Use of Trowell-Type Organ Culture to Study Regulation of Dental Stem Cells
Summary
The Trowell-type organ culture method has been used to unravel complex signaling networks that govern tooth development and, more recently, for studying regulation involved in stem cells of the continuously growing mouse incisor. Fluorescent-reporter animal models and live-imaging methods facilitate in-depth analyses of dental stem cells and their specific niche microenvironment.
Abstract
Organ development, function, and regeneration depend on stem cells, which reside within discrete anatomical spaces called stem cell niches. The continuously growing mouse incisor provides an excellent model to study tissue-specific stem cells. The epithelial tissue-specific stem cells of the incisor are located at the proximal end of the tooth in a niche called the cervical loop. They provide a continuous influx of cells to counterbalance the constant abrasion of the self-sharpening tip of the tooth. Presented here is a detailed protocol for the isolation and culture of the proximal end of the mouse incisor that houses stem cells and their niche. This is a modified Trowell-type organ culture protocol that enables in vitro culture of tissue pieces (explants), as well as the thick tissue slices at the liquid/air interface on a filter supported by a metal grid. The organ culture protocol described here enables tissue manipulations not feasible in vivo, and when combined with the use of a fluorescent reporter(s), it provides a platform for the identification and tracking of discrete cell populations in live tissues over time, including stem cells. Various regulatory molecules and pharmacological compounds can be tested in this system for their effect on stem cells and their niches. This ultimately provides a valuable tool to study stem cell regulation and maintenance.
Introduction
Mouse incisors grow continuously due to life-long preservation of the stem cells (SC) that support the unceasing production of tooth components. These include epithelial SCs, which generate enamel-producing ameloblasts, and mesenchymal stem cells (MSCs), which generate dentin-producing odontoblasts, among other cells1. The epithelial SCs in the continuously growing incisors were initially identified as label-retaining cells2,3 and have since been shown to express a number of well-known stemness genes, including Sox24. These cells share common features with epithelial SCs in other organs and reside within the SC niche called the cervical loop located on the labial side of the incisor. The niche is a dynamic entity composed of cells and extracellular matrix that control SC activity5. Lineage-tracing studies have demonstrated that Sox2+ epithelial SCs can regenerate the whole epithelial compartment of the tooth and that they are crucial for successional tooth formation6,7. MSCs with dentin reparative or regenerative potential are largely recruited from outside the organ through blood vessels and nerves8,9,10,11, therefore, providing a suitable model to study recruitment, migration, and housing of the MSC population.
To study SCs in vivo is not always feasible, since many of the genetic and/or pharmacological manipulations can affect organ homeostasis and/or have lethal consequences. Therefore, organ culture provides an excellent tool to study regulation of SCs and their niches in vitro. The organ culture system that utilizes a metal grid was initially developed by Trowell12 to study organ development and has been further modified by Saxen13 to study inductive signals in kidney development. Since then, this in vitro method of culturing the whole or part of the organ has been successfully applied in different fields. In the field of tooth development, this method has been widely used to study the epithelial-mesenchymal interactions that govern tooth development14 and successional tooth formation15. The work of the Thesleff laboratory has demonstrated the utility of this system for temporal analysis of tooth growth and morphogenesis, for analysis of the effect of various molecules and growth factors on tooth growth, and for time-lapse live imaging of tooth development16,17. More recently, this method has been utilized to study regulation of incisor SCs and their niche18,19, which is described in detail here.
Protocol
This protocol involves the use of animals and all the procedures were approved by Ethical Committees on the Use and Care of Animals and the Animal Facility at the University of Helsinki.
1. Preparation of the organ culture dish
- Perform all procedures in a laminar flow hood. Clean work surfaces with 70% ethanol and use autoclaved glass instruments and solutions. Sterilize scissors and other metal equipment in a glass-bead sterilizer.
- Prepare filters normally stored in 70% ethanol by washing them three times in 1x PBS to remove ethanol (Figure 1). Cut filters into rectangular pieces (3 x 3-5 x 5 mm).
NOTE: Washed filter pieces can be stored for several days in 1x PBS at 4 °C. - Prepare the culture medium (1:1 DMEM:F12 supplemented with 1% [v/v] 200 mM L-alanyl-L-glutamine dipeptide in 0.85% NaCl, 10% [v/v] FBS, 150 µg/mL ascorbic acid, and 0.2% [v/v] penicillin [10,000 I.U./mL] and streptomycin [10,000 µg/mL]). Store at 4 °C.
- Place the 30 mm metal grids (with 1-2 mm diameter holes that enable tissue imaging) in a 35 mm Petri dish. Add sufficient media to reach the grid surface without producing air bubbles. Pre-warm the prepared culture dish at 37 °C until the tissue is isolated and ready for culture (in a standard incubator with 5% CO2 and 90%-95% humidity).
2. Incisor dissection and isolation of the proximal end
- Sacrifice the animals following an approved animal care protocol.
- Decapitate the mouse and dissect the mandible. To do so, first remove the skin to expose the mandible and cut through the masseter muscles to separate it from the maxilla and the rest of the head.
- Once the mandible is isolated, remove the tongue and as much soft tissue as possible.
- Collect all the mandibles and keep them in a Petri dish containing PBS on ice, as this enhances the viability of the tissues.
- Transfer one mandible to a glass Petri dish and use disposable 20/26 G hypodermic needles to dissect the incisor under a stereomicroscope. Split the mandible at the midline, cutting through the symphysis. Clean the soft and muscle tissue away from the bone surface for better visualization.
NOTE: A glass Petri dish is essential, as this will not blunt the needles. - Mandibles obtained from animals younger than 10 days are softer and more fragile, therefore, use disposable 20/26 G hypodermic needles to open each half of the mandible longitudinally to expose the incisor tooth. For mice older than 10 days, use tweezers to grip the mandible and break the bone to expose the tooth.
- Gently detach the incisor from the surrounding bone and cut off the proximal end, which contains the cervical loop.
- Cut the proximal end and remove the mineralized enamel and dentin matrix.
- Keep the collected proximal ends in Dulbecco's PBS on ice until ready for culture.
3. Culture
- Carefully place a filter rectangle on the top of a grid in a pre-warmed culture dish.
- Use a stereomicroscope to properly orient the tissue pieces.
- Place in a standard incubator at 37 °C with 5% CO2 and 90%-95% humidity.
- Change the medium every other day and replace with fresh medium, carefully avoiding formation of air bubbles. Monitor tissue growth and photograph daily using a camera attached to the stereomicroscope.
4. Adding soluble factors to the culture
- Supplement the culture medium with soluble factors and molecules of interest to study their effect on regulation of SCs.
NOTE: The administration protocol for any molecule (growth factors, signaling molecules, blocking antibodies, pharmacological compounds such as inhibitors or activators, vectors, etc.) depends on its half-life and solubility. These parameters also determine the appropriate control to be used.
5. Molecular and histological analyses
- Remove the culture medium.
- Carefully add ice-cold methanol to the tissues to prevent detachment from the filters.
- Leave methanol for 5 min.
- Transfer filters carrying tissue explants to sampling tubes.
- Fix the explants in 4% paraformaldehyde in PBS for 10-24 h at 4 °C.
- Proceed with established protocols for histological processing (paraffin, frozen, etc.) or immunostainings.
6. Culture of tissue slices
NOTE: There are several variations to this protocol, which are all equally successful and are a matter of personal choice, depending on the speed and the skill of the user. These refer to the buffer used to collect and maintain tissue viability. For this purpose, Krebs buffer, PBS supplemented with 2% glucose and antibiotics, or PBS can be used. If Krebs buffer is used, it should be made 1 day in advance and kept at 4 °C.
- Before the experiment, prepare 4%-5% low-melting point agarose by dissolving 2-2.5 g of low-melting point agarose in 50 mL of boiling 2% glucose/PBS, after which the solution should be placed in a 45 °C water bath.
- Set up the vibratome, wash with 70% ethanol, and fill with ice-cold 2% glucose/PBS supplemented with antibiotics (100 U/mL penicillin, 100 mg/mL streptomycin).
- Dissect the proximal ends of the incisor and collect them in the ice-cold 2% glucose/PBS supplemented with antibiotics.
- Place one proximal end of the incisor in the mold containing 4%-5% low-melting point agarose. Under a stereomicroscope, orient the piece in the desired direction and leave on ice for the agarose to harden.
- Trim the hardened agarose block and place it in the vibratome. Cut thick slices (150-300 µm).
- Collect the slices in a Petri dish containing ice-cold 2% glucose/PBS supplemented with antibiotics.
- Use a spatula to transfer the thick slices on a filter rectangle placed on the pre-warmed grid prepared as in section 1.3.
- Incubate the thick slices in the incubator and proceed with imaging (Figure 2).
Representative Results
The epithelial SCs reside in a niche called the cervical loop, which is located at the proximal end of the incisor (Figure 3A). Cervical loops are morphologically distinct structures composed of inner and outer enamel epithelium that encase the stellate reticulum, a core of loosely arranged epithelial cells (Figure 3B,C). There are two cervical loops in each incisor (Figure 3A), but only the labial cervical loop contains SCs. The epithelial SCs are localized to the stellate reticulum and the adjacent enamel epithelium at the tip of the cervical loop20,21. They generate progeny characterized by Sfrp5 expression, which differentiate into highly proliferative transit-amplifying cells that produce various cells, including enamel-secreting ameloblasts2,7. Ameloblast differentiation from SCs can be recapitulated in the organ culture system described here2.
In recent years, reporter mice in which a fluorescent protein (such as GFP) is under the control of specific gene regulatory elements have become a widely used tool to identify and isolate cells from various tissues and to follow cell fate and lineage progression in vivo and in vitro22,23. The use of these animal models is beneficial for analyzing the effect of various regulatory molecules, since the intensity and the pattern of fluorescent reporter expression can be used as a readout of endogenous gene activity, or as a reporter of proliferation status (i.e., Fucci reporter mouse model).
The Sox2-GFP transgenic reporter mouse model24, in which the enhanced GFP (EGFP) expression is under the control of a 5.5 kb fragment of the upstream regulatory element of the Sox2 promoter, enables identification and visualization of Sox2-expressing incisor epithelial stem cells (Figure 4A-C). Generation of mice that carry several fluorescent reporters can be useful for identification of more than one cell population. For example, in cervical loops from Sox2-GFP;Fucci-mKO transgenic animals stem cells (GFP+, green, Figure 4D) and non-proliferative cells (Fucci-mKO+, red, Figure 4D) can be identified. In addition, Sox2-GFP expression can be used as a reporter when analyzing the effect of various molecules. Addition of the Wnt/β-catenin signaling activator BIO negatively affects Sox2-expressing stem cells and consequently GFP expression (Figure 4E).
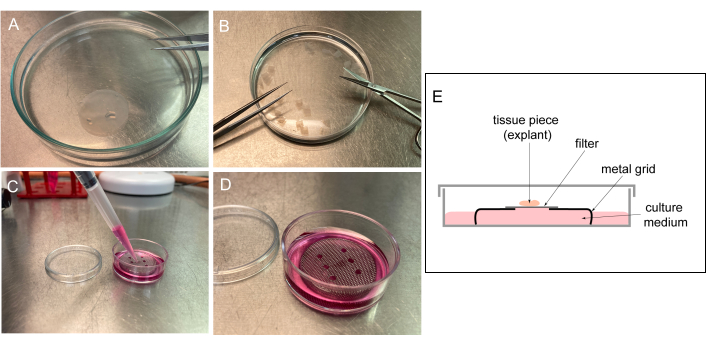
Figure 1: Preparation of the culture chamber. (A) Wash the filter in 1x PBS. (B) Cut the filter into rectangular pieces. (C) Prepare media in the hood and pipet it through the grid. Avoid air bubble formation. (D) Prewarm the pre-prepared culture dish at 37 °C prior to adding tissue explants on a filter placed on the grid. (E) Cartoon representation of the culture chamber. Please click here to view a larger version of this figure.
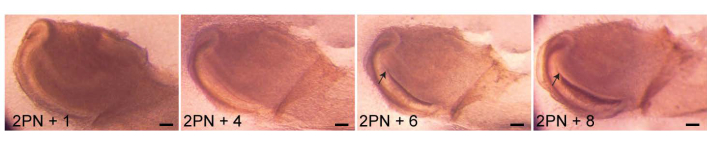
Figure 2: Culturing tissue slices. The proximal end of a 2-day-postnatal (2PN) mouse incisor was dissected and sectioned by vibratome. Slices (150 µm thick) were cultured and hard tissue formation (arrows) was observed after a 6-day culture. Scale 100 µm. Please click here to view a larger version of this figure.
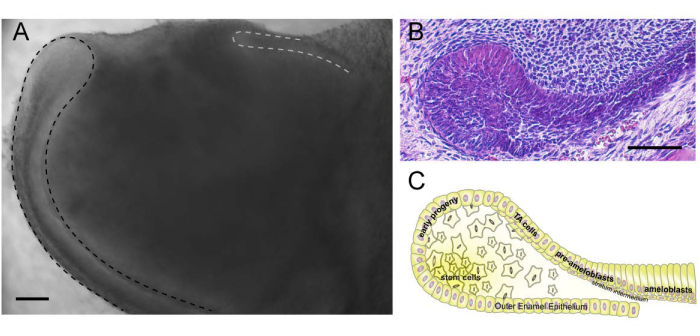
Figure 3: Cervical loop. (A) Proximal end of the incisor-containing labial (outlined by black dashed line) and lingual (outlined by grey dashed line) cervical loops. (B) Histological section of the labial cervical loop, which represents a niche for epithelial stem cells. (C) Cartoon representation of the labial cervical loop and its components. Please click here to view a larger version of this figure.
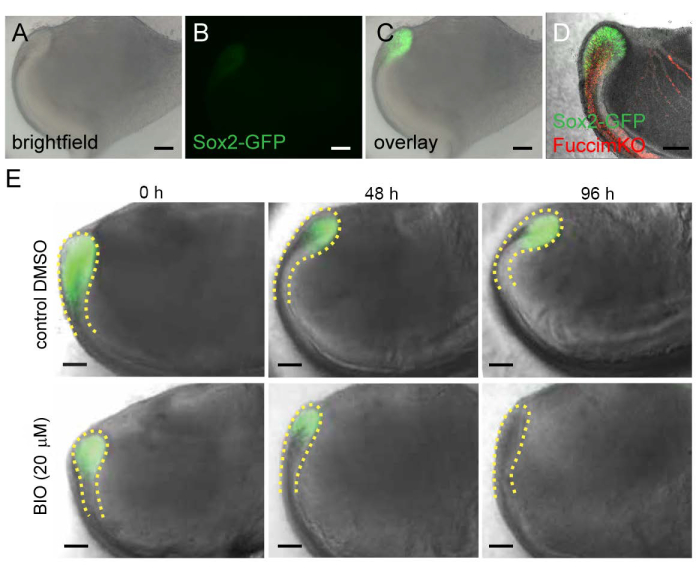
Figure 4: Use of fluorescent reporter mouse models in an organ culture system. (A) Brightfield, (B) fluorescent, and (C) overlay image of the proximal end of the incisor isolated from 2-day postnatal Sox2-GFP mice. (D) Sox2-GFP and Fucci-mKO expression in the proximal end of the incisor isolated from 2-day postnatal Sox2-GFP; Fucci-mKO mice. (E) Effect of BIO on the stem cells expressing Sox2-GFP and the stem cell niche (cervical loop, outlined by yellow dashed line). Scale 100 µm. Please click here to view a larger version of this figure.
Discussion
In vitro organ culture has been used extensively to study inductive potential and epithelial-mesenchymal interactions that govern organ growth and morphogenesis. The Thesleff laboratory has demonstrated how to adapt the Saxén modification of the Trowell-type organ culture and use it to study tooth development14. The reproducible conditions and advancements in fluorescent reporters have made this a useful method for monitoring tooth morphogenesis and the distinct cell populations within. This paper described how to apply this protocol to study epithelial stem cells and the microenvironment in which they reside.
A technique for isolating the proximal end of the incisor that contains the SC niche from postnatal pups and from older animals was described here. The success of this method depends on the isolation time (which directly impacts tissue viability) and on the ability to isolate an undamaged proximal end with an intact labial cervical loop. This is particularly challenging in older animals with mineralized bone. The protocol shows how to use controlled mechanical force to break the mineralized bone and isolate fully intact and viable soft tissue in a significantly shorter time when compared to other published protocols25. Once isolated, the tissue can be cultured in vitro, either as a whole or as a thick slice. The disadvantage of the thick tissue slicing method is that only a portion of the cervical loop is present in each slice; thus, obtaining a reproducibly identical portion of the cervical loop in different sections is not possible. Organ culture provides an easy and reproducible method to study the molecular and cellular mechanisms that regulate SCs and their niche over time. However, there are limitations to the total culture period, mainly due to the progressive loss of mesenchyme after the initial 3-4 days of culture.
The isolated tissue can also be further processed to obtain a single cell suspension enriched in incisor epithelial SCs26. Enzymatic separation of the cervical loop from the rest of the proximal end and the use of specific fluorescent reporters enables more detailed mRNA and protein analyses (such as qPCR, single-cell RNA sequencing) and a greater insight into specific cell populations within the niche.
In vitro culture provides a suitable platform for screening various genetic and pharmacological manipulations for their regulatory effect on tooth epithelial SCs and their niches. It should be noted that the success of the pharmacological treatments depends on the properties of the tested molecules, such as half-life, solubility, metabolic stability, dosage, and cell toxicity. In combination with a fluorescent reporter, the regulatory effect can be assigned to a specific cell population within a tissue, thus providing a powerful tool to uncover the molecular and cellular mechanisms that regulate tooth epithelial SCs and their niche.
With the use of specific fluorescent reporter mice, it is possible to identify and follow specific cell populations within live tissue over time. Recently, it has been demonstrated that in vitro organ culture can be used for live imaging of tooth development16. In addition, cell behavior can be imaged over a short period of time using in vitro culture of thick tissue slices7. As previously indicated, only a portion of the SC niche can be imaged and the imaging duration is brief. Ideally, the entire SC niche should be imaged. However, 4D time-lapse imaging of this structure is challenging, mainly due to the thickness of the niche and the limitations of currently available imaging methods. Nevertheless, continuous advances in imaging technology promise an exciting future in unravelling cellular behaviors that constitute the incisor SC niche.
Divulgaciones
The authors have nothing to disclose.
Acknowledgements
This study was supported by the Jane and Aatos Erkko Foundation.
Materials
| 1-mL plastic syringes | |||
| Disposable 20/26 gauge hypodermic needles | Terumo | ||
| DMEM | Gibco | 61965-026 | |
| Dulbecco's Phosphate-Buffered Saline | Gibco | 14287 | |
| Extra Fine Bonn Scissors | F.S.T. | 14084-08 | |
| F-12 | Gibco | 31765-027 | |
| FBS South American (CE) | LifeTechn. | 10270106 | divide in aliquotes, store at -20°C |
| Glass bead sterilizer, Steri 250 Seconds-Sterilizer | Simon Keller Ltd | 4AJ-6285884 | |
| GlutaMAX-1 (200 mM L-alanyl-L-glutamine dipeptide) | Gibco | 35050-038 | |
| Isopore Polycarb.Filters, 0,1 um 25-mm diameter | MerckMillipore | VCTP02500 | Store in 70% ethanol at room temperature. |
| L-Ascobic Acid | Sigma | A4544-25g | diluted 100 mg/ml in MilliQ, filter strerilized and divided in 20μl aliquotes, store at dark, -20°C |
| Low melting agarose | TopVision | R0801 | |
| Metal grids | Commercially available, or self-made from stainless-steel mesh (corrosion resistant, size of mesh 0.7 mm). Cut approximately 30 mm diameter disk and bend the edges to give 3 mm height. Use nails to make holes. | ||
| Micro forceps | Medicon | 07.60.03 | |
| Paraformaldehyde | Sigma-Aldrich | ||
| Penicillin-Streptomycin (10,000U/ml) sol. | Gibco | 15140-148 | |
| Petri dishes, Soda-Lime glass | DWK Life Sciences | 9170442 | |
| Petridish 35 mm, with vent | Duran | 237554008 | |
| Petridish 90 mm, no vent classic | Thermo Fisher | 101RT/C | |
| Small scissors |
Referencias
- Balic, A. Biology explaining tooth repair and regeneration: A mini-review. Gerontology. 64 (4), 382-388 (2018).
- Harada, H., et al. Localization of putative stem cells in dental epithelium and their association with Notch and FGF signaling. The Journal of Cell Biology. 147 (1), 105-120 (1999).
- Smith, C. E., Warshawsky, H. Cellular renewal in the enamel organ and the odontoblast layer of the rat incisor as followed by radioautography using 3H-thymidine. The Anatomical Record. 183 (4), 523-561 (1975).
- Balic, A., Thesleff, I. Tissue interactions regulating tooth development and renewal. Current Topics in Developmental Biology. 115, 157-186 (2015).
- Scadden, D. T. The stem-cell niche as an entity of action. Nature. 441 (7097), 1075-1079 (2006).
- Juuri, E., et al. Sox2 marks epithelial competence to generate teeth in mammals and reptiles. Development. 140 (7), 1424-1432 (2013).
- Juuri, E., et al. Sox2+ stem cells contribute to all epithelial lineages of the tooth via Sfrp5+ progenitors. Developmental Cell. 23 (2), 317-328 (2012).
- Feng, J., Mantesso, A., De Bari, C., Nishiyama, A., Sharpe, P. T. Dual origin of mesenchymal stem cells contributing to organ growth and repair. Proceedings of the National Academy of Sciences of the United States of America. 108 (16), 6503-6508 (2011).
- Kaukua, N., et al. Glial origin of mesenchymal stem cells in a tooth model system. Nature. 513 (7519), 551-554 (2014).
- Zhao, H., et al. Secretion of shh by a neurovascular bundle niche supports mesenchymal stem cell homeostasis in the adult mouse incisor. Cell Stem Cell. 14 (2), 160-173 (2014).
- Vidovic, I., et al. alphaSMA-expressing perivascular cells represent dental pulp progenitors in vivo. Journal of Dental Research. 96 (3), 323-330 (2017).
- Trowell, O. A. A modified technique for organ culture in vitro. Experimental Cell Research. 6 (1), 246-248 (1954).
- Saxen, L., Vainio, T., Toivonen, S. Effect of polyoma virus on mouse kidney rudiment in vitro. Journal of the National Cancer Institute. 29, 597-631 (1962).
- Thesleff, I., Sahlberg, C. Organ culture in the analysis of tissue interactions. Methods in Molecular Biology. 461, 23-30 (2008).
- Jarvinen, E., Shimomura-Kuroki, J., Balic, A., Jussila, M., Thesleff, I. Mesenchymal Wnt/beta-catenin signaling limits tooth number. Development. 145 (4), (2018).
- Ahtiainen, L., Uski, I., Thesleff, I., Mikkola, M. L. Early epithelial signaling center governs tooth budding morphogenesis. The Journal of Cell Biology. 214 (6), 753-767 (2016).
- Narhi, K., Thesleff, I. Explant culture of embryonic craniofacial tissues: analyzing effects of signaling molecules on gene expression. Methods in Molecular Biology. 666, 253-267 (2010).
- Yang, Z., Balic, A., Michon, F., Juuri, E., Thesleff, I. Mesenchymal Wnt/beta-Catenin signaling controls epithelial stem cell homeostasis in teeth by inhibiting the antiapoptotic effect of Fgf10. Stem Cells. 33 (5), 1670-1681 (2015).
- Binder, M., et al. Functionally distinctive Ptch receptors establish multimodal hedgehog signaling in the tooth epithelial stem cell niche. Stem Cells. 37 (9), 1238-1248 (2019).
- Harada, H., et al. Localization of putative stem cells in dental epithelium and their association with Notch and FGF signaling. The Journal of Cell Biology. 147 (1), 105-120 (1999).
- Seidel, K., et al. Hedgehog signaling regulates the generation of ameloblast progenitors in the continuously growing mouse incisor. Development. 137 (22), 3753-3761 (2010).
- Hadjantonakis, A. K., Nagy, A. FACS for the isolation of individual cells from transgenic mice harboring a fluorescent protein reporter. Genesis. 27 (3), 95-98 (2000).
- Yu, Y. A., Szalay, A. A., Wang, G., Oberg, K. Visualization of molecular and cellular events with green fluorescent proteins in developing embryos: a review. Luminescence. 18 (1), 1-18 (2003).
- D’Amour, K. A., Gage, F. H. Genetic and functional differences between multipotent neural and pluripotent embryonic stem cells. Proceedings of the National Academy of Sciences of the United States of America. 100, 11866-11872 (2003).
- Chavez, M. G., et al. Isolation and culture of dental epithelial stem cells from the adult mouse incisor. Journal of Visualized Experiments: JoVE. (87), (2014).
- Binder, M., et al. Novel strategies for expansion of tooth epithelial stem cells and ameloblast generation. Science Reports. 10 (1), 4963 (2020).

