Using Immunofluorescence to Detect PM2.5-induced DNA Damage in Zebrafish Embryo Hearts
Summary
This protocol uses an immunofluorescence assay to detect PM2.5-induced DNA damage in the dissected hearts of zebrafish embryos.
Abstract
Ambient fine particulate matter (PM2.5) exposure can lead to cardiac developmental toxicity but the underlying molecular mechanisms are still unclear. 8-hydroxy-2'deoxygenase (8-OHdG) is a marker of oxidative DNA damage and γH2AX is a sensitive marker for DNA double strand breaks. In this study, we aimed to detect PM2.5-induced 8-OHdG and γH2AX changes in the heart of zebrafish embryos using an immunofluorescence assay. Zebrafish embryos were treated with extractable organic matters (EOM) from PM2.5 at 5 μg/mL in the presence or absence of antioxidant N-acetyl-L-cysteine (NAC, 0.25 μM) at 2 h post fertilization (hpf). DMSO was used as a vehicle control. At 72 hpf, hearts were dissected from embryos using a syringe needle and fixed and permeabilized. After being blocked, samples were probed with primary antibodies against 8-OHdG and γH2AX. Samples were then washed and incubated with secondary antibodies. The resulting images were observed under fluorescence microscopy and quantified using ImageJ. The results show that EOM from PM2.5 significantly enhanced 8-OHdG and γH2AX signals in the heart of zebrafish embryos. However, NAC, acting as a reactive oxygen species (ROS) scavenger, partially counteracted the EOM-induced DNA damage. Here, we present an immunofluorescence protocol for investigating the role of DNA damage in PM2.5-induced heart defects that can be applied to the detection of environmental chemical-induced protein expression changes in the hearts of zebrafish embryos.
Introduction
Air pollution is now a serious environmental problem facing the world. Ambient fine particulate matter (PM2.5), which is one of the most important indicators of air quality, can carry a large number of harmful substances and enter the blood circulatory system, causing serious harm to human health1. Epidemiology studies have demonstrated that PM2.5 exposure can lead to an increased risk of congenital heart defects (CHDs)2,3. Evidence from animal experiments also showed that PM2.5 can cause abnormal cardiac development in zebrafish embryos and the offspring of mice but the molecular mechanisms of the cardiac developmental toxicity of PM2.5 is still largely unknown4,5,6.
DNA damage can cause cell cycle arrest and induce apoptosis, which may extensively destroy the potential of progenitor cells and, consequently, impair heart development7). It has been well documented that environmental pollutants, including PM2.5, have the potential to attack DNA through oxidative stress mechanisms8,9. Both human and zebrafish cardiac development are sensitive to oxidative stress10,11,12. 8-OHdG is an oxidative DNA damage marker, and γH2AX signal is a marker of DNA double strand breaks. N-acetyl-L-cysteine (NAC), a synthetic precursor of intracellular cysteine and glutathione, is widely used as an anti-oxidative compound. In this study, we use NAC to investigate the role of oxidative stress in PM2.5– induced DNA damage13.
Zebrafish as a model vertebrate has been widely used to study cardiac development and human cardiovascular diseases because mechanisms of cardiac development are highly conserved among vertebrates14,15. The advantages of using zebrafish as a model include their small size, strong reproductive ability, and low feeding cost. Of particular interest to these studies, zebrafish embryos do not depend on the circulatory system during early development and can survive severe heart malformation14. Moreover, their transparency allows the entire body to be directly observed under a microscope. Thus, zebrafish embryos provide an outstanding opportunity for assessing the molecular mechanisms involved in the induction of cardiac developmental toxicity as a result of exposure to various environmental chemicals5,16,17. We have previously reported that PM2.5-induced oxidative stress leads to DNA damage and apoptosis, resulting in heart malformations in zebrafish18. In this study, we provide a detailed protocol for investigating PM2.5-induced DNA damage in the heart of zebrafish embryos.
Protocol
Wild type zebrafish (AB) used in this study were obtained from the National Zebrafish Resource Center in Wuhan, China. All animal procedures outlined here have been reviewed and approved by the Animal Care Institution of The Ethics Committee of Soochow University.
1. PM2.5 sampling and organic compound extraction
NOTE: PM2.5 was collected in an urban area in Suzhou, China, August 1-7, 2015, as described previously5.
- Bake 47 mm quartz membrane filters in a 500 °C muffle furnace for 2 h to remove the organic components.
- Place a filter in a PM2.5 sampler for 24 h of uninterrupted sampling.
- Remove the filter and dry for 24 h at room temperature.
- Quantify the filter with an analytical balance.
- Extract organic components from the filter by Soxhlet extraction using dichloromethane as a solvent19.
- Dry the EOM by a rotary evaporation in a 60 °C water bath and nitrogen flow. Dissolve EOM in DMSO and store at -20 °C.
2. Zebrafish embryo collection and treatment
- Maintain the zebrafish at 28.5 ± 0.5 °C in a re-circulating aquaculture system with a 14 h light and 10 h dark photoperiod cycle.
- Place healthy adult zebrafish into a tank at a 2:1 male to female ratio.
- The next day, collect the embryos and wash them with system water (i.e., zebrafish breeding water).
- Select and randomly divide zebrafish embryos demonstrating a normal development (uniform size, full grains, and no egg coagulation) into 4 groups in individual glass Petri dishes with a diameter of 7 cm (about 50 embryos per dish).
- Treat the embryos with PM2.5 (5 mg/L) in the presence or absence of NAC at 0.25 μM from 2 hpf until 72 hpf. Use DMSO as a vehicle control to a final concentration at 0.1% (v/v).
3. Morphological observation of zebrafish embryos and cardiac dissection
- At 72 hpf, transfer embryos to glass slides and observe under a stereo microscope. Record heart malformations, such as pericardial edema, altered looping, and decreased size.
- Calculate malformation rates (the percentage of embryos with heart defects out of the total living embryos) and analyze differences between groups using one-way ANOVA followed by Turkey's Multiple Comparison Test (p < 0.05 = statistically significant).
- Anesthetize the embryos with 0.6 mg/mL MS-222 to immobilize them on glass slides.
- Record heart beats for 30 s and quantify heart rates using ImageJ software5,20.
- Dissect hearts from zebrafish embryos with a disposable syringe needle under a stereo microscope. Caution: Avoid destroying the heart shape.
4. Immunofluorescence Assay
- To use an immunofluorescence assay to detect PM2.5 induced DNA damage in the heart of zebrafish embryos, use a hydrophobic barrier pen to draw a circle on a clean glass side.
- Add 50 μL of 4% paraformaldehyde to 1.25 mL of Phosphate Buffered Saline (PBS) to make a fixative solution.
- Place 3 dissected hearts into one hydrophobic barrier pen circle and incubate for 20 min at room temperature.
- Decant the solution under the microscope and dry the samples at room temperature for at least 5 min, so that the hearts completely attach to the glass slides.
- Wash the slides three times in PBS with 0.1% Tween 20 (PBST) for 5 min per wash.
- Add 50 μL of bovine serum albumin (BSA) to 1000 μL of PBST to obtain a 5% BSA solution and incubate the slides in a humid chamber for 1 h to block non-specific antibody binding.
- Decant the solution and wash the samples three times with PBST for 5 min per wash.
- Dilute 2 μL of mouse monoclonal antibody against 8-OHdG and 2 μL of rabbit polyclonal antibody against γH2AX in 296 μL of PBST to obtain a working primary antibody cocktail solution.
- Incubate the heart samples with 50 μL of the primary antibody cocktail solution against 8-OHdG and γH2AX in a humidified chamber for at least one hour at room temperature or overnight at 4 °C (Overnight incubation can increase signal intensity).
- Decant the solution and wash the samples three times with PBST for 5 min per wash.
- Dilute 1 μL of FITC-labeled goat anti-mouse secondary antibody and 1 μL of cy3 goat anti-rabbit secondary antibody in 498 μL of PBST to obtain a working secondary antibody cocktail solution and incubate the samples with the secondary antibodies (1:500 in PBST) for 1 h at room temperature in the dark.
- Decant the solution and wash the samples three times with PBS for 5 min per wash protected from light.
- Add 20 μL of DAPI (4',6-diamidino-2-phenylindole) to the samples for nuclear staining for 30 min at room temperature.
- Apply a coverslip to slide and seal with nail polish to prevent drying and movement. Then image the samples under a fluorescence microscope and quantify the fluorescence signal of heart area using ImageJ software. Calculate the relative changes with the average of DMSO control samples. Determine the statistical significance of the data as in step 3.2.
Representative Results
This immunofluorescence assay is a sensitive and specific method for measuring protein expression changes in the hearts of zebrafish embryos exposed to environmental chemicals.
In this representative analysis, embryos exposed to PM2.5 in the absence or presence of the antioxidant NAC were evaluated for the presence the presence of heart malformations (Figure 1). As observed, EOM from PM2.5 caused a significant increase in cardiac teratogenesis, such as pericardial edema, altered looping, and decreased size, compared to DMSO control-treated hearts. The heartbeat rate was also significantly decreased in embryos exposed to EOM (Figure 1B). The addition of NAC significantly attenuated EOM-induced heart defects (Figure 1).
Here the immunofluorescence assay was used to measure 8-OHdG and γH2AX expression in zebrafish embryo to evaluate the extent of DNA damage in EOM-treated tissues. As shown in Figure 2, the levels of 8-OHdG and γH2AX expression were significantly increased in the hearts of zebrafish embryos treated with EOM group compared to the control, DMSO-treated hearts, indicating an increase in oxidative DNA damage and DNA double strand breaks, respectively. Furthermore, the EOM-induced DNA damage was partially counteracted by NAC supplementation (Figure 2).
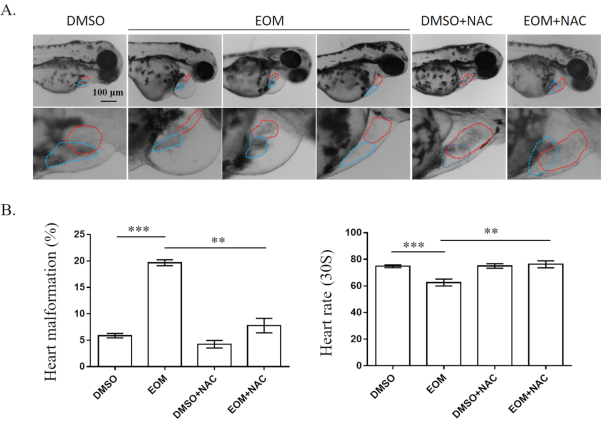
Figure 1. Cardiac defects of zebrafish embryos at 72 hpf. (A) Images of zebrafish embryos at 72 hpf. Dotted lines indicate atria (red) or ventricles (blue). Scale bar, 200 μm. (B) Heart malformation and heartbeat rates. Results are presented as mean ± SEM. At least 50 embryos in each group were examined. EOM: EOM at 5 mg/L; NAC: NAC at 0.25 μM.. **,P < 0.01; ***, p<0.001 Please click here to view a larger version of this figure.
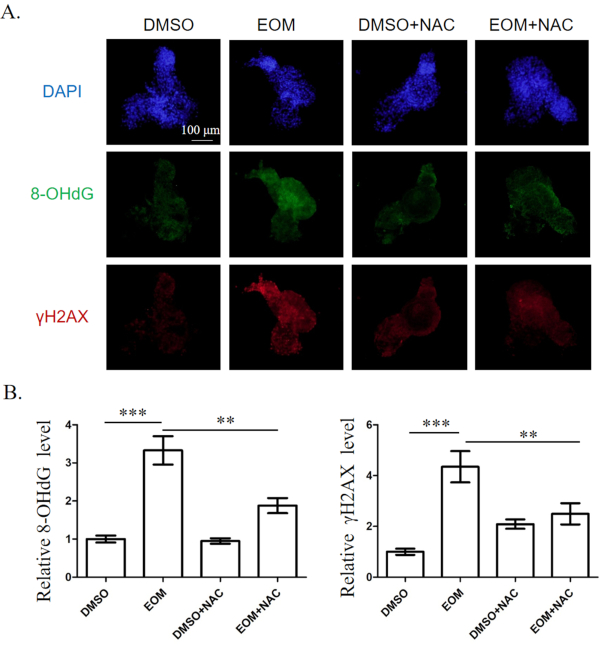
Figure 2. DNA damage in the heart of zebrafish embryos at 72 hpf. A) Immunofluorescence staining. Scale bar, 100 μm. B) Quantitative results. Results were presented as mean ± SEM. At least 15 hearts from each group were examined. EOM: EOM at 5 mg/L; NAC: NAC at 0.25 μM. **; P < 0.01; ***; p<0.001. Please click here to view a larger version of this figure.
Discussion
Although zebrafish is an excellent vertebrate model for studying the cardiac developmental toxicity of environmental chemicals, due to the small size of the embryo heart, it is difficult to obtain enough protein for western blot analysis. Therefore, we present a sensitive immunofluorescence method for quantifying the protein expression levels of DNA damage biomarkers in the hearts of zebrafish embryos exposed to PM2.5.
During dissection, it is important to keep the integrity of the heart intact. In our experience, it is relatively easy to perform the isolation at 72 hpf. In addition, the heart needs to be put into fixation solution as soon as possible after collection. Another critical step is drying the samples to make sure that the dissected hearts are completely attached to the glass slide. Otherwise, the samples may be washed off from the slide during labeling.
Dual immunofluorescence staining is performed to detect both 8-OHdG and γH2AX signals in the isolated hearts. This method not only saves labor and allows the use of a reduced sample size, but also facilitates co-localization of the two signals. Although this antibody-based method can not detect fluorescence signals in living embryos, this rapid protocol can be used to detect protein expression in isolated zebrafish embryo hearts.
It has been frequently reported that oxidative stress mediates PM2.5-induced DNA damage8,9. Excessive ROS production can lead to DNA damage and apoptosis during zebrafish embryonic development21,22,23. As we have previously reported18, an increased 8-OHdG and γH2AX signal expression is observed in the hearts of zebrafish embryos exposed to EOM, the expressions of which are significantly counteracted by treatment with the ROS scavenger NAC. It is noteworthy that NAC does not completely reverse PM2.5-induced DNA damage signal expression, indicating that oxidative stress may only contribute partially to the DNA damage observed in the hearts of zebrafish embryos exposed to PM2.5.
In conclusion, this method uses a sensitive technique for detecting PM2.5 induced DNA damage in the intact hearts of zebrafish embryos. In addition, the method can be applied to detect protein expression changes in the hearts of zebrafish embryos exposed to other environmental chemicals.
Divulgaciones
The authors have nothing to disclose.
Acknowledgements
This work was supported by the National Nature Sciences Foundation of China (Grant number: 81870239, 81741005, 81972999) and The Priority Academic Program Development of Jiangsu Higher Education Institutions.
Materials
| 8-OHdG Antibody | Santa Cruz Biotechnology, USA | sc-66036 | Primary antibody |
| Analytical balance | Sartorius,China | BSA124S | |
| BSA | Solarbio,Beijing,China | SW3015 | For blocking |
| DAPI | Abcam, USA | ab104139 | For nuclear counterstain. |
| DMSO | Solarbio,Beijing,China | D8371 | |
| Fluorescence microscope | Olympus, Japan | IX73 | For imaging fluorescence signals/ |
| Goat Anti-Rabbit IgG Cy3 | Carlsbad,USA | CW0159 | Secondary antibody |
| Goat Anti-Rabbit IgG FITC | Carlsbad,USA | RS0003 | Secondary antibody |
| N-Acetyl-L-cysteine(NAC) | Adamas-Beta, Shanghai, China | 616-91-1 | |
| Orbital shaker | QILINBEIER,China | TS-1 | |
| Paraformaldehyde | Sigma,China | P6148 | Make 4% paraformaldehyde for fixation. |
| Phosphate Buffered Saline | HyClone,USA | SH30256.01 | Prepare 0.1% Tween in PBS for washing. |
| PM2.5 sampler | TianHong,Wuhan, China | TH-150C | For 24-hr uninterrupted PM2.5 sampling. |
| Re-circulating aquaculture system | HaiSheng,Shanghai,China | The zebrafish was maintained in it. | |
| Soxhlet extractor | ZhengQiao,Shanghai, China | BSXT-02 | For organic components extraction. |
| Stereomicroscope | Nikon,Canada | SMZ645 | For heart dissection from zebrafish embryos. |
| Tricaine methanesulfonate (MS222) | Sigma,China | E10521 | To anesthetize zebrafish embryos |
| Tween 20 | Sigma,China | P1379 | |
| γH2AX Antibody | Abcam, USA | ab26350 | Primary antibody |
Referencias
- Zhang, B., et al. Maternal Exposure to Air Pollution and Risk of Congenital Heart Defects. European Journal of Pediatrics. 175, 1520 (2016).
- Huang, C. C., Chen, B. Y., Pan, S. C., Ho, Y. L., Guo, Y. L. Prenatal exposure to PM2.5 and Congenital Heart Diseases in Taiwan. The Science of the Total Environment. 655, 880-886 (2019).
- Mesquita, S. R., et al. Toxic assessment of urban atmospheric particle-bound PAHs: relevance of composition and particle size in Barcelona (Spain). Environmental Pollution. 184, 555-562 (2014).
- Zhang, H., et al. Crosstalk between AhR and wnt/beta-catenin signal pathways in the cardiac developmental toxicity of PM2.5 in zebrafish embryos. Toxicology. 355-356, 31-38 (2016).
- Duan, J., et al. Multi-organ toxicity induced by fine particulate matter PM2.5 in zebrafish (Danio rerio) model. Chemosphere. 180, 24-32 (2017).
- Lorda-Diez, C. I., et al. Cell senescence, apoptosis and DNA damage cooperate in the remodeling processes accounting for heart morphogenesis. Journal of Anatomy. 234, 815-829 (2019).
- Kouassi, K. S., et al. Oxidative damage induced in A549 cells by physically and chemically characterized air particulate matter (PM2.5) collected in Abidjan, Cote d’Ivoire. Journal of Applied Toxicology. 30, 310-320 (2010).
- Gualtieri, M., et al. Gene expression profiling of A549 cells exposed to Milan PM2.5. Toxicology Letters. 209, 136-145 (2012).
- Li, S. Y., Sigmon, V. K., Babcock, S. A., Ren, J. Advanced glycation endproduct induces ROS accumulation, apoptosis, MAP kinase activation and nuclear O-GlcNAcylation in human cardiac myocytes. Life Sciences. 80, 1051-1056 (2007).
- Yamashita, M. Apoptosis in zebrafish development. Comparative biochemistry and physiology. Part B, Biochemistry & Molecular Biology. 136, 731-742 (2003).
- Moazzen, H., et al. N-Acetylcysteine prevents congenital heart defects induced by pregestational diabetes. Cardiovascular Diabetology. 13, 46 (2014).
- Sun, S. Y. N-acetylcysteine, reactive oxygen species and beyond. Cancer Biology & Therapy. 9, 109-110 (2010).
- Tu, S., Chi, N. C. Zebrafish models in cardiac development and congenital heart birth defects. Differentiation. 84, 4-16 (2012).
- Asnani, A., Peterson, R. T. The zebrafish as a tool to identify novel therapies for human cardiovascular disease. Disease Models & Mechanisms. 7, 763-767 (2014).
- Li, M., et al. Toxic effects of polychlorinated biphenyls on cardiac development in zebrafish. Molecular Biology Reports. 41, 7973-7983 (2014).
- Massarsky, A., Prasad, G. L., Di Giulio, R. T. Total particulate matter from cigarette smoke disrupts vascular development in zebrafish brain (Danio rerio). Toxicology and Applied Pharmacology. 339, 85-96 (2018).
- Ren, F., et al. AHR-mediated ROS production contributes to the cardiac developmental toxicity of PM2.5 in zebrafish embryos. The Science of the Total Environment. 719, 135097 (2020).
- van Berlo, J. H., Molkentin, J. D. An emerging consensus on cardiac regeneration. Nature Medicine. 20, 1386-1393 (2014).
- Yue, C., et al. Protective effects of folic acid on PM2.5-induced cardiac developmental toxicity in zebrafish embryos by targeting AhR and Wnt/beta-catenin signal pathways. Environmental Toxicology. 32, 2316-2322 (2017).
- Zhao, X., Ren, X., Zhu, R., Luo, Z., Ren, B. Zinc oxide nanoparticles induce oxidative DNA damage and ROS-triggered mitochondria-mediated apoptosis in zebrafish embryos. Aquatic Toxicology. 180, 56-70 (2016).
- Zhao, X., Wang, S., Wu, Y., You, H., Lv, L. Acute ZnO nanoparticles exposure induces developmental toxicity, oxidative stress and DNA damage in embryo-larval zebrafish. Aquatic Toxicology. 136-137, 49-59 (2013).
- Zhu, L., et al. DNA damage and effects on glutathione-S-transferase activity induced by atrazine exposure in zebrafish (Danio rerio). Environmental Toxicology. 26, 480-488 (2011).

