Synthesis of Metal Nanoparticles Supported on Carbon Nanotube with Doped Co and N Atoms and its Catalytic Applications in Hydrogen Production
Summary
Here, we present a protocol to synthesize Co nanoparticles supported on carbon nanotubes with Co- and N- dopants for hydrogen productions.
Abstract
A method for facile synthesis of nanostructured catalysts supported on carbon nanotubes with atomically dispersed cobalt and nitrogen dopant is presented herein. The novel strategy is based on a facile one-pot pyrolysis treatment of cobalt (II) acetylacetonate and nitrogen-rich organic precursors under Ar atmosphere at 800 °C, resulting in the formation of Co- and N- co-doped carbon nanotube with earthworm-like morphology. The obtained catalyst was found to have a high density of defect sites, as confirmed by Raman spectroscopy. Here, cobalt (II) nanoparticles were stabilized on the atomically dispersed cobalt- and nitrogen-doped carbon nanotubes. The catalyst was confirmed to be effective in the catalytic hydrolysis of ammonia borane, in which the turnover frequency was 5.87 mol H2·molCo-1·min-1, and the specific hydrogen generation rate was determined to be 2447 mL H2·gCo-1·min-1. A synergistic function between the Co nanoparticle and the doped carbon nanotubes was proposed for the first time in the catalytic hydrolysis of ammonia borane reaction under a mild condition. The resulting hydrogen production with its high energy density and minimal refueling time could be suitable for future development as energy sources for mobile and stationary applications such as road trucks and forklifts in transport and logistics.
Introduction
Developing low-cost and highly efficient catalysts for renewable energy production remains one of the most critical and challenging problems to relieve the energy crisis. However, it is far from practical applications due to several concerns, such as large-scale production methods with reliable performance, high production cost, and long-standing stability to extend the service life of catalysts. Industry sectors, like transport and logistics, require energy production for vehicles and equipment with long operation hours, high powered energy supply, and minimal refueling time in achieving efficient operations1,2,3. Therefore, effective strategies have been extensively exploited to address the above technical challenges. For example, by regulating the electronic structure of the metal active sites and catalyst supports, designing the specific architecture of the metal nano-catalysts, fine-tuning metal compositions, functional group modification of anchored support, and varying the morphology to increase the number of intrinsic active sites. In the past few decades, nanoparticles (NPs) have dominated the fields of various heterogeneous catalysis, and the catalytic activities can be effectively tuned by varying the size of the NPs. Only until in recent years, highly dispersed single-atom catalysts (SACs) emerged to have excellent properties towards many catalytic reactions due to their unique electronic structure and coordination environment. Particularly, SACs have already demonstrated superior performances in energy conversion such as electrochemical reactions (HER, ORR, OER) and electrochemical energy systems (e.g., supercapacitors, rechargeable batteries)4,5,6. While both NPs and SACs have their respective advantages and limitations in catalytic applications, there do exist reactions that require both NPs and SACs in order to boost catalytic reactivity. For example, Ru NPs supported on Ni- and N-co-doped carbon nanotube superstructure could facilitate the high catalytic wet air oxidation of acetic acid7. This synergistic effect was also demonstrated by Pd1+NPs/TiO2 catalysts for highly selective ketone and aldehydes hydrogenation at room temperatures8. In order to accelerate the field of synergistic NPs and SACs catalysis and explore more on their catalytic applications, a facile way of catalyst synthesis is highly desirable, and the introduction of high loadings of the atomically dispersed active site remains a challenge due to the high tendency of the aggregation of SACs9.
Several methods have been utilized to synthesize SACs for applications in the hydrogenation of nitroarenes10, oxygen reduction reaction and hydrogen evolution reaction11,12, lithium-oxygen batteries13.The most common strategy is the bottom-up approach, in which the metal precursors were absorbed, reduced, and immobilized on the defects of the corresponding support. Mononuclear metal complexes could also be first attached to the functional group of supports, followed by subsequent removal of the organic ligands, thus creating active metal sites for the catalytic process. Atomic layer deposition (ALD) is probably the most frequently used procedure for bottom-up fabrication by depositing a thin layer of film on the substrate with repeated exposure of reactants. Although the catalyst size could be precisely controlled and the reactivity could be greatly improved14, the purity of the substrate was rather demanding, and the metal loading was relatively low, thus resulting in high production costs for practical applications. Various methods such as direct impregnation, co-precipitation, and deposition-precipitation, have been employed to immobilize metal nanoparticles onto the support surfaces, such as metal oxide and nitride, through surface charging effects. However, increasing metal loading usually leads to significant agglomeration and cluster formation of the metal atoms or nanoparticles. Therefore, usually, a very diluted metal solution is required, thus leading to low SACs loadings of the catalysts15. Amine ligands such as phenanthroline have been employed to undergo pyrolysis with metal precursors to prepare atomically dispersed metal catalysts with highly active Co-Nx active sites for the selective dehydrogenation of formic acid. However, the metal loading was relatively low (2-3 wt%) due to the limited number of available N atoms in the amine precursors16.
In the past few decades, hydrogen has been regarded as a potential alternative to replace fossil fuels or hydrocarbons, such as coal, natural gas, and gasoline, due to the advantage of zero-emission of the former. Until now, about 94% of commercial hydrogen is still produced from the reforming process of fossil fuels, in which the process releases a great deal of greenhouse gas17. Therefore, hydrogen production from renewable resources such as water electrolysis is a way to solve the problem of depleted fossil resources and severe carbon emissions. However, the low hydrogen production efficiency has hindered their wider applications. Thus, to overcome this kinetic energy barrier for water-splitting, numerous efficient electrocatalysts have been discovered in the past decade18. Another issue is the storage problem due to the gaseous and explosive nature of hydrogen gas at ambient conditions. Physical storage methods such as compression will require the hydrogen to be compressed up to 700-800 bar, and cryogenic storage by liquefaction will require low temperature at -253 °C19. Although commercialized hydrogen fuel cell-powered vehicles have been successfully demonstrated, the storage problem is yet to be solved if the technology is to be used in wider applications, such as miniature devices and mini-fuel cells. Thus, storage methods of using chemical H materials have been one of the hot focuses in hydrogen energy research. Some examples of chemical H storage materials are ammonia borane (AB)20, formic acid (FA)21, ammonia gas22, sodium alanate23, and magnesium hydride24. Among these, AB has a low molecular weight (30.7 g·mol-1), high gravimetric and volumetric densities (196 gH2·kg-1 and 146 gH2·L-1, respectively). Besides, it is an air and moisture stable compound, non-toxic, and highly soluble in water. Metal nanoparticles on various supported materials have been widely used to release the three equivalents of hydrogen from AB, such as platinum- (Pt-), palladium- (Pd-), ruthenium- (Ru-), cobalt- (Co-), and nickel- (Ni-) based catalysts. Co-based heterogeneous catalysts supported on carbon materials are especially attracting much attention due to their low cost, high abundance, and ease of recovery. Several synthetic strategies have been reported, such as the Co NPs supported on branched polyethylenimine-decorated graphene oxide25. The 3D structure with a large surface area ensures the stabilization of Co NPs maintaining at the 2-3 nm size range and prevented the aggregation of NPs. Another strategy is to use N-doped carbon materials to support Co NPs with small sizes. Using Co(salen)26 and Co-MOF27 (metal organic framework) as the precursors, Co NPs of 9.0 nm and 3.5 nm supported on N-doped porous carbon materials have been prepared respectively. The stability towards AB hydrolysis are high and the reactivity can maintain over 95% of the initial activity after 10 reaction runs. Recently, catalysts with hollow micro/nanostructures have been exploited for AB hydrolysis. These materials are conventionally prepared by hydrothermal methods and have been widely used for lithium-ion batteries, supercapacitors, chemical sensors, and heterogeneous catalysis research. Thus, the copper-cobalt synergy towards AB hydrolysis has been demonstrated by the hollow CuMoO4-CoMoO428, which gives a high TOF of 104.7 min-1. Other highly structural-dependent examples include the core-shell CuO-NiO/Co3O429, the CoxCu1−xCo2O4@CoyCu1−yCo2O4 yolk-shell type30, and the Ni0.4Cu0.6Co2O4 nanoarrays31 were also found to be active towards AB hydrolysis. Another type of emerging materials known as heterostructured catalysts, such as MXenes and layered double hydroxides (LDHs), are increasingly being exploited for electrocatalytic and photocatalytic reaction32,33,34,35. These materials such as the NiFe-layered double hydroxide36,37 and the CoB-N materials having N-doped carbon-cobalt boride heterointerfaces38 are especially active for oxygen evolution and reduction reaction. In principle, they could be further exploited for hydrogen evolution reactions from hydrogen storage materials such as ammonia borane39. Maximizing the interaction between the catalysts and substrates is also another strategy for AB hydrolysis. Chiang et al. have utilized the surface oxide group of graphene oxide to form an initiated complex species with AB40, thus Ni0.8Pt0.2/GO and rGO demonstrated excellent reactivity towards AB hydrolysis. The use of α-MoC as support for Co and Ni bimetallic catalysts assisted the activation of water molecules and achieved high TOF towards AB hydrolysis, which is four times higher than the commercial Pt/C catalyst41.
Taking advantage of high N contents of the dicyandiamide and related C3N4 materials, a protocol for achieving a facile synthesis of cobalt NPs supported on highly dispersed Co- and N-doped carbon nanotubes is presented herein. The gradual in-situ formation of Co NPs from the formed atomically dispersed Co during the pyrolysis of C3N4 materials ensure that 1) Co NPs and Co dopants are highly dispersed; 2) Co NPs can be strongly anchored on the doped carbon supports and 3) Co NPs size can be carefully controlled by the temperature and time of the pyrolysis. The as-prepared Co/Co-N-CNT, as a result of the strongly anchored Co NPs and the ability of the Co dopants to lower the adsorption energy of water molecules, was found to have superior stability towards the hydrolysis of AB for hydrogen production. The details of the synthetic protocol of the catalysts and the measurement of the hydrogen production will be the focal point of this report.
Protocol
CAUTION: Readers are advised to carefully check the properties and toxicities of the chemicals described in this paper for the proper chemical handling from the relevant material safety data sheets (MSDS). Some of the chemicals used are detrimental to health, and special cares are to be taken. The impact of nanomaterials on human health is unknown and could pose safety and health risks. Inhalation and contact through the skin with these materials should be avoided. Safety precautions shall also be exercised, such as releasing the waste gas during the catalyst synthesis to the fume hood and catalyst performance evaluation with proper venting of the hydrogen gas. Personal protective equipment is advised to be worn at all times. Hydrogen is a potentially explosive gas with a very broad flammability range from 4%-74% in air. Care shall be taken to allow the hydrogen gas to vent properly to the atmosphere.
1. Synthesis of melem-C 3N4 materials
- Weigh out 280 g of dicyandiamide (density = 1.4 g·cm-3) into an 800 mL beaker.
- Place the beaker with the above solid into a muffle furnace and slowly raise the temperature from room temperature to 350 °C with a ramp of 5 °C·min-1.
- Keep the temperature at 350 °C for 2 h, cool down the furnace by natural cooling.
- Grind the obtained white solids into fine powder as the C3N4 materials in the melem form (DCD-350).
NOTE: The yield is 175 g.
2. Annealing the melem-C 3N4 and Co(acac)2 mixtures at different temperature
- Mix 10.0 g of melem-C3N4 with 0.218 g of Co(acac)2 [Co : melem-C3N4 = 1:200 (weight ratio)]. Grind and mix the two solids until the homogeneous color is observed.
- Add 6 mL of citric acid solution (water: ethanol = 1:1, citric acid = 10 g·L-1) to the homogeneous mixture and further grind the materials.
- Dry the materials in an oven at 60 °C for 6 h.
- Place the materials into a square-shaped crucible and then put it into a tubular furnace.
- Heat the materials at a heating rate of 2.6 °C·min-1 from room temperature to 800 °C and keep for 2 h under an Ar flow of 100 mL·min-1.
- Slowly cool down the furnace by natural cooling.
- Weight out the catalyst samples. Here, the yield was 0.65 g.
3. Measuring hydrogen release from ammonia borane hydrolysis
- Set up the water-filled inverted cylinder system (Supplementary Figure 1).
- Set up the 0.1 M H2SO4 washing solution.
- Connect the Schlenk flask with the washing solution and the water-filled inverted cylinder.
- Set the water bath temperature to 40 °C.
- Place 0.04 g of the catalyst into the Schlenk flask.
- Prepare a solution of ammonia borane in water, with 0.04 g of ammonia borane in 0.948 mL of water (concentration = 0.04 g·mL-1).
- Inject 1 mL of the NH3BH3 solution (40 mg·mL-1) to the reactor to initiate the hydrolysis reaction.
- Monitor the drop in the water level as the reaction proceeds. Carefully record the production volume at designated times, e.g., each 5 s intervals.
- Plot the graph of volume of H2 production vs. time in minutes.
4. Kinetic studies
- Determination of the activation energy
- Set the water bath temperature at 40 °C.
- Place 0.04 g of the catalyst and 10 mL of water into the Schlenk flask and immerse into the water bath. Sonicate the solution at 40 kHz in an ultrasonic bath for 6 min.
- Inject 1 mL of the NH3BH3 solution (40 mg·mL-1) to the reactor to initiate the hydrolysis reaction.
- Record the time for completion of the hydrogen release.
- Repeat steps 4.1.1-4.1.4 setting the water bath temperature at 35 °C.
- Repeat the above experiment at 30 °C and 25 °C, respectively.
- Plot the specific rate constant versus time on a graph using the following equation. A plot of ln k and 1/T should yield a straight line.
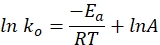
where ko denotes the specific rate constant (mol H2·gCo-1·min-1), R is the ideal gas constant (8.314 kJ·mol-1), T represents the reaction temperature (K), and A is the pre-exponential factor (mol H2 gCo-1·min-1).
- Determination of the turnover frequency and specific hydrogen generation rate
- Calculate the turnover frequency according to the following equation:
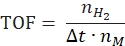
where nH2 is the moles of hydrogen produced, Δt is the time required for complete hydrogen release and nM is the molar amount of metal in the catalyst. - Calculate the specific hydrogen generation rate according to the following equation42,43:
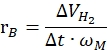
Where ΔVH2 is the volume of hydrogen produced, t is the time required for the initiating and stabilizing stages (e.g., the time where 70 mL of hydrogen generated for 40 mg ammonia borane, the time where 140 mL of hydrogen generated for 80 mg ammonia borane) and ωM is the mass of metal in the catalyst.
- Calculate the turnover frequency according to the following equation:
- Determination of the relationship between [ammonia borane] and reaction rate
- Set the water bath temperature at 40 °C.
- Place 40 mg of the catalyst and 10 mL of water into the Schlenk flask and immerse into the water bath. Sonicate the solution at 40 kHz in an ultrasonic bath for 6 min.
- Inject 1 mL of the NH3BH3 solution (40 mg·mL-1) to the reactor to initiate the hydrolysis reaction.
- Record the time for completion of the hydrogen release.
- Repeat step 4.3.3 injecting 2 mL of the NH3BH3 solution (i.e., 80 mg per 2 mL) to the reactor to initiate the hydrolysis reaction.
- Repeat steps 4.3.1-4.3.4 with 0.5 mL and 0.25 mL of the NH3BH3 solution (40 mg/mL) respectively to record the time for completion of the hydrogen release.
- Calculate the reaction rate according to the following equation44:
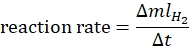
where ΔmlH2 is the volume of hydrogen produced, Δt is the time required for 70 mL of hydrogen release. - Plot the ln rate vs. ln[ammonia borane] and determine the slope of the graph.
- Determination of the relationship between [catalysts] and production rate
- Set the water bath temperature at 40 °C.
- Place 40 mg of the catalyst and 10 mL of water into the Schlenk flask and immerse into the water bath. Sonicate the solution at 40 kHz in an ultrasonic bath for 6 min.
- Inject 1 mL of the NH3BH3 solution (40 mg·mL-1) to the reactor to initiate the hydrolysis reaction.
- Record the time for completion of the hydrogen release.
- Repeat steps 4.4.1-4.4.4 varying the amount of catalyst (20 mg, 40 mg, 60 mg, 80 mg) and inject 1 mL of the NH3BH3 solution (i.e., 40 mg·mL-1) into the reactor to initiate the hydrolysis reaction.
- Record the time for completion of the hydrogen release for using the above various catalyst amounts.
- Calculate the reaction rate according to the following equation44:
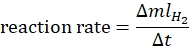
where ΔmlH2 is the volume of hydrogen produced, Δt is the time required for 70 mL of hydrogen release. - Plot the ln rate vs. ln[catalyst] and determine the slope of the graph.
5. Cycling performance test
- Set the water bath temperature at 40 °C.
- Place 0.04 g of the catalyst and 10 mL of water into the Schlenk flask and immerse into the water bath. Sonicate the solution at 40 kHz in an ultrasonic bath for 6 min.
- Inject 1 mL of the NH3BH3 solution (40 mg·mL-1) to the reactor to initiate the hydrolysis reaction.
- Record the time for completion of the hydrogen release.
- Filter off the catalyst, washed with water (5 mL) three times, and then dry the catalyst in the oven (60 °C) for 3 h.
- Place the catalyst in 10 mL of water and sonicate the solution at 40 kHz in an ultrasonic bath.
- Repeat steps 5.1.3-5.1.5 for ten times.
- Plot the hydrogen production volume, TOF and specific generation rate vs. cycles, respectively.
6. Leaching experiment for metal NPs to obtain pure metal SAs CNT
- Set the oil bath temperature at 80 °C.
- Place 0.15 g of the catalyst and 50 mL of 0.5 M H2SO4 into the Schlenk flask and immerse into the oil bath.
- Stir the reaction for 2 h.
- Filter off the solid using a Buchner funnel and wash the solid with deionized water (3x in 10 mL each). Dilute the leachate further to 250 mL in a 250 mL volumetric flask.
- Collect the metal nanoparticles-leached solids (which contain only Co-doped CNT) and dry at 60 °C in an oven.
7. Metal content determination using Inductively Coupled Plasma Optical Emission Spectroscopy (ICP-OES)
- Determination of total cobalt metal content
- Place approximately 0.02 g of as-prepared catalyst from section 2 into 50 mL of 2 M acid solution (HCl: HNO3 = 3:1 mole ratio)45,46 in a polytetrafluoroethylene-lined stainless-steel autoclave.
- Place the polytetrafluoroethylene-lined container into the stainless steel bomb and secure the cap.
- Place the bomb into an oven, set the temperature to 180 °C, and heat the bomb for 12 h.
- Remove the bomb and empty the contents. Filter the solid and dilute the solute in a 250 mL volumetric flask with 200 mL of deionized water.
NOTE: The purpose of the dilution is to adjust the concentration of the ICP samples, which will fit into the metal standard concentration range, i.e., 0-40 ppm. - Run the ICP-OES test of the solution and calculate the total amount of Co in wt%.
- Determination of the cobalt atoms content on the CNT
- Place approximately 0.02 g of as-prepared catalyst from step 6.5 into 50 mL of 2 M acid solution (HCl: HNO3 = 3:1 mole ratio)45,46 in a polytetrafluoroethylene-lined stainless-steel autoclave.
- Place the polytetrafluoroethylene-lined container into the stainless-steel bomb and secure the cap.
- Place the bomb into an oven, set the temperature to 180 °C, and heat the bomb for 12 h.
- Remove the bomb and empty the contents. Filter the solid and dilute the solute in a 250 mL volumetric flask with 200 mL of deionized water.
NOTE: The purpose of the dilution is to adjust the concentration of the ICP samples, which will fit into the metal standard concentration range, i.e., 0-40 ppm. - Run the ICP-OES test of the solution and calculate the amount of Co atom contents in wt%.
- Determination of the cobalt nanoparticles (NPs) content
- The difference between the metal content of 7.1.5 and 7.2.5 is the wt% of Co NPs.
Representative Results
X-ray diffraction patterns (XRD) have been obtained to determine the crystallinity and size of the cobalt NPs. As shown in Figure 1, diffraction peaks corresponding to the (111), (200) and (220) planes (at 2θ of 44.2°, 51.5°, and 75.8° respectively) of the cubic phase of metallic cobalt were present in agreement with the JCPDS (Joint Committee for Powder Diffraction Standards) power diffraction file (card # 15-0806)47. The broad peak at 2θ of around 26° corresponding to the graphitic carbon (N-CNTs) can be indexed to JCPDS card # 75-1621. The strong and sharp diffraction peaks indicate a well-defined crystalline structure.
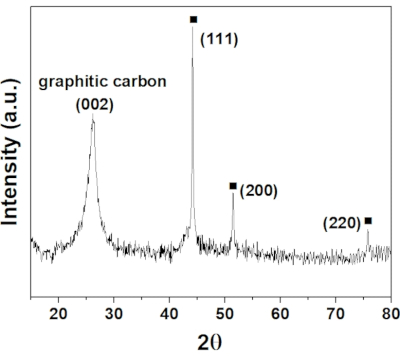
Figure 1: XRD pattern of 0.5 wt% Co/CoNx-CNT support. The black square symbol represents the crystal plane of Co. Please click here to view a larger version of this figure.
The structural defects and transformation can be illustrated in the Raman spectrum, as shown in Figure 2. The D band, which was attributed to the structural deformation of the sp3-hybridized carbon vibrations, can be assigned to the peak at 1338 cm-1. The G band, which was due to the E2g scattering vibrational mode of first-ordered scattering in a hexagonal lattice by the sp2-hybridized carbon domain, can be assigned to the peak at 1585 cm-1. The ratio of ID/IG was determined to be 1.13, indicating that there was a high degree of defect density in the sample. The defects could be caused by the anchored Co and N dopants in the carbon structure of the as-prepared catalyst48,49. In addition, there were three peaks detected at 475.4 cm-1, 519.3 cm-1, and 674.0 cm-1 which can be assigned to the cobalt nitride phase50. This indicated that partial nitridation of the Co NPs occurred during the NH3(g) formation from g-C3N4 decomposition at around 700 °C49. There was no observable change in the appearance of the spectrum after the AB hydrolysis reaction, suggesting the high stability of the as-prepared catalyst.
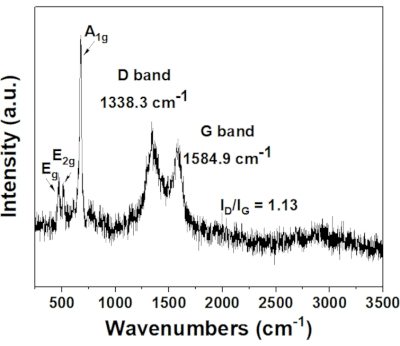
Figure 2: Raman spectrum of 0.5 wt% Co/CoNx-CNT support. ID/IG represents the ratio of the height of the peaks of D band and G band, respectively. Please click here to view a larger version of this figure.
As shown in Figure 3A, the orbital peaks of the XPS survey spectrum signified the presence of N, C, and Co elements. The high-resolution electron spectroscopy for chemical analysis (ESCA) of each element further indicates the chemical states of the elements. Three characteristics peaks, namely, the metallic Co, Co-N, and Co2+, are shown by the spatial resolution of the Co 2p3/2 XPS profile as in Figure 3B. The peaks corresponding to metallic Co, Co2+ (probably due to partial surface oxidation of the Co nanoparticles) and Co-NX were located at 778.2 eV, 779.8 eV, and 781.1 eV, respectively, while the Co 2p1/2 XPS indicated the presence of Co2+ located at 795.8 eV. The deconvolution of the N1s high-resolution profile in Figure 3C showed that four peaks centered at 397.8 eV, 398.9 eV, 400.6 eV, and 402.9 eV were corresponding to C-N-C, Co-N, C-N-H, and graphitic N, respectively. The relatively stronger peak at 397.8 eV could be attributed to the presence of the strong interaction of metallic cobalt with nitrogen atoms49,51, which could be either cobalt nanoparticles or/and cobalt dopants. As shown in Figure 3D, the ESCA spectrum of C1s can be resolved into three main peaks, indicating the different hybridization of carbon atoms during the formation of the carbon nanotube structures. The peaks centered at around 285 eV, 286 eV, and 290 eV could be attributed to C-C sp3, C-C sp2, and C=N, respectively.
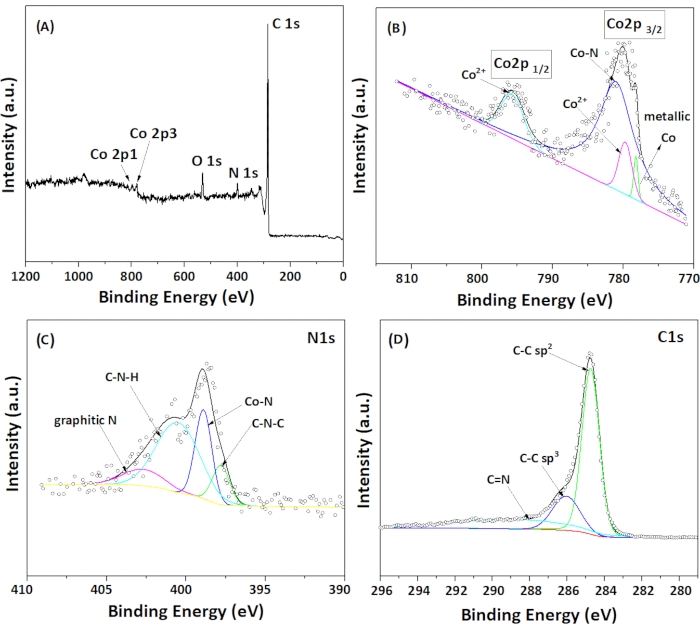
Figure 3: Typical XPS spectra of 0.5 wt% Co/CoNx-CNT support. (A) XPS survey, (B) Co2p, (C) N1s and (D) C1s. Please click here to view a larger version of this figure.
The specific surface area and pore size distribution of the Co/Co-N-CNT support were measured from a nitrogen adsorption-desorption isotherm at liquid nitrogen temperature (77 K). As shown in Figure 4A, the adsorption-desorption isotherm demonstrated a clear hysteresis look of type IV according to IUPAC nomenclature, with a specific surface area SBET of 42.02 m2·g-1. The total pore volume of the pores less than 391.6 nm diameter at P/Po is 0.25 cm3·g-1. The average pore size distribution was determined to be 3.6 nm on the basis of the Barrett-Joyner-Halenda (BJH) methods, as shown in Figure 4B.
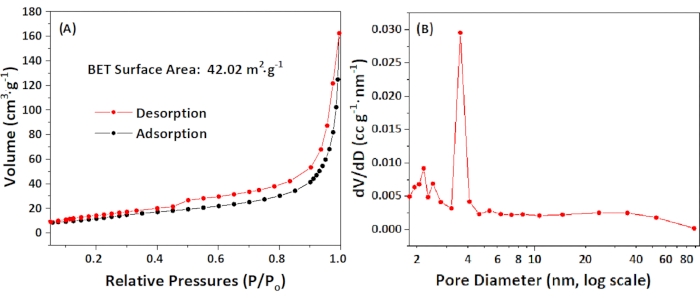
Figure 4: Adsorption-desorption isotherm and size distribution. (A) Adsorption-desorption isotherm of 0.5 wt% Co/CoNx-CNT materials. (B) Size distribution of 0.5 wt% Co/Co SACs-N-CNT determined from Barrett-Joyner-Halenda (BJH) method. Please click here to view a larger version of this figure.
The structural and compositional characterization results of the Co/Co-N-CNT sample annealed at 800 °C were given in Figure 5. Here, the length of the convoluted carbon nanofiber was up to 5 μm and the tubular nanostructures could be clearly observed in Figures 5A–B and Figure 6. The Co nanoparticle, resulting from the catalyzing growth of the nanofiber52,53, was located at the tip of the N-doped nanofiber. This could be clearly seen from TEM (JEM-2100Plus, JEOL) images in Figures 5A–B and the EDS mapping was also shown in Figure 5C. Such Co nanoparticle was wrapped by a few layers of graphitic carbon, as shown in Figure 5D and Figure 5F. The d-spacing of the graphitic carbon interlayer was around 3.46 Å, which was assigned to the (002) lattice plane of graphitic carbon. The crystalline structure of the Co nanoparticle was characterized by selected area electron diffraction (SAED) pattern from the 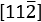 zone axis, as revealed in Figure 5E. The lattice planes of (111),
zone axis, as revealed in Figure 5E. The lattice planes of (111), 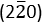 and
and 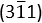 of the crystalline Co were indexed accordingly. The main body of the carbon nanofiber is endowed with graphitic carbon layers of different orientations, as shown in Figures 5G and Figure 5I. The diffraction rings in Figure 5H confirmed the typical planes {002}, {101} and {110} of the graphitic carbon. Image obtained with scanning electron microscopy is shown in Figure 6.
of the crystalline Co were indexed accordingly. The main body of the carbon nanofiber is endowed with graphitic carbon layers of different orientations, as shown in Figures 5G and Figure 5I. The diffraction rings in Figure 5H confirmed the typical planes {002}, {101} and {110} of the graphitic carbon. Image obtained with scanning electron microscopy is shown in Figure 6.
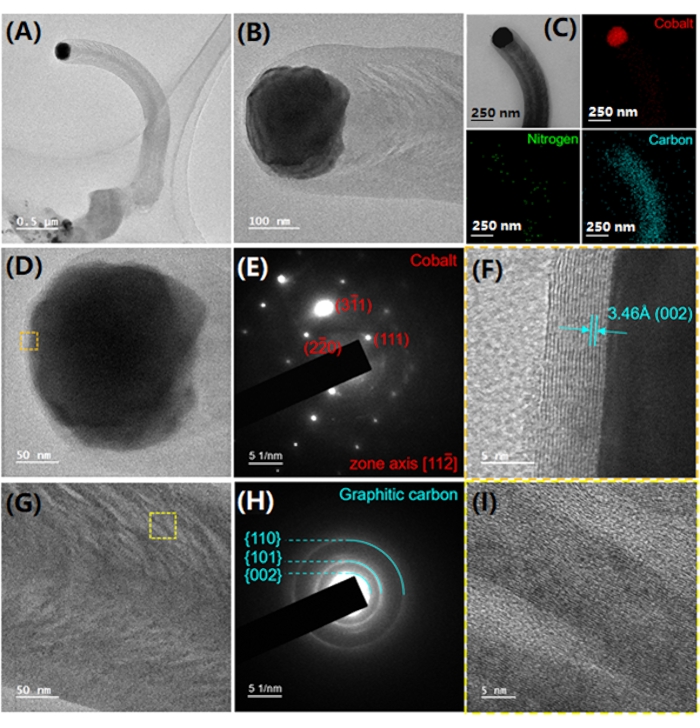
Figure 5: Transmission Emission Microscopy. (A, B) TEM images and (C) EDS mapping of the Co/Co-N-CNT. (D) TEM image and (E) SAED pattern of the Co nanoparticle at the tip of the CNT. (F) HRTEM image of the graphitized carbon layers wrapping the Co nanoparticle. (G) TEM image, (H) SAED pattern, and (I) HRTEM image of the CNT. The dashed orange and yellow squares indicate the location of (F) in (D) and (I) in (G), respectively. Please click here to view a larger version of this figure.

Figure 6: Scanning Electron Microscopy. FE-SEM images of 0.5 wt% Co/CoNx-CNT support. Please click here to view a larger version of this figure.
The total metal content determined by ICP-OES was found to be 25.1 wt%, with 9.7 wt% of Co NPs and 15.4 wt% of Co dopants on the CNT, as shown in Table 1. The presence of Co NPs was probably due to the high loading of metal precursor Co(acac)2 used in the synthesis.
| Actual ICP-OES results | Total Co (wt%) | Co Nanoparticles (wt%) | Co Single Atoms (wt%) |
| 0.5 wt% (nominal) Co/Co-N-CNT | 25.1 | 9.7 | 15.4 |
Table 1: The total metal content determined by ICP-OES.
The catalytic performance of the catalyst towards the hydrolysis of AB was given in Figure 7. The catalyst first underwent an activation process, probably due to the gradual formation of the HO-Co-N2 active phase from Co SAs54. Finally, when the activation process was stabilized, the reaction finished in 3.8 min to release 2.94 equivalents of hydrogen, close to the theoretical value (3.0 equivalents of hydrogen). The turnover frequency (TOF) was determined to be 5.87 mol H2·molCo-1·min-1, and the specific hydrogen generation rate was determined to be 2447 mL H2·gCo-1·min-1.
To understand the rate law of the reaction, the correlation of the rate and the amount of catalyst and ammonia borane was estimated, respectively. As shown in Figure 8, the ln rate Vs. ln [AB] and ln [catalyst] were plotted, respectively. The rate showed a slight dependence on ammonia borane, and the reaction order of the catalyst was about 0.4, which was quite different from most other reported rate law in the hydrolysis of ammonia borane but is similar to the one reported on the AB hydrolysis by CoP nanoparticles, reported as 0.6 due to the longer induction time of AB on the catalyst55. The rate law is thus determined to be rate = k[catalyst]1.3[AB]0.4. The activation energy (Ea) was determined to be 42.8 kJ·mol-1, as shown in Figure 9.
To verify the high stability of the as-prepared catalyst, cycling performance was also tested for 15 cycles. Ammonia borane was continuously allowed to react with the catalyst, and it was found that until the 10th time of AB addition, there was no obvious decline in the catalytic performance, as shown in Figure 10A–C, confirming that the synthesic strategy used in this study could achieve strong anchorage of nanoparticles on the CNT support. The NPs enclosed by the graphitic carbon of CNT could effectively prevent metal NPs from agglomeration. Finally, the formation of the HO-Co-N2 complex species54 on the CNT at optimal pH effectively lowered the adsorption energy of water molecules onto the CNT for a hydrolysis reaction. During the activation process, as more ammonia borane was added, the reaction media reached an optimal pH in which all of the Co dopants were activated. This was demonstrated by the gradual increase in the specific hydrogen generation rate from 2447 ml H2·g Co-1 min-1 to over 3500 ml H2·gCo-1·min-1 at the 7th catalytic reaction. The XRD results showed that (Supplementary Figure 2) the state of Co nanoparticles remains unchanged after the recycles. The detailed mechanism will be discussed in the following section.
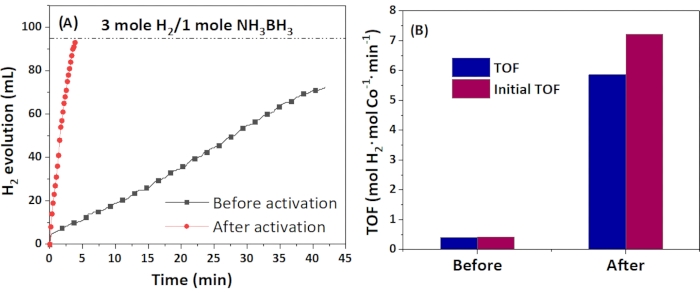
Figure 7: The catalytic performance of 0.5 wt% Co/Co SACs during AB hydrolysis. (A) Before and after activation, (B) TOF ratio at these two conditions. Please click here to view a larger version of this figure.
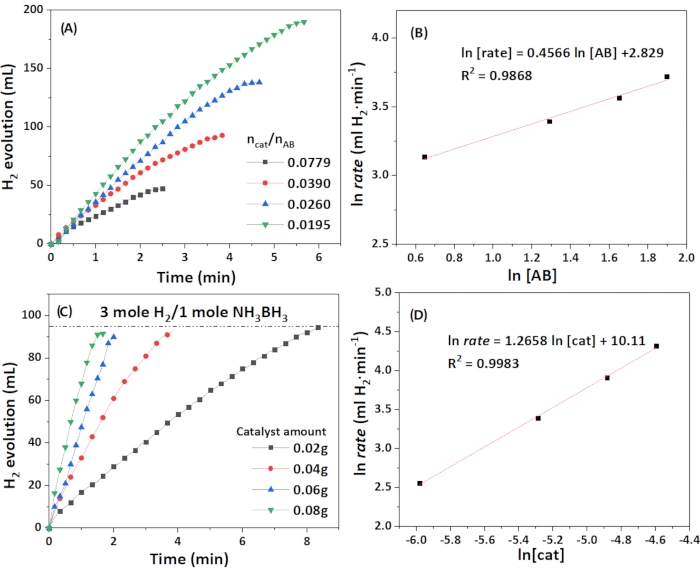
Figure 8: Kinetics studies. (A) Plots of the time of dehydrogenation of AB at various AB concentrations. (B) Plot of the rate of H2 generation at various concentrations of AB in natural logarithmic scale. (C) Plots of the time of dehydrogenation of AB at various amounts of catalysts. (D) Plot of the rate of H2 generation at various concentrations of catalyst in natural logarithmic scale. Please click here to view a larger version of this figure.
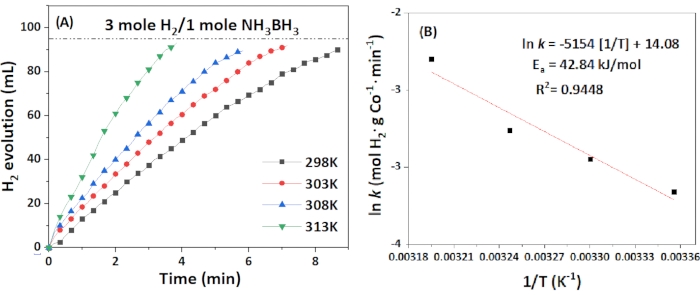
Figure 9: Kinetics studies. (A) Plots of the time of dehydrogenation of AB at various temperatures; (B) Arrhenius plots derived from the obtained kinetic data. Please click here to view a larger version of this figure.
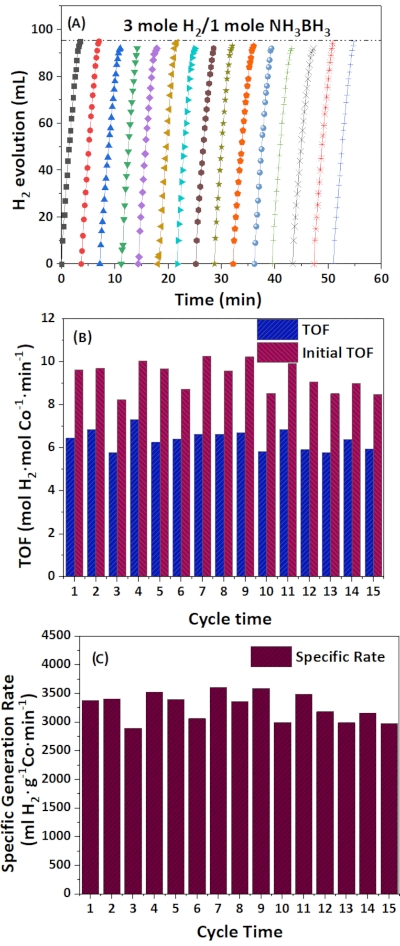
Figure 10: Cyclic performance studies. (A) Recycling of 0.5 wt% Co/Co SACs catalyst (40 mg) in water (10 mL), with the addition of AB (1.30 mmol) to the system at 313 K in each cycle. (B) TOF ratio at each recycle. (C) Specific generation rate. Please click here to view a larger version of this figure.
Supplementary Figure 1: Setup for the measurement of the volume of hydrogen release from the hydrolysis of ammonia borane. The measurement was recorded after the ammonia borane solution was fully injected into the reaction flask. Sulfuric acid was used to wash any residual ammonia gas which may be produced during the hydrolysis reaction. The volume displaced was recorded on a 10 s interval as the water level dropped inside the inverted cylinder. Please click here to download this File.
Supplementary Figure 2: XRD pattern of 0.5 wt% Co/CoNx-CNT support after 15 recycles. The state of Co nanoparticles remains unchanged. Please click here to download this File.
Discussion
The pyrolysis method has become one of the powerful strategies in the synthesis of one-dimensional nanomaterial on various heteroatom-doped solid supports with controlled sizes of NPs. For example, the nanospace-confined pyrolysis strategy was reported by Guo et al.56. Briefly, the pre-treated MWCNTs, cobalt, and phosphorus precursors were pyrolyzed at 800 °C under N2 atmosphere, and the CoP NPs supported on N-CNT can be obtained. The presence of the micro-pores can act as the pathway for Co and P precursors to permeate during the pyrolysis and coordinate with the C and N atoms around the pores. This strategy could effectively control the size of NPs and inhibit their aggregation. Controlled growth of NPs could also be prepared by the transformation of SAs through slow aggregation. Previously, Ni-SA-N-CNT was also prepared by one-pot pyrolysis method with Ni precursor and dicyandiamide52. Ni SAs loading up to 20.3% was obtained at 800 °C under Ar, and it was found that at 900 °C small amount of Ni NPs started to form. With slight modification of the procedure, for our Co SAs synthesis, it was found that as early as 700 °C, Co NPs were already observed, and it was found that pyrolysis at 800 °C for 2 h, 9.7 wt% of Co NPs were formed with about 15.4 wt% of Co SAs remaining. In addition, Co nanoparticle was uniformly wrapped by a few layers of graphitic carbon, as shown in Figure 5D and Figure 5F. The few layers of graphitic carbon, which was considered a protective coating for nanoparticles, could effectively inhibit the further growth of the NPs and prevent further agglomeration after the ammonia borane hydrolysis reactions.
Given the fact that High-angle annular dark-field (HAADF) imaging may not always be available to detect the atomic dopants, a combined technique of XRD and ICP-OES will be useful for preliminary screening of the presence of atomic dopants in the catalyst samples. The NPs were usually well-observed as strong and sharp peaks, while SAs were generally shown as broad and weak peaks in the XRD spectrum; therefore, closely monitoring the XRD spectrum ensures the formation of the Co NPs. In addition, since NPs could be easily leached out by reacting with dilute acid solution16, as shown in section 6 of the protocol, the amount of the respective NPs and SAs could be estimated unequivocally. This protocol ensures the catalysts have the Co dopant components before sending them to HAADF for imaging.
Different from the reported "one-pot" pyrolysis method to prepare SAs CNT, DCD-350 was first prepared by decomposition of the dicyandiamide in a muffle furnace at 350 °C to prepare the melem-C3N4 materials (m-C3N4). The reason was due to the formation of a large amount of gas and solid materials during the decomposition of dicyandiamide, presumably NH3(g) and ammonium salts. Therefore, the one-pot was slightly modified to a two-step procedure to avoid too many solids being deposited in the tubular furnace at elevated temperatures, which may cause blocking of the gas outlet of the furnace.
The formation mechanism of the earthworm-shaped CNT was believed to undergo a Co metal-catalyzed reaction with the further decomposition of the m-C3N4. After the m-C3N4 and Co precursor was completely mixed, at 650 °C, the m-C3N4 slowly transformed to the g-C3N4 form. At 700 °C, Co SAs within the g-C3N4 molecules began to be thermally activated and became more mobile. The thermal movement of Co SAs then creates internal stresses, which curl up the layered Co-g-C3N4 layers to create a seamless graphitic cylindrical network, exhibited as an earthworm-shaped CNT, as shown in the FE-SEM images in Figure 6. Increasing the nominal ratio of Co precursor to DCD-350 from 1:200 to 1:33.3 greatly impacts the final morphology of the CNT catalysts. It is believed that during the nitridation treatment of Co-SA/C-CNT, more defect density can be created which was caused by the anchored implementation of Co SAs and N dopants in the carbon structure49. Since the ratio of Co precursor to DCD-350 of 1:200 could lead to more NH3 generation during pyrolysis; as a result, the D band and G band observed in Figure 2 was greater than 1 while for the ratio of 1:33.3, D: G dropped below 1. This could explain the defect of the nanotube was due to nitridation. In addition, the presence of the Co nitride peaks indicated the presence of partial nitrification of the Co NPs; however, no such peaks were observed for the catalyst with the ratio of 1:33.3. Therefore, the ratio of Co precursor to m-C3N4 can be used to control defect density, while the Raman spectrum can be used to monitor this.
The synergy of NPs and SAs on catalyst support was very specific to reaction types, and therefore understanding the AB hydrolysis mechanism can provide insight into effective catalyst design and explore more useful applications for NPs-SAs catalysts. Several AB hydrolysis mechanisms were proposed by different research groups. Fu et al. proposed the formation of an intermediate BH3OH–NH4+ on the surface of the Ni2P catalysts and followed by the subsequent attack of H2O to produce hydrogen molecules57. Xu et al. reported that the AB molecule interacts with the metal NPs surface to form an activated complex, and this should be the rate-determining step (RDS)58. The ammonia borane molecule on the surface of the metal NPs was first attacked by an H2O molecule, followed by the concerted dissociation of the B-N bond. Hydrogen was then released with the concomitant production of borate ion on the metal NPs surface (-B(OH)H2*, -B(OH)2H*and -B(OH)3*). Jagirdar et al. reported the formation of a transient M-H bond after the attack of H2O on the boron atom of AB, followed by the reaction of another H2O molecule on the M-H bond to release hydrogen molecule59. Using D2O as the solvent to study the kinetic isotope effect, Fu et al. confirmed that half of the released hydrogen came from H2O, and half of the hydrogen comes from AB55. Chen et al. first proposed the SN2 mechanism, where H2O and AB are first adsorbed on the catalyst surface of NiCo2P2. Under the attack of OH* molecule on the B atom to produce a series of intermediates such as B(OH)H3*, B(OH)2H2*and B(OH)3H* (i.e., OH* + BH3NH3* → B(OH)3H* + NH3*). The B(OH)3H* species then dissociate to release H*, and B(OH)H2* recombine with NH3* to form the NH3B(OH)H2*, and the process is repeated until all three equivalent of hydrogen were released. Although the mechanism by different catalysts may slightly differ, the involvement of H2O molecules was critical to the rate of the hydrolysis reactions. It is proposed that as the hydrolysis of AB proceeds, the reaction media become slightly alkaline, which facilitated the formation of the HO*-Co-N2 species by the oxidative addition of H2O molecules, as shown in Figure 11. This species could lower the adsorption energy of H2O molecules. At the same time, the abundant Co-Nx active site could donate electrons to the Co NPs, making them more active to activate AB through oxidative addition60,61. The H atom from both the AB and H2O could then undergo reductive elimination to release H2. Future work will be conducted to modulate the alkalinity of the reaction media to optimize the AB and H2O adsorption kinetics for achieving better catalytic performance.
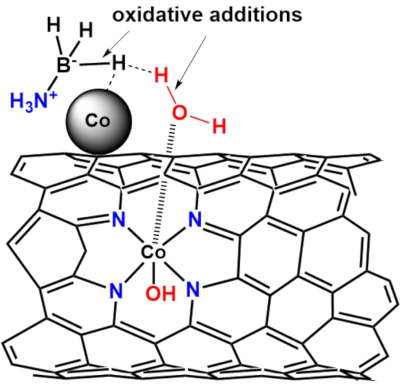
Figure 11: Proposed mechanism for the initiation step of ammonia borane hydrolysis by Co/Co-N-CNT catalyst. It was proposed that water molecules were first adsorbed on the activated Co single atoms, followed by interacting with the activated B-H bonds of the ammonia borane molecules. Please click here to view a larger version of this figure.
In summary, a type of heterogeneous catalyst composed of both Co NPs and Co SAs supported on earthworm-like CNT nanostructure was demonstrated with a facile synthesis strategy. The nano-catalyst with an abundant amount of both NPs and SAs and high defect density was successfully prepared. Above all, the as-prepared nano-catalyst exhibited excellent activity and stability towards the hydrolysis reaction of ammonia borane for hydrogen gas production, demonstrating the successful catalyst design through combined functions by both NPs and SAs. The demonstrated high stability could facilitate hydrogen production with more reliable performance for the high-powered and stable requirement of industrial applications, such as cargo transportation through truck and forklift in the transport and logistics sector.
Divulgaciones
The authors have nothing to disclose.
Acknowledgements
This work was fully funded by Hong Kong University Grants Committee – Institutional Development Scheme (IDS) Collaborative Research Grant, grant number UGC/IDS(C)14/B(E)01/19, the Faculty Development Scheme (FDS), grant number UGC/FDS25/E08/20 and partially funded by the Institutional Development Scheme (IDS), grant number UGC/IDS(R)25/20.
.
Materials
| Dicyandiamide | Sigma Aldrich | D76609 | |
| Borane-ammonia complex | Aladdin | B131882-100g | |
| Citric acid, 99% | Sigma Aldrich | C0759 | |
| Cobalt metal standard solution, traceable to SRM from NIST Co(NO3)2 in HNO3 0.5 mol/l 1000 mg/l Co Certipur | Sigma Aldrich | 1.19785 | |
| Cobalt(II) acetylacetonate, ≥ 99% | Sigma Aldrich | 727970 | |
| Hydrochloric acid, ACS reagent | Sigma Aldrich | 320331-2.5L | |
| ICP-OES | ICP-OES with dichroic spectral combiner (Agilent 5110) | ||
| Muffle furnace | High Performance Hybrid Muffle furnace, Chamber: (360 x 250 x 320) mm, Exterior: (610 x 545 x 500) mm, Power(3100W), Vulcan 3-1750) | ||
| Nitric acid, puriss. p.a., 65.0-67.0% | Sigma Aldrich | 84378 | |
| Sulphuric acid, ACS reagent 95-98% | Sigma Aldrich | 258105 | |
| Tubular furnace | OTF-1200X with tube size of 60 mm outer diameter (Hefei Kejing) | ||
| Ultrasonic bath | 10L Digital Single Frequency 40 kHz Ultrasonic Cleaner (Biobase) |
Referencias
- Di Ilio, G., Di Giorgio, P., Tribioli, L., Bella, G., Jannelli, E. Preliminary design of a fuel cell/battery hybrid powertrain for a heavy-duty yard truck for port logistics. Energy Conversion and Management. , 243 (2021).
- Imdahl, C., et al. Potentials of hydrogen technologies for sustainable factory systems. 28th CIRP Conference on Life Cycle Engineering. , 583-588 (2021).
- Keller, A. V., Karpukhin, K. E., Kolbasov, A. F., Kozlov, V. N. Analysis of hydrogen use as an energy carrier in transport. IOP Conference Series: Materials Science and Engineering. 1159, 012087 (2021).
- Sun, B. -. W., Li, H. -. J., Yu, H. -. Y., Qian, D. -. J., Chen, M. In situ synthesis of polymetallic Co-doped g-C3N4 photocatalyst with increased defect sites and superior charge carrier properties. Carbon. 117, 1-11 (2017).
- Zhang, Y., et al. Biomass chitosan derived cobalt/nitrogen-doped carbon nanotubes for the electrocatalytic oxygen reduction reaction. Journal of Materials Chemistry A. 6 (14), 5740-5745 (2018).
- Sun, J. -. F., et al. Isolated single atoms anchored on N-doped carbon materials as a highly efficient catalyst for electrochemical and organic reactions. ACS Sustainable Chemistry & Engineering. 8 (39), 14630-14656 (2020).
- Jin, C., et al. Single-atom nickel confined nanotube superstructure as support for catalytic wet air oxidation of acetic acid. Communications Chemistry. 2 (1), (2019).
- Kuai, L., et al. Titania supported synergistic palladium single atoms and nanoparticles for room temperature ketone and aldehydes hydrogenation. Nature Communications. 11 (1), 48 (2020).
- Yang, X. -. F., et al. Single-atom catalysts: A new frontier in heterogeneous catalysis. Accounts of Chemical Research. 46 (8), 1740-1748 (2013).
- Sun, X., et al. Single cobalt sites in mesoporous N-doped carbon matrix for selective catalytic hydrogenation of nitroarenes. Journal of Catalysis. 357, 20-28 (2018).
- Sun, T., et al. Single-atomic cobalt sites embedded in hierarchically ordered porous nitrogen-doped carbon as a superior bifunctional electrocatalyst. Proceedings of the National Academy of Sciences of the United States of America. 115 (50), 12692-12697 (2018).
- Wan, G., et al. Engineering single-atom cobalt catalysts toward improved electrocatalysis. Small. 14 (15), 1704319 (2018).
- Wang, P., et al. Atomically dispersed cobalt catalyst anchored on nitrogen-doped carbon nanosheets for lithium-oxygen batteries. Nature Communications. 11 (1), 1576 (2020).
- Yan, H., et al. Bottom-up precise synthesis of stable platinum dimers on graphene. Nature Communications. 8 (1), 1070 (2017).
- Qiao, B., et al. Single-atom catalysis of CO oxidation using Pt1/FeOx. Nature Chemistry. 3 (8), 634-641 (2011).
- Tang, C., et al. A stable nanocobalt catalyst with highly dispersed CoNx active sites for the selective dehydrogenation of formic acid. Angewandte Chemie International Edition. 56 (52), 16616-16620 (2017).
- Gnanapragasam, N. V., Rosen, M. A. A review of hydrogen production using coal, biomass and other solid fuels. Biofuels. 8 (6), 725-745 (2017).
- Wang, S., Lu, A., Zhong, C. J. Hydrogen production from water electrolysis: role of catalysts. Nano Convergence. 8 (1), 4 (2021).
- Demirci, U. B. About the technological readiness of the H2 generation by hydrolysis of B(-N)-H compounds. Energy Technology. 6 (3), 470-486 (2018).
- Wu, H., et al. Metal-catalyzed hydrolysis of ammonia borane: Mechanism, catalysts, and challenges. International Journal of Hydrogen Energy. 45 (55), 30325-30340 (2020).
- Singh, A. K., Singh, S., Kumar, A. Hydrogen energy future with formic acid: a renewable chemical hydrogen storage system. Catalysis Science & Technology. 6 (1), 12-40 (2016).
- Grinberg, A., Shter, G. E., Grader, G. S. Nitrogen-based alternative fuels: Progress and future prospects. Energy Technology. 4 (1), 7-18 (2016).
- Ley, M. B., Meggouh, M., Moury, R., Peinecke, K., Felderhoff, M. Development of hydrogen storage tank systems based on complex metal hydrides. Materials. 8 (9), 5891-5921 (2015).
- Wang, H., Lin, H. J., Cai, W. T., Ouyang, L. Z., Zhu, M. Tuning kinetics and thermodynamics of hydrogen storage in light metal element based systems – A review of recent progress). Journal of Alloys and Compounds. 658, 280-300 (2016).
- Li, M., Hu, J., Lu, H. A stable and efficient 3D cobalt-graphene composite catalyst for the hydrolysis of ammonia borane. Catalysis Science & Technology. 6 (19), 7186-7192 (2016).
- Wang, H., Zhao, Y., Cheng, F., Tao, Z., Chen, J. Cobalt nanoparticles embedded in porous N-doped carbon as long-life catalysts for hydrolysis of ammonia borane. Catalysis Science & Technology. 6 (10), 3443-3448 (2016).
- Zhou, L., et al. Ultrasmall cobalt nanoparticles supported on nitrogen-doped porous carbon nanowires for hydrogen evolution from ammonia borane. Materials Horizons. 4 (2), 268-273 (2017).
- Feng, Y., et al. Sea-urchin-like hollow CuMoO4-CoMoO4 hybrid microspheres, a noble-metal-like robust catalyst for the fast hydrogen production from ammonia borane. ACS Applied Energy Materials. 4 (1), 633-642 (2021).
- Liao, J., et al. CuO-NiO/Co3O4 hybrid nanoplates as highly active catalyst for ammonia borane hydrolysis. International Journal of Hydrogen Energy. 45 (15), 8168-8176 (2020).
- Lu, D., et al. A simple and scalable route to synthesize Cox Cu1-x Co2O4@CoyCu1-yCo2O4 yolk-shell microspheres, a high-performance catalyst to hydrolyze ammonia borane for hydrogen production. Small. 15 (10), 1805460 (2019).
- Feng, Y., et al. Durable and high performing Ti supported Ni0.4Cu0.6Co2O4 nanoleaf-like array catalysts for hydrogen production. Renewable Energy. 169, 660-669 (2021).
- Prabhu, P., Jose, V., Lee, J. M. Heterostructured catalysts for electrocatalytic and photocatalytic carbon dioxide reduction. Advanced Functional Materials. 30 (24), (2020).
- Wang, H., et al. Electronic modulation of non-van der Waals 2D electrocatalysts for efficient energy conversion. Advanced Materials. 33 (26), 2008422 (2021).
- Wang, H., Lee, J. -. M. Recent advances in structural engineering of MXene electrocatalysts. Journal of Materials Chemistry A. 8 (21), 10604-10624 (2020).
- Prabhu, P., Lee, J. M. Metallenes as functional materials in electrocatalysis. Chemical Society Reviews. 50 (12), 6700-6719 (2021).
- Lin, Y., et al. Co-induced electronic optimization of hierarchical NiFe LDH for oxygen evolution. Small. 16 (38), 2002426 (2020).
- Li, M., et al. Gd-induced electronic structure engineering of a NiFe-layered double hydroxide for efficient oxygen evolution. Journal of Materials Chemistry A. 9 (5), 2999-3006 (2021).
- Jose, V., et al. Highly efficient oxygen reduction reaction activity of N-doped carbon-cobalt boride heterointerfaces. Advanced Energy Materials. 11 (17), (2021).
- Qiu, X., et al. Hydrogen generation from ammonia borane hydrolysis catalyzed by ruthenium nanoparticles supported on Co-Ni layered double oxides. Sustainable Energy & Fuels. 5 (8), 2301-2312 (2021).
- Prabu, S., Chiang, K. -. Y. Improved catalytic effect and metal nanoparticle stability using graphene oxide surface coating and reduced graphene oxide for hydrogen generation from ammonia-borane dehydrogenation. Materials Advances. 1 (6), 1952-1962 (2020).
- Ge, Y., et al. Maximizing the synergistic effect of CoNi catalyst on α-MoC for robust hydrogen production. Journal of the American Chemical Society. 143 (2), 628-633 (2020).
- Duan, S., et al. Magnetic Co@g-C3N4 core-shells on rGO sheets for momentum transfer with catalytic activity toward continuous-flow hydrogen generation. Langmuir. 32 (25), 6272-6281 (2016).
- Zhang, H., et al. Birdcage-type CoOx-carbon catalyst derived from metal-organic frameworks for enhanced hydrogen generation. ACS Sustainable Chemistry & Engineering. 7 (11), 9782-9792 (2019).
- Semiz, L. Dehydrogenation of ammonia borane by dealloyed ruthenium catalysts. Inorganic and Nano-Metal Chemistry. 51 (1), 20-26 (2020).
- Bulut, A., et al. Carbon dispersed copper-cobalt alloy nanoparticles: A cost-effective heterogeneous catalyst with exceptional performance in the hydrolytic dehydrogenation of ammonia-borane. Applied Catalysis B: Environmental. 180, 121-129 (2016).
- Akbayrak, S., Tonbul, Y., Özkar, S. Ceria supported rhodium nanoparticles: Superb catalytic activity in hydrogen generation from the hydrolysis of ammonia borane. Applied Catalysis B: Environmental. 198, 162-170 (2016).
- International Centre for Diffraction Data. Powder diffraction file PDF-2 data base international center for diffraction data JCPDS-ICDD 1999 in JCPDS database. International Centre for Diffraction Data. , (2021).
- Zhang, J., Zhao, Z., Xia, Z., Dai, L. A metal-free bifunctional electrocatalyst for oxygen reduction and oxygen evolution reactions. Nature Nanotechnology. 10 (5), 444-452 (2015).
- Cao, B., et al. Tailoring the d-band center of N-doped carbon nanotube arrays with Co4N nanoparticles and single-atom Co for a superior hydrogen evolution reaction. NPG Asia Materials. 13 (1), (2021).
- Varga, T., et al. Co4N/nitrogen-doped graphene: A non-noble metal oxygen reduction electrocatalyst for alkaline fuel cells. Applied Catalysis B: Environmental. 237, 826-834 (2018).
- Li, H., Gan, S., Wang, H., Han, D., Niu, L. Intercorrelated superhybrid of AgBr supported on graphitic-C3N4-decorated nitrogen-doped graphene: High engineering photocatalytic activities for water purification and CO2 reduction. Advanced Materials. 27 (43), 6906-6913 (2015).
- Zhao, S., et al. One-pot pyrolysis method to fabricate carbon nanotube supported Ni single-atom catalysts with ultrahigh loading. ACS Applied Energy Materials. 1 (10), 5286-5297 (2018).
- Dilpazir, S., et al. Cobalt single atoms immobilized N-doped carbon nanotubes for enhanced bifunctional catalysis toward oxygen reduction and oxygen evolution reactions. ACS Applied Energy Materials. 1 (7), 3283-3291 (2018).
- Cao, L., et al. Identification of single-atom active sites in carbon-based cobalt catalysts during electrocatalytic hydrogen evolution. Nature Catalysis. 2 (2), 134-141 (2018).
- Fu, Z. C., et al. Highly efficient hydrolysis of ammonia borane by anion (-OH, F-, Cl-)-tuned interactions between reactant molecules and CoP nanoparticles. Chemical Communications. 53 (4), 705-708 (2017).
- Hou, C. -. C., et al. Tailoring three-dimensional porous cobalt phosphides templated from bimetallic metal-organic frameworks as precious metal-free catalysts towards the dehydrogenation of ammonia-borane. Journal of Materials Chemistry A. 7 (14), 8277-8283 (2019).
- Peng, C. Y., et al. Nanostructured Ni2P as a robust catalyst for the hydrolytic dehydrogenation of ammonia-borane. Angewandte Chemie International Edition English. 54 (52), 15725-15729 (2015).
- Xu, Q., Chandra, M. Catalytic activities of non-noble metals for hydrogen generation from aqueous ammonia-borane at room temperature. Journal of Power Sources. 163 (1), 364-370 (2006).
- Kalidindi, S. B., Sanyal, U., Jagirdar, B. R. Nanostructured Cu and Cu@Cu2O core shell catalysts for hydrogen generation from ammonia-borane. Physical Chemistry – Chemical Physics. 10, 5870-5874 (2008).
- Ning, X., Yu, H., Peng, F., Wang, H. Pt nanoparticles interacting with graphitic nitrogen of N-doped carbon nanotubes: Effect of electronic properties on activity for aerobic oxidation of glycerol and electro-oxidation of CO. Journal of Catalysis. 325, 136-144 (2015).
- Li, Z., et al. Covalent triazine framework supported non-noble metal nanoparticles with superior activity for catalytic hydrolysis of ammonia borane: from mechanistic study to catalyst design. Chemical Science. 8 (1), 781-788 (2017).

