goldbloom, L. M. Organizing principles of cortical integration in the rat neostriatum: Corticostriate map of the body surface is an ordered lattice of curved laminae and radial points. Journal of Comparative Neurology. 392 (4), 468-488 (1998).
- Yael, D., Tahary, O., Gurovich, B., Belelovsky, K., Bar-Gad, I. Disinhibition of the nucleus accumbens leads to macro-scale hyperactivity consisting of micro-scale behavioral segments encoded by striatal activity. The Journal of Neuroscience. , 3120 (2019).
- Obeso, J. A., Rothwell, J. C., Marsden, C. D. The spectrum of cortical myoclonus. From focal reflex jerks to spontaneous motor epilepsy. Brain. 108, 124-193 (1985).
- Bronfeld, M., et al. Bicuculline-induced chorea manifests in focal rather than globalized abnormalities in the activation of the external and internal globus pallidus. Journal of Neurophysiology. 104 (6), 3261-3275 (2010).

