A Model for Epilepsy of Infectious Etiology using Theiler’s Murine Encephalomyelitis Virus
Summary
Intracerebral infection with the Theiler’s murine encephalomyelitis virus (TMEV) in C57BL/6 mice replicates many of the early and chronic clinical symptoms of viral encephalitis and subsequent epilepsy in human patients. This paper describes the virus infection, symptoms, and histopathology of the TMEV model.
Abstract
One of the main causes of epilepsy is an infection of the central nervous system (CNS); approximately 8% of patients who survive such an infection develop epilepsy as a consequence, with rates being significantly higher in less economically developed countries. This work provides an overview of modeling epilepsy of infectious etiology and using it as a platform for novel antiseizure compound testing. A protocol of epilepsy induction by non-stereotactic intracerebral injection of Theiler’s murine encephalomyelitis virus (TMEV) in C57BL/6 mice is presented, which replicates many of the early and chronic clinical symptoms of viral encephalitis and subsequent epilepsy in human patients. The clinical evaluation of mice during encephalitis to monitor seizure activity and detect the potential antiseizure effects of novel compounds is described. Furthermore, histopathological consequences of viral encephalitis and seizures such as hippocampal damage and neuroinflammation are shown, as well as long-term consequences such as spontaneous epileptic seizures. The TMEV model is one of the first translational, infection-driven, experimental platforms to allow for the investigation of the mechanisms of epilepsy development as a consequence of CNS infection. Thus, it also serves to identify potential therapeutic targets and compounds for patients at risk of developing epilepsy following a CNS infection.
Introduction
One of the frequent consequences of viral encephalitis is epileptic seizures. Many viral infections trigger symptomatic seizures during the acute phase of the infection; the risk for such seizures is increased by over 20% among the general public1,2,3. Patients who survive the infection also have an increased risk of 4%-20% of developing chronic epilepsy in the months to years after infection1,4. The Theiler's murine encephalomyelitis virus (TMEV) has been identified as a suitable virus to study acute and chronic seizures in a mouse model of viral encephalitis5,6,7. TMEV is a non-enveloped, positive-sense, single-stranded RNA virus of the Picornaviridae family and has traditionally been used to study demyelination in the spinal cord of SJL mice, which C57BL/6 (B6) mice are protected from because they have the ability to clear the virus rapidly after infection. However, TMEV induces acute seizures in 50%-75% of male and female B6 mice within the first week postinfection (pi), while about 25%-40% develop chronic epilepsy weeks to months pi2,5,6,8,9. Apart from seizures, the mice also display the common histopathology of an epileptic hippocampus with neurodegeneration and gliosis5,6,8,10,11,12. Furthermore, TMEV-infected B6 mice perform significantly worse in behavioral tests for learning and memory and have cognitive comorbidity, which is also seen in clinical patients with epilepsy13,14,15.
Traditionally, models of epilepsy and seizures utilize either the application of chemoconvulsant substances or electrical stimulation to induce seizures; however, these models lack construct validity and often display more severe seizures and brain damage than are seen in clinical patients16. There is no model that is appropriate for every research question17. Using the TMEV model is especially interesting if the predisposing factors of seizure development after an infection of the CNS are researched or if compounds are screened for their antiseizure efficiency.
Since the TMEV model has been established and used across several different laboratories internationally, the authors have identified many details that allow for successful implementation of the model, e.g., the specificity of different virus and mouse strains. The most reliable seizure induction was generated with the Daniel's strain of TMEV and B6J mice2,5,6,8,9. The model is currently used by the National Institute of Neurological Disorders and Stroke (NINDS) as a platform to identify new drugs against epilepsy and seizures18,19. This paper includes the detailed protocol of virus induction and clinical monitoring to allow other researchers to utilize this model of viral encephalitis to further the understanding of the disease mechanisms, as well as for drug testing.
The following protocol reflects a study designed for compound testing in this model, although numerous other types of studies can be performed. Mice are anesthetized briefly before injection with the Daniel's strain of TMEV in the temporal region of the right hemisphere (posterior and medial to the right eye). Depending on the research question, if non-infected control animals are required, mice receive sterile phosphate-buffered saline (PBS, pH 7.4, including KH2PO4 [1.06 mM], NaCl [155.17 mM], and Na2HPO4·7H2O [2.97 mM]) instead of TMEV. Previous experience in TMEV-infected mice has indicated that handling-induced seizures occur between day 3 and day 7 postinfection. The frequency of the injection, route, and time of testing of experimental compounds vary according to their properties. It is recommended to perform the virus inoculation on a Friday, which allows day 3-7 seizure monitoring to happen the following week, Monday-Friday. During the seizure monitoring week, experimental compounds can be administered (i.p.) twice daily (at least 4 h apart) unless otherwise suggested by the compound's kinetics or mechanism of action. Seizure monitoring during treatment can be performed at a previously determined time point. Injection and observation times vary depending on individual compounds. Animals are injected with the test compound or with a vehicle in lieu of the drug compound. These two groups can be handled and observed analogously to the experimental group. During the experiment, the one person handling the mice and scoring the seizures should be blinded to the treatment.
Protocol
All described procedures were authorized by the respective authorities. Animals are maintained following the recommendations in the "Guide for Care and Use of Laboratory Animals" (National Research Council) and in accordance with Public Health Service policy and the Institutional Animal Care and Use Committee at the University of Utah, animal protocol (number: 21-11009, Dept. of Pharmacology and Toxicology) and at Freie Universität Berlin (protocol: G0015/21, LAGeSo Berlin, Institute of Pharmacology and Toxicology), respectively. The results shown in Figure 3 and Figure 5 were approved under 33.9-42502-04-11/0516 and 33.9-42502-04-15/1892 (LAVES Oldenburg, Dept. of Pharmacology, Toxicology, and Pharmacy, University of Veterinary Medicine Hannover).
1. Considerations and preparations for designing the study
- Virus
- The Daniel's strain of TMEV was kindly provided by Robert Fujinami from the University of Utah. Originally, it was isolated from a mouse in the Harvard colony20. Look up the specific classification and regulations for biological agents in the respective countries before handling TMEV, and discuss with the institutional biosafety personnel to ensure compliance with regulations. Typically, TMEV is classified as BSL 2, depending on the strain and genetic modifications.The standard dose necessary to induce seizures in a majority of the animals is 3 x 105 plaque-forming units (PFU).
NOTE: The dose might have to be adapted, for example, if transgenic mice are used. Overall, titers between 2 x 104 PFU and 2.44 x 107 PFU have been used to successfully induce seizures. Control animals receive sterile PBS and do not experience seizures. The virus does not infect humans; however, PPE (lab coat, gloves, safety glasses) should be worn by experimenters at all times, and mouse carcasses and bedding should be autoclaved before disposal.
- The Daniel's strain of TMEV was kindly provided by Robert Fujinami from the University of Utah. Originally, it was isolated from a mouse in the Harvard colony20. Look up the specific classification and regulations for biological agents in the respective countries before handling TMEV, and discuss with the institutional biosafety personnel to ensure compliance with regulations. Typically, TMEV is classified as BSL 2, depending on the strain and genetic modifications.The standard dose necessary to induce seizures in a majority of the animals is 3 x 105 plaque-forming units (PFU).
- Animals
- For inducing and investigating seizures, use B6J mice, as other mouse strains do not necessarily display seizures, e.g., SJL/J, FVB/N, or Balb/c mice5. There is no difference between female and male mice in acute seizure frequency5. Perform the experiments on adolescent to adult mice (starting at 5-6 weeks of age).
- Diet
- Diet has been determined as one source of lab-to-lab variability in disease severity; hence, consider the diet as a potential factor for variation22.
- Group size
- Since not all animals develop acute or chronic seizures in this model, use about 20 mice/group for compound testing.
- Animal welfare
- If any mouse demonstrates significant adverse effects from the infection or administration of the investigational compound (e.g., lethargy, poor grooming, excessive redness, and purulent discharge from the wound) after an observation time determined by the local IACUC guidelines (e.g., 48 h), euthanize the mouse humanely.
- Euthanize any mouse demonstrating extreme body weight loss (>20%) during the infection period humanely.
- If animals do not eat properly, give them access to supplemental pellets moistened with pediatric electrolyte solution or similar.
2. Virus inoculation
- Sterilization of the injection syringe
- Remove the cap of the insulin syringe. Add polyethylene tubing as a collar around the needle to ensure an adequate injection depth of 2.5 mm. Submerge the syringe in ethanol for 30 min. Place the syringe under UV light for 30 min.
- Put the cap back onto the needle. Wrap the syringe in a sterilizing pouch and tape it closed. Label with the date of preparation.
- Virus injection
- Get aliquots of Daniel's strain of TMEV from the −80 °C freezer. Thaw the virus and keep it on ice. Avoid thawing and re-freezing the virus. Load the syringe with the virus suspension (3 x 105 PFU diluted in 20 µL of PBS or DMEM culture medium).
CAUTION: The virus is infectious to mice but not to humans. - Clean the bench with disinfectant and work under a fume absorber. Virus inoculation is not sterile but is as clean as possible.
- Transfer the mouse into the anesthesia induction chamber, and use 2% isoflurane in oxygen to induce anesthesia. Reaching surgical tolerance takes a few minutes; adjust the concentration based on the animal's breathing and depth of anesthesia.
- Transfer the anesthetized mouse from the anesthesia box under the hood. Check surgical tolerance, e.g., by toe pinch. The whole procedure is performed in less than 30 s, so the animal does not need to be inhaling isoflurane during the injection. Add eye ointment to prevent drying of the cornea.
- Clean the head of the animal with an alcohol pad. Tilt the mouse's head slightly to the left so that the injection site is pointing upwards.
- Pull the skin a little backward, insert the needle into the head, and inject 20 µL intracortically to a depth of 2.5 mm in the temporal region of the right hemisphere (posterior and medial to the right eye).
- Perform the injection unilaterally in the same hemisphere in all animals. Use the eye and ear as landmarks for the location of the parietal cortex. The placement has been previously verified histologically; see Figure 1. Injection coordinates according to stereotaxic injection in relation to bregma are −2.0 (AP); +3.0 (ML); −1.5 (DV).
- Leave the syringe in place for 5-15 s. Write down if there is any leakage from injection or if air bubbles are seen – in that case, prepare a new syringe. When carefully pulling the syringe out, rotate it slightly. Apply eye ointment to prevent dryness while under anesthesia.
- OPTIONAL: Code the animal on the tail or the ear depending on local procedures (optional but easy as the animal is unconscious).
- Transfer the animal to a new cage, which is placed half-on/half-off a heating pad (35-40 °C) during recovery from the anesthesia. Do not leave the animals unattended until they have regained sufficient consciousness to maintain sternal recumbency.
NOTE: Animals can be group-housed again after recovery (infected animals are not mixed with mock-infected animals). Infected mice should not be housed in the same room as non-infected mice. - Keep track of the weight of the mice for the first 7 days pi as they lose weight after infection and might require additional feeding.
- Get aliquots of Daniel's strain of TMEV from the −80 °C freezer. Thaw the virus and keep it on ice. Avoid thawing and re-freezing the virus. Load the syringe with the virus suspension (3 x 105 PFU diluted in 20 µL of PBS or DMEM culture medium).
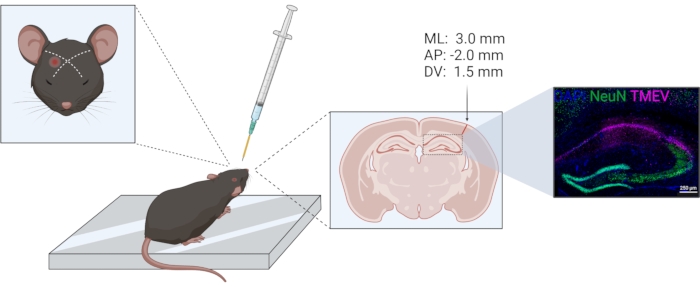
Figure 1: Schematic of TMEV injection procedure. From left to right: For a right parietal cortex injection, the needle is injected slightly lateral of an imaginary line between the eye and opposite ear. The collar for depth control is indicated in yellow. The injection tract can be seen in the coronal brain section, marked by the arrow. The injection site corresponds to the coordinates given above the arrow. The right image shows the distribution of the Theiler virus (violet) within the CA1 of the hippocampal formation. The figure was prepared using biorender.com. Please click here to view a larger version of this figure.
3. Compound testing
- Compound preparation
- Review the formulation instructions. Prepare vehicle solution as per recommendations or provided instructions. As an example, the procedure for the antiepileptic drug levetiracetam (LEV) is presented. LEV reduces seizure burden to 30%-40% of vehicle levels at a dose of 350 mg/kg. The vehicle used in this study is 0.5% methylcellulose.
- Calculate doses depending on the compound. Weigh out the drug according to the calculation. For treating 1 kg of mice, weigh out 350 mg of LEV.
- Prepare a stock solution with the appropriate volume of vehicle solution, as directed by the formulation instructions (e.g., sonication, vehicle excipients, etc.). Considering an injection volume of 0.01 mL/g of mouse, the injection volume for 1 kg would be 10 mL with 350 mg of LEV, resulting in a solution of 35 mg/mL19. Report the calculation and the dose in mg/kg and the volume in mL.
NOTE: An exemplary protocol can be found on page 2 of Supplementary Material 1.
- Compound administration
- Randomize cages to the vehicle or compound group. Use mock-infected mice for studies on the mechanisms of epilepsy development or for validation purposes but not for routine drug screening.
- Report environment conditions such as temperature, humidity, time of day, etc.
- Vortex the solution with the compound as well as the vehicle solution. Aspirate the vehicle or compound in a syringe and color code the syringes to prevent mix-up.
- Weigh the animals. Ensure that B6J mice are >18 g on day 3 pi (not rounded). Report the weight.
- Administer the compound (e.g., by intraperitoneal injection or other appropriate routes). Write the time and route of administration.
- Monitor the animals for any behavioral changes after compound administration, especially after the first injections. If seizures occur, report seizure intensity according to the Racine scale23; see step 3.2.
NOTE: An exemplary protocol for compound administration can be found in Supplementary Material 1, page 3, while seizures observed during administration would be recorded on Supplementary Material 1, pages 4-5.
- Seizure monitoring of handling-induced seizures
- Perform this procedure by an experimenter blinded to the treatment. Bring all the cages to the bench. Observe the animals for seizures 2x daily during the light phase.
- Score the seizure activity by a modified Racine scale23: 0 = no change in behavior, 1 = mouth and facial movements, 2 = head nodding, 3 = unilateral forelimb clonus, 4 = bilateral forelimb clonus with rearing, 5 = generalized tonic-clonic activity with loss of postural tone, sometimes jumping, 6 = prolonged and excessive jumping and hyperactivity. Report the number and intensity of seizures.
- Slide a pen across the cage to make some noise.
- Transfer each animal to another box and back.
- Gently shake the cage using a back-and-forth motion, taking care not to shake the cage so vigorously that animals will hit the sides or the top of the cage and are in danger of sustaining physical injury.
- Monitor all animals in the cage for seizures. At any time, if a mouse has a seizure, transfer it back to the home cage and write down the level of the seizure without further seizure stimulation by noise or handling.
- For animals that have not seized spontaneously or after gentle cage shaking, trigger seizures by more intense handling: Carefully turn over the mouse by flipping it at its tail from left to right.
NOTE: Animals with seizures are hyperexcitable, and can be jumpy. - Observe each animal for seizure behavior again. Repeat the process for subsequent cages.
NOTE: An exemplary protocol for seizure monitoring can be found in Supplementary Material 1, pages 6-7.
- Data report on compound effects
- Analyze the daily body weights using repeated measures (RM) ANOVA.
- Use the daily cumulative seizure burden values for a graphical representation of the data. Present the efficacy data (number of animals with no Racine stage 3-5 seizures) as the number protected/the number tested in each respective group (usually N = 20). Therefore, compare the data between the vehicle- and drug-treated groups for responses (seizing or non-seizing) using a Fisher's exact test.
NOTE: An animal is considered "protected" from seizures if having a seizure of stage 2 or less and non-protected animals have seizures of stages 3-5. - Analyze the tolerability data similarly, as the number of animals with behavioral impairments or toxic effects/the number tested in each group. Note any adverse effects, including deaths.
- If less than 50% of the mice injected with the vehicle seize acutely, do not consider the data.
Representative Results
Compound effects on acute seizures
Behavioral seizures are recorded if they occur during drug injection (DI) or during the subsequent AM/PM seizure handling/monitoring sessions. Seizures observed during handling seizures can be presented as a heat map, which is shown in Figure 2 for LEV (350 mg/kg). For the analysis of seizure burden, the average of the final (day 7) cumulative seizure burden values for the vehicle-treated group are taken and compared by the Mann-Whitney U test to the compound treated group (seizure burden values include those collected at the post-injection observation). For compound efficacy, a Fisher's exact test determines if there is a statistical difference in efficacy between vehicle- and drug-treated groups. Similarly, tolerability data are analyzed with Fisher's exact test. Bodyweight analysis is performed by repeated-measures ANOVA to determine changes over the course of the experiment, as well as differences between compound- and vehicle-treated mice.
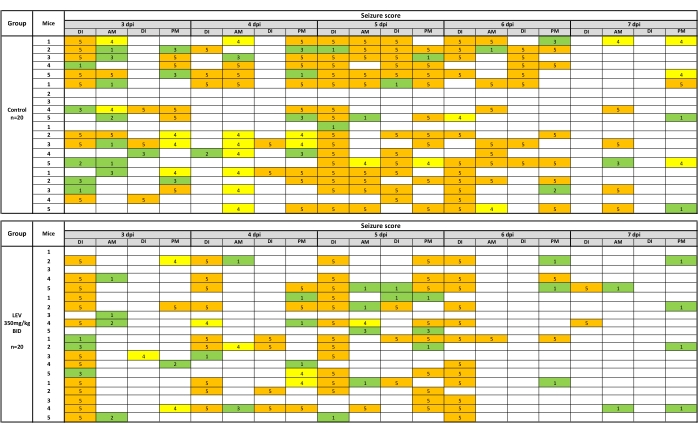
Figure 2: A heat map of behavioral seizures following testing with vehicle (0.5% methylcellulose) or LEV (350 mg/kg). Twice daily injections occurred 1 h prior to handling and seizure observations. Behavioral seizures were recorded if they occurred during drug injection (DI) or during the subsequent AM/PM seizure handling/monitoring sessions. LEV (350 mg/kg) significantly reduces seizures observed during handling sessions (35.9% of vehicle seizure burden) over the 5 day observation period. The heat map is created by picturing seizures stage 1-3 in green, 4 in yellow, and 5 in orange. This new figure was created from a published dataset19. Please click here to view a larger version of this figure.
Chronic epilepsy
Apart from the reported seizure burden in the first week pi, there are several readouts that could be useful depending on the study and hypothesis. If animals are kept long-term, a subset of infected animals will develop spontaneous epileptic seizures at several weeks pi, which occur less frequently than acute seizures and, thus, require EEG recording. In order to record EEG from mice, electrodes need to be implanted in a stereotaxic surgery24. Figure 3 shows various epileptic events in the chronic phase by EEG recording in mice infected with TMEV8.
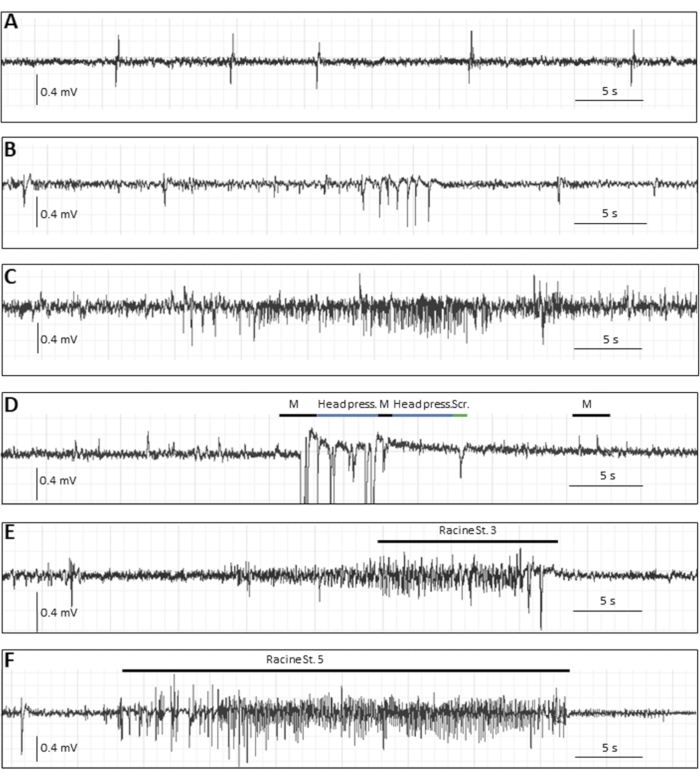
Figure 3: Typical EEG traces in the chronic phase (14 weeks pi) following DA virus infection in mice. (A–C) Representative EEG events in which no behavioral motor correlate was seen, i.e., (A) single spikes, (B) spike clusters, (C) as well as an electrographic, presumably focal seizure. (D–F) Representative EEG events with behavioral correlates. The mouse illustrated in D had seizure-like events with myoclonic twitches (indicated by "M", accompanied by movement artifacts), stereotyped movements (indicated by "head press", when the mouse pressed the head flatly on the ground), or behavioral arrest; "Scr" describes a movement artifact of scratching, (E) and (F) show typical EEG alterations during generalized convulsive seizures: (E) a Racine stage 3 seizure with a duration of 22 s and 1 Hz, and (F) a stage 5 seizure with a duration of 34 s and 5.4 Hz. This figure has been published before8 and is reprinted with permission from Elsevier. Please click here to view a larger version of this figure.
Histology
Since epilepsy and seizures are usually accompanied by hippocampal pathology in patients, which is recapitulated in experimental models, most laboratories also analyze hippocampal changes or the effect of a potential antiseizure treatment on pathology. Commonly analyzed parameters include hippocampal neurodegeneration and shrinking, as well as inflammation by labeling for specific immune cell populations. For such analyses, at the end of the experiment, mice are deeply anesthetized until breathing arrest occurs and the heart rate significantly slows down or shows arrhythmia. Blood is removed by intracardial perfusion of PBS followed by 4% paraformaldehyde (PFA)25 to fixate the tissue. The tissue is then processed by cryosectioning and (immune-)staining26, followed by microscopic analyses.
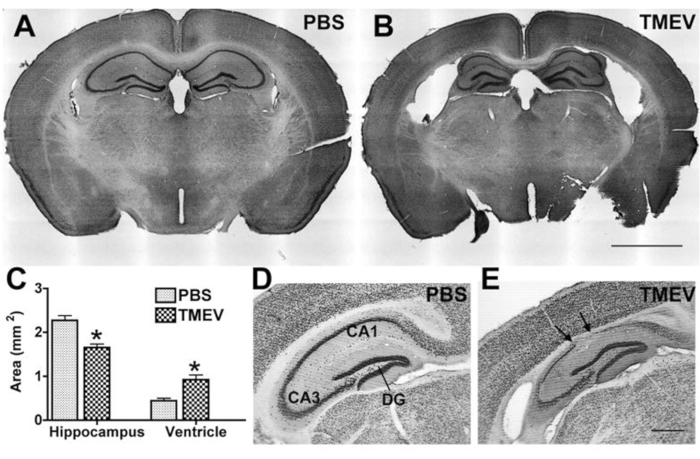
Figure 4: Hippocampal degeneration in epileptic TMEV-infected mice. (A,B) Cresyl violet-stained coronal sections show normal cytoarchitecture in a (A) control (PBS) mouse and hippocampal degeneration in a (B) TMEV mouse at 2 months pi. Note: Enlarged lateral ventricles, collapse of the alveus, and thinning of the pyramidal cell layer. (C) Quantification of this damage shows a significant decrease in hippocampal area and a corresponding increase in the ventricular area of TMEV mice (N = 7) vs. PBS mice (N = 6; data are mean ± SEM; p < 0.001; Student's t-test). (D,E) NeuN labeling further illustrates the magnitude of neuronal cell loss in sections taken at 6 months pi. Arrows indicate regions with complete pyramidal cell loss. (E) The dentate gyrus appears to be relatively intact even in epileptic mice. Scale bar = (A,B) 2 mm; (D,E) 0.5 mm. This figure has been published before6 and is reprinted with permission from Oxford University Press. Please click here to view a larger version of this figure.
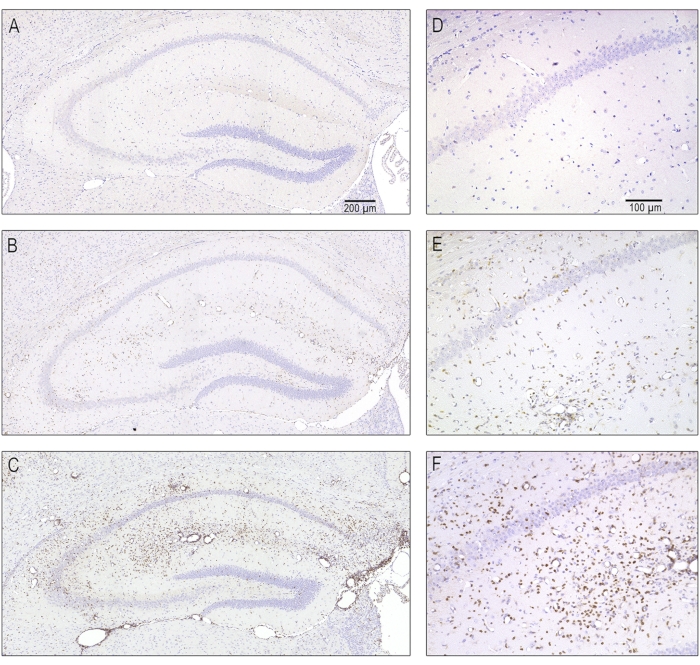
Figure 5: Representative photomicrographs of different severity of T cell infiltration due to acute encephalitis at 7 days postinfection. Serial sections containing the ipsilateral dorsal hippocampus were stained with antibodies against CD3 in order to label T-lymphocytes. (A,D) A normal hippocampus without T cell infiltration as it appeared in mock-infected animals. (B, E) Moderate T cell infiltration as is seen in the majority of TMEV-infected mice. (C, F) A severe infiltration of T lymphocytes, which was only seen in some of the infected mice. This figure has been published before8 and is reprinted with permission from Elsevier. Please click here to view a larger version of this figure.
Supplementary File 1: The supplementary file consists of the form used for compound testing in the TMEV model. Page 1 gives an overview of the experimental setup. Information on the compound, vehicle, and compound solution preparation is recorded on pages 1-2. Compound application is recorded on page 3. The scoring sheets on pages 4-5 are used for recording the seizures observed and quantified by the experimenter performing the compound injection. On page 4, data can be collected for cages 1-4 of 5 mice each, and on page 5, cages 5-8 can be recorded, which is the standard number of animals for compound testing in our hands. The scoring sheets on pages 6-7 are used by the blinded observer who is performing the observation, handling, and gentle cage shaking 1 h after every compound injection. Again, seizure scores can be noted for eight cages in total on these exemplary score sheets. The last page 8 can be used for recording any other observations or notes. Please click here to view a larger version of this figure.
Discussion
This is the first infection-based rodent model for epilepsy that allows investigation of acute and chronic seizure development. It will help to identify drug targets and new compounds for disease prevention or modification for one of the most common etiologies of epilepsy.
As described above, careful consideration of the batch and viral titer may be necessary to ensure that an adequate proportion of TMEV-treated mice demonstrate handling-induced seizures. If animals have fewer seizures than usual, use a batch of N = 20 animals to verify the virus efficiency. If its activity is decreased (less than 50%), it is time to make new aliquots and test them with N = 20 animals. If the new aliquots are not more efficient, a new batch of the virus should be purified. For some transgenic mouse lines, it may be necessary to use a lower viral titer; therefore, the viral titer should be diluted as needed after preliminary experiments. Most data available on B6 mice originated from Jackson Laboratories (Bar Harbor, ME, USA or Charles River, Sulzfeld, Germany); however, similar seizure rates in B6 mice obtained from Harlan (Eystrup, Germany) have been confirmed8. Seizure rates of transgenic animals with a B6 background are comparable to wild-type B6 mice but could differ if the genetic changes have an influence on virus invasion, inflammatory response, or neurodegeneration21. Acute seizures are observed spontaneously but triggered by handling and noise, so it is of utmost importance to handle all animals in a similar fashion when seizure rates are being compared. Twice daily handling sessions have previously provided a high seizure burden and a greater proportion of mice demonstrating seizures during days 3-7 following infection6,8,19. Additional handling sessions (day 1 and day 2) may also be employed to increase seizure burden. Further, animals can be observed prior to each handling session to ensure that spontaneous seizures are not occurring. A loud lab environment, for example, may produce seizures, which may, in turn, render animals refractory to handling-induced seizures during testing times.
While TMEV infection produces handling-induced seizures in the majority of mice, it is unknown why some animals are resistant to this treatment. As described above, it may be that electrographic seizures (with minimal or no associated behaviors) occur and are not normally quantified without concomitant EEG recording. It may also be that small differences in injection location facilitate reduced viral effect in the brain; however, seizures have been reported after cortical and striatal infection5,6,8,9 due to tropism of the virus to the hippocampus. For drug screening studies in this model, to identify a reduction in seizures (e.g., a 50% reduction in seizure burden), a larger number of animals is required for each group (e.g., N = 20). Furthermore, the variability in seizure behaviors in this model necessitates larger differences in drug vs. vehicle effects to identify a significant seizure reduction. Therefore, one limitation of this model is the requirement for larger group sizes. Nevertheless, sufficient group sizes also allow for the identification of anti-seizure and anti-inflammatory effects in this model19.
The vast majority of observable seizures in this model occur during the acute infection period. Despite the occurrence of hippocampal degeneration, immune cell activation, and cognitive deficits observed in mice treated with TMEV, only a small portion of animals treated eventually develop chronic, spontaneous seizures. This low overall seizure burden would require a large number of infected mice in order to properly study spontaneous seizures in this model, which is beyond the scope and capability of many projects. Depth electrode implantation and EEG monitoring would also increase the burden on the experimental animals. While depth electrodes may aid in the identification of spontaneous seizure activity, changes in hippocampal anatomy following infection may make consistent electrode placement a challenge.
The urgent need of identifying new treatments for epilepsy requires the development of models that can be used as a quick screening method for antiseizure efficacy. This model provides features to address this urgent request. Moreover, the fact that it does not require any stereotactical surgery makes it a suitable and easy-to-perform model for the investigation of antiseizure compounds.
Divulgaciones
The authors have nothing to disclose.
Acknowledgements
SB is supported by a starting grant of the FU Berlin. KSW is supported by R37 NS065434 and the ALSAM Foundation. LAB is supported by a D-SPAN award 1F99NS125773-01. We thank Robert Fujinami, Ph.D. for providing us with the Theiler virus and the University of Utah Cell Imaging Core Facility for microscopy support.
Materials
| Absorbent paper | – | – | any |
| Analytical balance | Mettler Toledo (Columbus, OH, U.S.A.) | 30216542 | 0. 1 mg–220 g |
| Animal balance | Ohaus (Parsippany, NJ, U.S.A.) | STX2202 | 0.01 g–2200 g |
| BD Lo-Dose U-100 Insulin Syringes | BD (Mississauga, ON, Canada) | BD329461 | Lo-Dose sterile syringes with permanent BD Micro-Fine IV needle – 1 mL |
| Daniel's strain of TMEV | kindly provided by Robert Fujinami (University of Utah) | – | 3 x 105 plaque-forming units aliquot(s) |
| Disinfectant, e.g. VennoVet 1 super | Menno Chemie Vertriebsgesellschaft GmbH, Germany | – | Recommended by campus veterinarians with less than or equal to 5% alcohol |
| Fisherbrand medium sterile Alcohol prep pad C7 | Thermo Fisher Scientific (Waltham, MA, U.S.A.) | 22-363-750 | |
| Fluriso | VETone (Boise, ID, U.S.A) | 502017 | Isoflurane 250 mL, 2%–5% |
| Fume absorber | Labconco (Kansas City, MO, U.S.A.) | – | – |
| General Protection Disposable SMS White Lab Coats | Thermo Fisher Scientific (Waltham, MA, U.S.A.) | 17-100-810A | |
| GraphPad Prism version 9 | (La Jolla, CA, U.S.A.) | ||
| Ice bucket | – | – | any |
| Microsoft Excel Microsoft | (Redmond, WA, U.S.A.) | ||
| Microsoft Word Microsoft | (Redmond, WA, U.S.A.) | ||
| Mouse cage | – | – | any mouse cage holding at least 5 mice |
| PrecisionGlide needles | BD (Mississauga, ON, Canada) | 329652 | BD Slip Tip with PrecisionGlide Needle Insulin Syringes – 26 G x 3/8 – 0.45 mm x 10 mm |
| Self-Sealing Sterilizing Pouch | Fisher Scientific (Hampton, NY, U.S.A.) | NC9241087 | 12.6 x 25.5 cm |
| Small glass flask | – | – | any, volume 25 mL |
| sterile PBS | Thermo Fisher Scientific (Waltham, MA, U.S.A.) | 10010056 | |
| Stir bar | Carl Roth GmbH & CO. KG | X171.1 | size according to volume of solution |
| Stir plate | Carl Roth GmbH & CO. KG | AAN2.1 | |
| Syringe Luer-Lok | BD (Mississauga, ON, Canada) | 309628 | 1 mL syringe only |
| Ultrasonic Cleaner, Heater/Mechanical Timer | Cole-Parmer (Vernon Hills, IL, U.S.A.) | EW-08895-23 | Bath sonicator – 0.5 gal, 115 V |
| Vehicle solution | – | – | depending on compound vehicle |
| Vortex REAX | Heidolph Instruments GmbH & Co. KG, Germany | 541-10000-00 |
Referencias
- Getts, D. R., Balcar, V. J., Matsumoto, I., Müller, M., King, N. J. Viruses and the immune system: their roles in seizure cascade development. Journal of Neurochemistry. 104 (5), 1167-1176 (2008).
- Libbey, J. E., Fujinami, R. S. Neurotropic viral infections leading to epilepsy: Focus on Theiler’s murine encephalomyelitis virus. Future Virology. 6 (11), 1339-1350 (2011).
- Vezzani, A., et al. Infections, inflammation and epilepsy. Acta Neuropathologica. 131 (2), 211-234 (2016).
- Misra, U. K., Tan, C. T., Kalita, J. Viral encephalitis and epilepsy. Epilepsia. 49, 13-18 (2008).
- Libbey, J. E., et al. Seizures following picornavirus infection). Epilepsia. 49 (6), 1066-1074 (2008).
- Stewart, K. A., Wilcox, K. S., Fujinami, R. S., White, H. S. Development of postinfection epilepsy after Theiler’s virus infection of C57BL/6 mice. Journal of Neuropathology and Experimental Neurology. 69 (12), 1210-1219 (2010).
- Stewart, A. M., et al. Perspectives of zebrafish models of epilepsy: What, how and where next. Brain Research Bulletin. 87 (2-3), 135-143 (2012).
- Bröer, S., et al. Brain inflammation, neurodegeneration and seizure development following picornavirus infection markedly differ among virus and mouse strains and substrains. Experimental Neurology. 279, 57-74 (2016).
- Bröer, S., et al. Viral mouse models of multiple sclerosis and epilepsy: Marked differences in neuropathogenesis following infection with two naturally occurring variants of Theiler’s virus BeAn strain. Neurobiology of Disease. 99, 121-132 (2017).
- Loewen, J. L., Barker-Haliski, M. L., Dahle, E. J., White, H. S., Wilcox, K. S. Neuronal Injury, gliosis, and glial proliferation in two models of temporal lobe epilepsy. Journal of Neuropathology and Experimental Neurology. 75 (4), 366-378 (2016).
- Bell, L. A., Wallis, G. J., Wilcox, K. S. Reactivity and increased proliferation of NG2 cells following central nervous system infection with Theiler’s murine encephalomyelitis virus. Journal of Neuroinflammation. 17 (1), 369 (2020).
- Stewart, K. A., Wilcox, K. S., Fujinami, R. S., White, H. S. Theiler’s virus infection chronically alters seizure susceptibility. Epilepsia. 51 (8), 1418-1428 (2010).
- Buenz, E. J., Rodriguez, M., Howe, C. L. Disrupted spatial memory is a consequence of picornavirus infection. Neurobiology of Disease. 24 (2), 266-273 (2006).
- Tramoni-Negre, E., Lambert, I., Bartolomei, F., Felician, O. Long-term memory deficits in temporal lobe epilepsy. Revue Neurologique. 173 (7-8), 490-497 (2017).
- Ponds, R. W., Hendriks, M. Cognitive rehabilitation of memory problems in patients with epilepsy. Seizure. 15 (4), 267-273 (2006).
- Sloviter, R. S. Experimental status epilepticus in animals: What are we modeling. Epilepsia. 12, 11-13 (2009).
- Löscher, W. Animal models of seizures and epilepsy: Past, present, and future role for the discovery of antiseizure drugs. Neurochemical Research. 42 (7), 1873-1888 (2017).
- Kehne, J. H., Klein, B. D., Raeissi, S., Sharma, S. The National Institute of Neurological Disorders and Stroke (NINDS) Epilepsy Therapy Screening Program (ETSP). Neurochemical Research. 42 (7), 1894-1903 (2017).
- Metcalf, C. S., et al. Screening of prototype antiseizure and anti-inflammatory compounds in the Theiler’s murine encephalomyelitis virus model of epilepsy. Epilepsia Open. 7 (1), 46-58 (2021).
- Daniels, J. B., Pappenheimer, A. M., Richardson, S. Observations on encephalomyelitis of mice (DA strain). Journal of Experimental Medicine. 96 (6), 517-530 (1952).
- Käufer, C., et al. Chemokine receptors CCR2 and CX3CR1 regulate viral encephalitis-induced hippocampal damage but not seizures. Proceedings of the National Academy of Sciences of the United States of America. 115 (38), 8929-8938 (2018).
- Libbey, J. E., et al. The effects of diet on the severity of central nervous system disease: One part of lab-to-lab variability. Nutrition. 32 (7-8), 877-883 (2016).
- Racine, R. J. Modification of seizure activity by electrical stimulation. II. Motor seizure. Electroencephalography and Clinical Neurophysiology. 32 (3), 281-294 (1972).
- Kim, J. E., Cho, K. O. The pilocarpine model of temporal lobe epilepsy and EEG monitoring using radiotelemetry system in mice. Journal of Visualized Experiments. (132), e56831 (2018).
- Gage, G. J., Kipke, D. R., Shain, W. Whole animal perfusion fixation for rodents. Journal of Visualized Experiments. (65), e3564 (2012).
- Tu, L., et al. Free-floating immunostaining of mouse brains. Journal of Visualized Experiments. (176), e62876 (2021).

