Complete and Partial Resuscitative Endovascular Balloon Occlusion of the Aorta for Hemorrhagic Shock
Summary
A commercial catheter was designed to facilitate true partial resuscitative endovascular balloon occlusion of the aorta (REBOA) and address the complications associated with complete aortic occlusion. Initial clinical reports indicate that partial REBOA improves transition to reperfusion, reduction of distal ischemia, and extension of safe occlusion time compared to complete occlusion.
Abstract
Resuscitative endovascular balloon occlusion of the aorta (REBOA) devices grew out of a military-civilian partnership to develop new capabilities for hemorrhage control. With the advent of purpose-built devices, REBOA has become increasingly common in civilian trauma and acute care settings. Currently available REBOA catheters were designed as complete aortic occlusion devices. However, the therapeutic window for complete aortic occlusion is time-limited due to ischemia-reperfusion injury. The partial procedure allows blood flow past the level of occlusion while maintaining targeted proximal pressure, which has been shown to reduce distal ischemia and adjunctive resuscitation requirements in preclinical studies with prolonged occlusion times as compared to traditional complete occlusion.
pREBOA-PRO is the first catheter designed to enable partial and complete aortic occlusion and is currently in limited market release at seven Level I trauma centers in North America. This paper will focus on procedural considerations for REBOA, including patient selection criteria and a comparison of complete and partial aortic occlusion in a simulator, along with highlighting critical steps to improve clinical outcomes. Additionally, this paper reviews a contrast-enhanced CT scan from a trauma patient that shows distal perfusion after 2 h of partial aortic occlusion using this newly designed catheter and discusses representative results from the limited market release to highlight the profound effect of technological innovation on outcomes in vascular emergencies.
Introduction
Resuscitative endovascular balloon occlusion of the aorta (REBOA) devices originated from a military-civilian research and development effort to control exsanguination from non-compressible torso hemorrhage as an alternative approach to open aortic occlusion. With the advent of purpose-built devices and advances in REBOA technology, including guidewire-free catheters and compatibility with 7 Fr sheaths available in the second-generation devices, REBOA has become increasingly common in civilian trauma and acute care settings. As is common with military trauma innovations, REBOA has found use in civilian trauma, highlighting the benefit of military trauma innovation to civilian trauma patients. The use of this technique and purpose-built devices has also found application in non-traumatic bleeding, with reports of use in peripartum hemorrhage, gastrointestinal bleeding, tumor resection, and iatrogenic bleeding.
REBOA involves the retrograde advancement of a balloon catheter through the common femoral artery (CFA) to fully occlude the descending aorta allowing the surgeon the time needed to obtain definitive hemorrhage control. Based on the location of the hemorrhage, the balloon can be inflated in the supradiaphragmatic aortic Zone I, extending from the left subclavian artery to the celiac trunk, or in aortic Zone III, which extends from the lowest renal artery to the aortic bifurcation. The most critical limitation of complete aortic occlusion with this procedure is prolonged occlusion times. Longer occlusion generates progressive ischemic effects on downstream tissues and organs and ischemia-reperfusion injuries. Clinical practice guidelines recommend that occlusion times should not exceed 30-60 min for complete Zone I occlusion1,2, as longer occlusion times are associated with an increased risk of ischemic complications and associated reperfusion sequelae, including rebound hypotension and ischemia-reperfusion injury3.
Partial aortic occlusion, in which low volume aortic flow is permitted distal to occlusion, has been proposed as a technique to alleviate the ischemic consequences of complete occlusion. Preclinical literature indicates that compared to complete occlusion, partial occlusion reduces biomarker evidence of organ injury (e.g., lactate, K+, creatinine, pH), reduces adjunct resuscitation requirements (e.g., norepinephrine, bicarbonate)4,5,6, and increases survival with prolonged occlusion times6. Additionally, preliminary clinical literature has demonstrated the benefits of partial occlusion and the feasibility of implementing partial occlusion in a clinical setting. Specifically, University of Maryland Shock Trauma performed a retrospective review of patients treated with Zone 1 partial or complete REBOA. Compared to complete occlusion, partial occlusion significantly reduced vasoactive support requirements and increased the number of patients discharged home in cases requiring >30 min of occlusion, with a trend toward reduction of organ failure and reduced organ support needs7. This suggests that partial REBOA may help to mitigate ischemia and reperfusion injuries, especially in cases requiring prolonged occlusion times7,8. These benefits may also extend to scenarios that are more prone to requiring longer occlusion times, such as austere environments9 and military trauma field care and en route care10.
Due to the nature of compliant balloon technology, standard endovascular balloon occlusion functions in a binary fashion; a small change in balloon volume triggers a significant change in blood flow around the balloon. The result is that the vessel is either completely occluded and distal flow drops to zero, or it is not occluded and near-normal flow resumes. Although gradual transition is possible with existing catheters in the hands of experienced and well-resourced users11, it is difficult to achieve with this current technology as it requires frequent manipulation to maintain the desired level of partial aortic occlusion. The third-generation pREBOA-PRO catheter is currently FDA-cleared and in limited market release at seven Level I trauma centers in North America. It is the first catheter specifically designed to address the limitations of existing technology by enabling partial occlusion with a unique semicompliant balloon design incorporating flow channels that allow for precise control of aortic occlusion to facilitate the balance between hemorrhage control, hemodynamic stability, and distal perfusion. Additionally, the improved control may allow for a gradual transition to reperfusion, presumably avoiding precipitous changes in hemodynamics that complicate resuscitation efforts. This protocol will focus on procedural considerations for partial REBOA, including patient selection criteria and a comparison of complete and partial aortic occlusion with the pREBOA-PRO catheter (herein referred to as a dedicated partial REBOA catheter) in a simulator. Critical steps associated with optimized clinical outcomes using the dedicated partial REBOA catheter will be highlighted. Additionally, a contrast-enhanced CT scan from a trauma patient showing distal perfusion after 2 h of partial aortic occlusion using the dedicated partial REBOA catheter will be reviewed and representative results from the initial uses are discussed.
Protocol
Surgeons are using an FDA-approved REBOA device in trauma patients when it is medically necessary. These data were reviewed by the IRB committee of Grant Medical Center/OhioHealth and were determined to be exempt from human subjects' research. Since no patient information is obtained, written consent from the patients is not required. See the Table of Materials for details about the materials and equipment used in this protocol.
1. Common femoral arterial access
- Locate the common femoral artery (CFA) using percutaneous landmarks and ultrasound guidance12.
- Look for one or more of the following signs in the patient: i) systolic blood pressure (SBP) <90 mmHg; ii) transient or non-responder to transfusion; iii) profound refractory shock; iv) out-of-hospital cardiac arrest with return of spontaneous circulation.
NOTE: Establishing CFA access also allows for quick upsizing if occlusion becomes necessary, reducing the time it takes to achieve aortic occlusion13,14.
- Look for one or more of the following signs in the patient: i) systolic blood pressure (SBP) <90 mmHg; ii) transient or non-responder to transfusion; iii) profound refractory shock; iv) out-of-hospital cardiac arrest with return of spontaneous circulation.
- For safe arterial access, use the modified Seldinger technique: use a needle to puncture the ventral arterial wall of the CFA at a 45° angle. Insert a compatible guidewire through the needle into the artery and remove the needle. Place the 4 Fr sheath with dilator firmly in place over the wire and into the artery. Remove the wire and dilator, leaving the sheath in place15.
- Transduce the CFA arterial line, verify the waveform, and transduce the pressure to confirm arterial placement.
2. REBOA procedure
- When REBOA is indicated, have the team prepare the second arterial line. Label one arterial line Proximal and the other arterial line Distal.
NOTE: Dual channel arterial lines help guide resuscitation and optimize partial occlusion (Figure 1).- Identify that REBOA is needed when any of the following conditions are met.
- Look for patients with penetrating or blunt injury who are hypotensive (SBP < 90) and do not respond sufficiently to initial resuscitation with 1 or 2 units of whole blood (or 1:1:1 component therapy) in the trauma bay.
- Look for patients with non-traumatic hemorrhage who are profoundly hypotensive and require blood transfusion to maintain SBP > 90.
- Look for patients who are in arrest but are not beyond salvage according to ATLS guidelines for patients in hypovolemic cardiac arrest1.
- Identify that REBOA is needed when any of the following conditions are met.
- Using Seldinger technique with an 0.035 inch wire, upsize to a compatible 7 French sheath.
- Prepare the catheter according to the manufacturer's instructions:
- Using a 30 mL syringe with normal saline, prime the safety valve while leaving the orange peel-away in place.
NOTE: It is not necessary to inspect the balloon prior to insertion as all balloons are tested during the manufacturing process. Readvancement of the peel-away over the balloon for insertion is difficult and creates unnecessary, time-consuming steps to reassemble the device. - Pull the vacuum to evacuate the air and close the stopcock.
- Advance the orange peel-away to straighten and cover atraumatic P-tip for insertion.
- Connect the proximal arterial line to the ART port on the device (Figure 2) and flush. Connect the distal arterial line to the side arm of the sheath.
- Using a 30 mL syringe with normal saline, prime the safety valve while leaving the orange peel-away in place.
- Perform REBOA
- Insert the orange peel-away into the hemostasis valve on sheath ~5 mm until it stops, and advance the dedicated partial occlusion catheter to the desired aortic zone using the zone markers and measurements on the catheter.
- Remove the orange peel-away from the hemostasis valve.
- Flush both arterial lines after placement of the catheter.
- If available, use imaging, such as x-ray or fluoroscopy, to confirm placement of the balloon prior to inflation. Use radiopaque markers integrated into the catheter on either end of the balloon as references for placement.
- Using the patient's physiological response as a guide, slowly inflate the balloon with saline using a 30 mL syringe. Inflate to support a proximal systolic blood pressure target of 100-130 mmHg.
- To perform partial REBOA, verify the presence of pulsatile flow on the distal arterial waveform measured from the sheath to ensure partial occlusion.
NOTE: Non-pulsatile flow indicates complete aortic occlusion is being performed, even if the SBP is non-zero. Titration of distal pressure is a secondary consideration when performing partial occlusion, with a target SBP of 20-50 mmHg when possible. - If complete occlusion is required, continue inflating until non-pulsatile flow is observed on the distal arterial line.
- To perform partial REBOA, verify the presence of pulsatile flow on the distal arterial waveform measured from the sheath to ensure partial occlusion.
- Secure the device near the sheath with the securement clip.
- Provide definitive hemorrhage control.
- Identify and control the source of hemorrhage using appropriate surgical techniques and imaging when clinically indicated. Use clinical judgment to determine the value and risks of additional imaging.
NOTE: Contrast-enhanced imaging is feasible while leaving the balloon at partial occlusion to maintain hemodynamic stability while allowing blood and contrast flow past the balloon16 (shown in Video 1). Imaging is associated with a 47% increase in survival at 24 h and a 65% increase in survival at 28 days, despite longer time to initiate surgical hemostasis17. - Monitor the patient's vital signs throughout the procedure.
NOTE: If a radial arterial line is obtained in the operating room, the SBP will likely be higher than the integrated central aortic pressure monitoring from the catheter due to pulse pressure amplification18. - Obtain laboratory measures as clinically indicated, including blood gas analysis.
NOTE: Do not draw blood from the arterial port on the catheter as the catheter's length requires a large flush volume and may clot if not completely flushed after a blood draw.
- Identify and control the source of hemorrhage using appropriate surgical techniques and imaging when clinically indicated. Use clinical judgment to determine the value and risks of additional imaging.
- Remove REBOA.
- Deflate the balloon slowly and monitor the patient's response.
NOTE: Gradual deflation facilitates an improved transition to reperfusion. - If needed, advance a guidewire through the catheter and leave it in place for additional procedures such as endovascular coils for hemorrhage control.
NOTE: A guidewire in the catheter is incompatible with pressure monitoring. - Deflate the balloon using a 30 mL syringe and pull a strong vacuum to ensure complete evacuation of balloon volume; then close the stopcock. Remove the catheter and begin rotating at the 20 cm mark to wrap the balloon around the catheter shaft so it fits through the sheath more easily.
- Do not use excessive force to remove the catheter through the sheath. If resistance is encountered, readvance the catheter to a safe zone of occlusion and reinflate briefly to redistribute the balloon material. Perform the previous removal step again, ensuring a strong vacuum and frequent twisting upon removal.
NOTE: This balloon has more surface area than previous balloons due to the flow channels, so the fit through the sheath will be tighter.
- Do not use excessive force to remove the catheter through the sheath. If resistance is encountered, readvance the catheter to a safe zone of occlusion and reinflate briefly to redistribute the balloon material. Perform the previous removal step again, ensuring a strong vacuum and frequent twisting upon removal.
- Deflate the balloon slowly and monitor the patient's response.
3. Post-REBOA sheath management and removal
- Remove the sheath as soon as possible following the occlusion procedure.
NOTE: If there is no plan for subsequent procedure(s) with the 7 French sheath or an emergent care requirement that must be completed as soon as possible, proceed with removal of the sheath. If it is necessary to leave the sheath in place, the sheath must be managed until it is removed. - Sheath management
- While the sheath is in place, transduce and infuse it with crystalloid or flush it regularly to minimize the risk of thrombosis.
- Conduct hourly vascular checks to assess bilateral pulses; consider Doppler ultrasound and ankle-brachial index measurements19.
NOTE: If the patient is transferred, ensure sheath management is handed over and discuss who will remove the sheath.
- Sheath removal
- Assess coagulopathy using conventional coagulation assays such as prothrombin time, or viscoelastic assays, including thrombelastography (TEG) and rotational thrombelastometry (ROTEM)20.
NOTE: If the patient is coagulopathic, consider reversing coagulopathy prior to sheath removal or surgical repair of the arteriotomy after sheath removal. - Check bilateral lower extremity pulses to verify full and equal pulses.
NOTE: If diminished pulse is noted, notify the attending trauma surgeon, and consider duplex ultrasound or CT angiogram to determine the best course of action to restore function. - Remove the sheath and close by direct pressure for 30 min or use a closure device per institution policy and physician preference. Ensure the patient is placed on bedrest with affected leg straight for 6 h following sheath removal.
- Perform regular monitoring of the access site21: visualize the site, assess neurovascular function, and monitor vascular function with distal pulse checks and Doppler ultrasound. Monitor the patient every hour for 4 h, and then every 6 h for the next 24 h. If diminished vascular or neurovascular function is noted during any of these checks, immediately notify the attending trauma surgeon, and consider duplex ultrasound or CT angiogram to determine the best course of action to restore function.
NOTE: Consider duplex ultrasound 24 h after sheath removal to proactively assess vascular function after sheath removal.
- Assess coagulopathy using conventional coagulation assays such as prothrombin time, or viscoelastic assays, including thrombelastography (TEG) and rotational thrombelastometry (ROTEM)20.
Representative Results
Currently, the described dedicated partial REBOA catheter is the only FDA-approved catheter designed specifically to enable partial occlusion and is in limited market release at 7 Level I trauma centers in North America. The data provided are qualitative surgeon impressions from experienced REBOA users, but no quantitative measurements are obtained as part of this effort. Caution is warranted when interpreting these initial results; similar outcomes may not be observed when using alternative REBOA devices to deliver partial occlusion.
The third-generation, dedicated, partial REBOA catheter is designed specifically to enable partial occlusion while maintaining many of the key features of the second-generation, complete-occlusion catheters, including integrated blood pressure monitoring and image-free and wire-free use. Additionally, it provides significantly improved precision control of occlusion to deliver easily titratable partial aortic occlusion and improve the transition to reperfusion (Video 2). One major clinical benefit of the dedicated partial REBOA catheter is the ability to precisely control the extent of aortic occlusion to allow perfusion past the balloon, as seen on the CT angiogram performed after 2 h of partial aortic occlusion (Video 1). On the CT, partial occlusion is being performed during contrast-enhanced imaging. Distal perfusion should be monitored in real time by ensuring that distal pulses are present via the arterial line or other appropriate method.
Although zone 1 provides maximal hemodynamic support22, complete occlusion in zone 1 has been associated with short occlusion times to mitigate physiologic hazards. The data show that partial occlusion has increased the use of zone 1 occlusion (80% compared to 67% reported in the AORTA database from 2017 to the present), and that partial REBOA is associated with specific features compared to complete occlusion. As shown in Table 1, compared to complete occlusion, partial occlusion significantly increases the following observed benefits: improved transition to reperfusion (56.9% vs 0%), extension of safe occlusion time (47.1% vs 5.6%), reduction of distal ischemia (39.2% vs 5.6%), with a trend toward reduction of proximal hypertension (21.6% vs 0%). As expected, the observations surrounding reduced interoperative bleeding and reduced blood use are not different between the two occlusion strategies. It should be noted that there may be differences in baseline patient physiology (e.g., SBP, injury severity) between the two occlusion strategies, as the surgeons report patient intolerance of partial REBOA as the main reason for using complete REBOA exclusively (85.7%, n = 12 of 14 respondents).
Additionally, in cases requiring extended (≥30 min) occlusion time, surgeons have observed reduction of distal ischemia (47.5% vs 8.7%) and extension of safe occlusion time (50.0% vs 21.7%) at significantly higher rates than when occlusion times are <30 min (Figure 3). There were six cases where occlusion time was not reported and were eliminated from this analysis. One limitation of these initial observed benefits is the use of subjective observations based on clinical experience and expertise of the surgeons, as these items are not quantitatively measured. Quantification of physiological responses to partial occlusion in the clinical setting would benefit the field.
In summary, partial REBOA significantly increases the observation of improved transition to reperfusion, extension of safe occlusion time, and reduction of distal ischemia compared to complete occlusion (Table 1). Additionally, surgeons report reduction of distal ischemia and extension of safe occlusion time significantly more often in cases requiring ≥30 min of occlusion compared to cases of shorter occlusion length (Figure 3). These benefits are associated with partial REBOA, which is characterized by blood flow distal to aortic occlusion (Video 1).
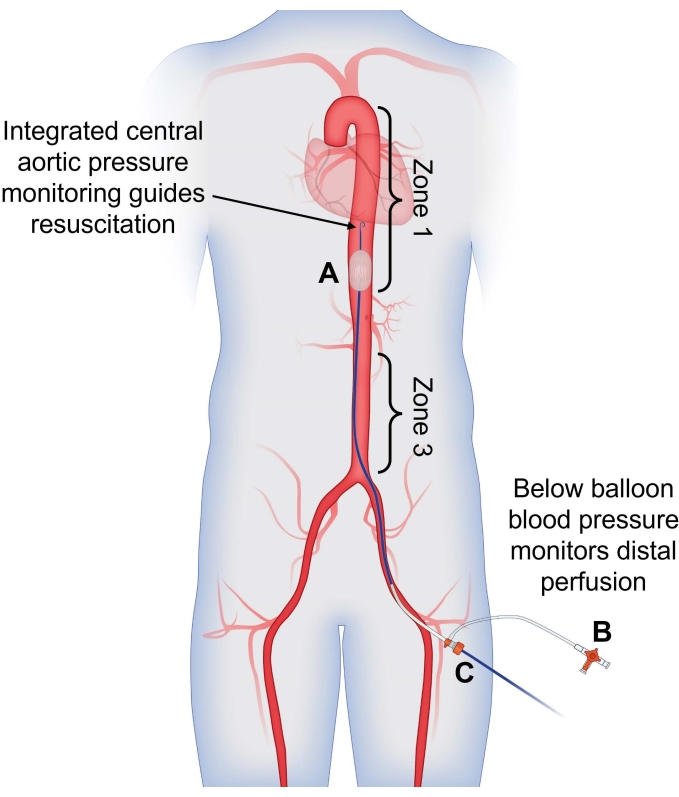
Figure 1: Use of dual arterial blood pressure monitoring to guide partial REBOA. (A) Partial REBOA is performed using the flow channels incorporated into the semicompliant balloon. Integrated central aortic pressure monitoring from the tip of the catheter is used to guide resuscitation and assess the patient's response to partial occlusion, providing a blood pressure reading above the site of occlusion. (B) Arterial pressure measured from the CFA sheath is used to assess distal perfusion; presence of pulsatile arterial flow indicates that partial occlusion is being performed. (C) The catheter is placed through the CFA sheath for a singular access site with dual-channel pressure-monitoring capabilities. Abbreviations: REBOA = Resuscitative endovascular balloon occlusion of the aorta; CFA = common femoral artery. Please click here to view a larger version of this figure.
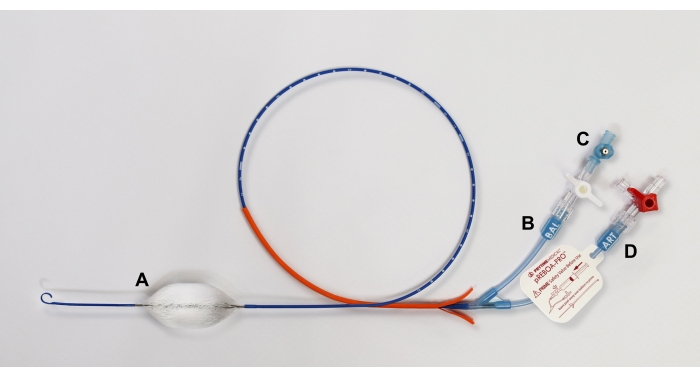
Figure 2: Diagram of pREBOA-PRO catheter and its features. The catheter is characterized by (A) a semicompliant prune balloon that forms flow channels when apposed to the aortic wall, enabling partial REBOA. The catheter includes two extension lines: (B) BAL for balloon inflation with (C) an integrated safety valve to prevent overinflation of the balloon and (D) ART with an integrated arterial line to measure central aortic pressure. Abbreviation: REBOA = Resuscitative endovascular balloon occlusion of the aorta. Please click here to view a larger version of this figure.
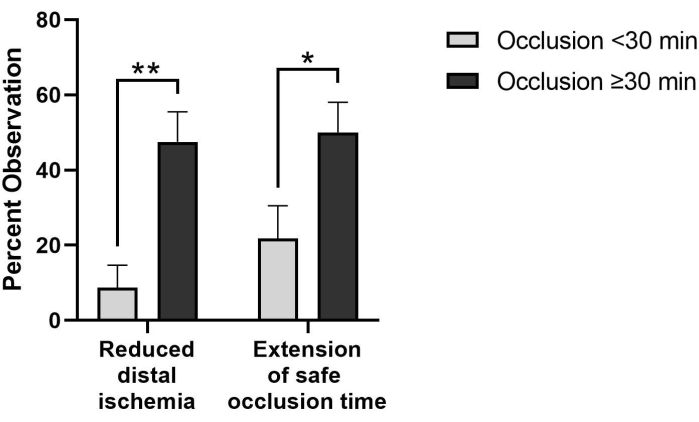
Figure 3: Perfusion-related benefits are observed more frequently in pREBOA-PRO cases requiring extended occlusion times (≥30 min, n = 40) compared to shorter occlusion (<30 min, n = 23). In cases with occlusion times greater than 30 min, reduced distal ischemia and extension of safe occlusion time are observed significantly more frequently than in cases with shorter occlusion times. * indicates p < 0.05, ** indicates p < 0.01 via Fisher's exact test. Data represent mean ± SEM. Please click here to view a larger version of this figure.
Video 1: Partial REBOA observed on CT angiogram with perfusion distal to aortic occlusion after 2 h of partial REBOA in zone 1. The patient was in a motor vehicle collision and was persistently hypotensive upon presentation to the trauma bay with a positive FAST exam, suggestive of abdominal hemorrhage. Partial REBOA was deployed in zone 1, and the patient was taken to the operating room for exploratory laparotomy. As no significant sources of bleeding were identified, a full-body contrast-enhanced CT scan was performed after 2 h of partial aortic occlusion. Partial occlusion was performed during the scan, which allowed contrast to pass, while maintaining hemodynamic stability. Abbreviations: REBOA = Resuscitative endovascular balloon occlusion of the aorta; CT = computed tomography; FAST = focused assessment with sonography in trauma. Please click here to download this Video.
Video 2: Side-by-side comparison of REBOA balloon deflation demonstrating improved transition to reperfusion. Both REBOA balloons are inflated to complete occlusion in a silicone tube with 19 mm inner diameter, simulating zone 1 aortic occlusion. Complete aortic occlusion, evidenced by a non-pulsatile waveform from the distal blood pressure, can be observed on the monitors. The balloons are deflated simultaneously at 0.2 cc/s using a syringe puller. The ER-REBOA catheter (left) has a transition volume of 2.3 cc, while pREBOA-PRO (right) has a transition volume of 9.4 cc. The increased transition volume of the dedicated partial occlusion device provides increased control of aortic occlusion and reperfusion. Abbreviation: REBOA = Resuscitative endovascular balloon occlusion of the aorta. Please click here to download this Video.
| Observed benefit: | Partial REBOA N=51 % (n) | Complete REBOA N=18 % (n) | p value | |
| Improved transition to reperfusion | 56.9 (29) | 0 (0) | *p=0.00001 | |
| Extension of safe occlusion time | 47.1 (24) | 5.6 (1) | *p=0.001 | |
| Reduced distal ischemia | 39.2 (20) | 5.6 (1) | *p=0.007 | |
| Reduced proximal hypertension | 21.6 (11) | 0 (0) | +p=0.05 | |
| Reduced interoperative bleeding | 45.1 (23) | 22.2 (4) | p=0.10 | |
| Reduced blood use | 33.3 (17) | 11.1 (2) | p=0.12 | |
Table 1: Observed benefits in cases utilizing partial REBOA at any point during the case compared to cases utilizing complete aortic occlusion only. Fisher's exact test was used to determine whether response rates for observed benefits differed by aortic occlusion strategy (partial or complete). Exact p values are reported, with * indicating p < 0.05 and + indicating p = 0.05.
Discussion
Critical steps
Partial REBOA is a technique that can balance effective control of emergent hemorrhage with mitigation of distal ischemia and reperfusion injury and reduction of supraphysiologic proximal blood pressure upon occlusion. Prior to the dedicated partial REBOA catheter, partial occlusion could only be performed by skilled users11 and required frequent manipulation of balloon volume to maintain the desired level of partial aortic occlusion. The new balloon occlusion technology has integrated flow channels that when apposed to the wall of the aorta, allow a controlled amount of perfusion past the balloon. Dual-channel pressure-monitoring proximal (integrated into the catheter) and distal (side arm of the sheath) allows for pressure-guided therapy during key phases of resuscitation (Figure 1) and safer, more effective delivery of partial REBOA to optimize distal perfusion.
This catheter is currently in limited market release at seven Level I trauma centers in North America, which has illuminated some critical steps in the implementation of occlusion procedures, several of which occur prior to utilizing the device. The first step is to establish and implement hospital or departmental guidelines and/or protocols for obtaining early arterial access on all hypotensive trauma patients. Central aortic blood pressure provides superior hemodynamic monitoring for patients in shock compared to a blood pressure cuff23, which helps to guide resuscitation and assess the need for intervention more accurately. Arterial access also facilitates quick upsizing to a 7 French sheath if progression to REBOA is indicated13,14. The next step is to implement a guideline that outlines patient selection criteria and indications for use of REBOA. It is important to consider early intervention, as higher SBPs upon initiation are associated with improved outcomes24. Guidelines for post REBOA sheath management and routine access site monitoring should also be implemented. Training for all surgeons and staff should be conducted so that they are familiar with their roles and responsibilities during the REBOA procedure. Since dual arterial lines are necessary for blood pressure monitoring proximal (integrated into the catheter) and distal (integrated in the side arm of the sheath) to the site of occlusion, staff should be trained in the timely preparation of dual arterial lines.
Modifications and troubleshooting of the method
Modifications from this protocol in certain areas are acceptable if there are standardized guidelines/protocols in place at the institution. For instance, there is some variance in preferred initial sheath size for obtaining arterial access, with most utilizing a 4, 5, or 7 French sheath or an 18 G micropuncture kit. Arterial lines smaller than 18 G are not recommended because they are not compatible with the 0.035 inch guidewire required to upsize to a 7 French sheath if REBOA is indicated13. Arterial line alternatives, such as handheld pressure transducer devices25, may also be considered, particularly in low-resource or austere environments.
Limitations of the method
Since the dedicated partial REBOA catheter allows for precise control of aortic occlusion, the degree of occlusion should be titrated to support a desired proximal blood pressure in the range of SBP 100-130 mmHg. A limitation of the partial occlusion technique is that some patients will not be able to tolerate partial REBOA and will require complete aortic occlusion to achieve hemodynamic stability. Although this catheter supports complete aortic occlusion, certain benefits of partial occlusion will not be observed in these instances (Table 1). Indicators of distal flow should be verified during occlusion to ensure partial occlusion is being performed.
True partial REBOA
Partial REBOA is an evolution of existing REBOA technology that addresses the intractable clinical problem of distal ischemia and ischemia-reperfusion injury associated with complete aortic occlusion. A dedicated partial REBOA catheter facilitates partial occlusion with minimal provider titration4. There has been increasing utilization of partial REBOA (73% of 80 cases at the Centers of Excellence) as compared to the AORTA database (3% of the 125 cases with occlusion strategy specified, data as of November 2021). The major changes arising from partial occlusion are improved transition to reperfusion and reduction of distal ischemia, especially when occlusion times >30 min are required to complete definitive hemorrhage control. One major change observed in the initial uses from the surgeons at the Centers of Excellence is that there is a significant increase in Zone 1 occlusion with the dedicated partial REBOA catheter (73% of 80 cases at the centers) compared to the AORTA database (67% of 686 cases from the emergence of the 7 French-compatible devices in 2017 to present). Partial REBOA offers the maximal hemodynamic support benefits of Zone 1 occlusion22, while reducing concerns regarding limited safe occlusion time due to distal ischemia4,5,6,7,8.
Although preclinical benefits of partial occlusion have been demonstrated, objective clinical measurements have yet to be obtained. Subjective, surgeon-reported feedback indicates that partial REBOA facilitates specific benefits that are not observed with complete occlusion, including improved transition to reperfusion, extension of safe occlusion time, reduction of distal ischemia, and reduced proximal hypertension (Table 1). Furthermore, surgeons report observed benefits past 30 min, including reduction of distal ischemia and extension of safe occlusion time in 48% and 50% of cases with ≥30 min of occlusion, respectively (Figure 3). Although the observed benefits with a dedicated partial REBOA catheter have yet to be quantified clinically, the limited-market release data indicates the ability to perform partial REBOA represents a significant improvement over previous occlusion capabilities and addresses the clinical need to optimize distal perfusion, especially in cases with prolonged occlusion time.
Divulgaciones
The authors have nothing to disclose.
Acknowledgements
The data presented in this article were obtained as part of a process and quality improvement initiative in collaboration with Prytime Medical to optimize the new partial REBOA technology.
Materials
| IV Pole | Any | Arterial Line Setup | |
| Jelly | Any | Ultrasound for Vascular Access | |
| Micropuncture kit | Cook | MPIS-405-SST | 21 G needle for vascular access, 4 Fr x 10 cm arterial sheath |
| Non-compliant pressure tubing, 2 | Any | Arterial Line Setup | |
| Normal saline, 2x 500 mL | Any | Arterial Line Setup | |
| pREBOA-PRO Catheter | Prytime Medical | PRP7226PRO | Partial REBOA Catheter |
| Pressure bag, 2 | Any | Arterial Line Setup | |
| Probe cover | Any | Ultrasound for Vascular Access | |
| Probe for vascular access | Any | Ultrasound for Vascular Access | |
| REBOA Catheter Convenience Set | Prytime Medical | KT1835C (US) KT1835E (EU) KT1835CAN (Can) |
18 G needle, 7 Fr introducer sheath, 4x 10 cc saline syringe, 30 cc syringe, scalpel, 2-0 suture, three quarter drape, catheter securement device |
| Transducers with extension lines, 2 | Any | Arterial Line Setup | |
| Ultrasound machine | Any | Ultrasound for Vascular Access | |
| Vital sign monitor – dual channel BP capable | Any | Arterial Line Setup, displays blood pressure from ART lines above and below the balloon |
Referencias
- Cannon, J., et al. Resuscitative Endovascular Balloon Occlusion of the Aorta (REBOA) for hemorrhagic shock. Military Medicine. 183, 55-59 (2018).
- Bulger, E. M., et al. Clinical use of resuscitative endovascular balloon occlusion of the aorta (REBOA) in civilian trauma systems in the USA, 2019: a joint statement from the American College of Surgeons Committee on Trauma, the American College of Emergency Physicians, the National Association of Emergency Medical Services Physicians and the National Association of Emergency Medical Technicians. Trauma Surgery & Acute Care Open. 4 (1), 000376 (2019).
- Moore, L. J., et al. Prospective observational evaluation of the ER-REBOA catheter at 6 U.S. trauma centers. Annals of Surgery. 275 (2), 520-526 (2020).
- Kemp, M. T., et al. A novel partial resuscitative endovascular balloon aortic occlusion device that can be deployed in zone 1 for more than 2 hours with minimal provider titration. Journal of Trauma and Acute Care Surgery. 90 (3), 426-433 (2021).
- Sadeghi, M., et al. Blood pressure targeting by partial REBOA is possible in severe hemorrhagic shock in pigs and produces less circulatory, metabolic and inflammatory sequelae than total REBOA. Injury. 49 (12), 2132-2141 (2018).
- Forte, D., et al. Validation of a novel partial Reboa device in a swine hemorrhagic shock model: Fine tuning flow to optimize bleeding control and reperfusion injury. Journal of Trauma and Acute Care Surgery. 89 (1), 58-67 (2020).
- Madurska, M. J., et al. A feasibility study of partial REBOA data in a high-volume trauma center. European Journal of Trauma and Emergency Surgery. 48 (1), 299-305 (2021).
- Russo, R. M., White, J. M., Baer, D. G. Partial REBOA: A systematic review of the preclinical and clinical literature. Journal of Surgical Research. 262, 101-114 (2021).
- de Schoutheete, J. C., et al. Three cases of resuscitative endovascular balloon occlusion of the aorta (REBOA) in austere pre-hospital environment-technical and methodological aspects. World Journal of Emergency Surgery. 13, 54 (2018).
- Reva, V. A., et al. Field and en route resuscitative endovascular occlusion of the aorta: A feasible military reality. Journal of Trauma and Acute Care Surgery. 83, 170-176 (2017).
- DuBose, J. J. How I do it: Partial resuscitative endovascular balloon occlusion of the aorta (P-REBOA). Journal of Trauma and Acute Care Surgery. 83 (1), 197-199 (2017).
- Bangalore, S., Bhatt, D. L. Femoral arterial access and closure. Circulation. 124 (5), 147-156 (2011).
- Vernamonti, J. P., et al. Step Up’ approach to the application of REBOA technology in a rural trauma system. Trauma Surgery & Acute Care Open. 4 (1), 000335 (2019).
- Romagnoli, A., et al. Time to aortic occlusion: It’s all about access. Journal of Trauma and Acute Care Surgery. 83 (6), 1161-1164 (2017).
- Stannard, A., Eliason, J. L., Rasmussen, T. E. Resuscitative endovascular balloon occlusion of the aorta (REBOA) as an adjunct for hemorrhagic shock. Journal of Trauma. 71 (6), 1869-1872 (2011).
- Madurska, M. J., Jansen, J. O., Reva, V. A., Mirghani, M., Morrison, J. J. The compatibility of computed tomography scanning and partial REBOA: A large animal pilot study. Journal of Trauma and Acute Care Surgery. 83 (3), 557-561 (2017).
- Otsuka, H., et al. Is resuscitative endovascular balloon occlusion of the aorta for computed tomography diagnosis feasible or not? A Japanese single-center, retrospective, observational study. Journal of Trauma and Acute Care Surgery. 91 (2), 287-294 (2021).
- McEniery, C. M., Cockcroft, J. R., Roman, M. J., Franklin, S. S., Wilkinson, I. B. Central blood pressure: current evidence and clinical importance. European Heart Journal. 35 (26), 1719-1725 (2014).
- Aboyans, V., et al. Measurement and interpretation of the ankle-brachial index: a scientific statement from the American Heart Association. Circulation. 126 (24), 2890-2909 (2012).
- Gonzalez, E., Moore, E. E., Moore, H. B. Management of trauma-induced coagulopathy with thrombelastography. Critical Care Clinics. 33 (1), 119-134 (2017).
- Romagnoli, A., Brenner, M. . Principles of REBOA. in Hot Topics in Acute Care Surgery and Trauma. Endovascular Resuscitation and Trauma Management: Bleeding and Haemodynamic Control. , (2019).
- Beyer, C. A., Johnson, M. A., Galante, J. M., DuBose, J. J. Zones matter: Hemodynamic effects of zone 1 vs zone 3 resuscitative endovascular balloon occlusion of the aorta placement in trauma patients. Injury. 50 (4), 855-858 (2019).
- Meidert, A. S., et al. Oscillometric versus invasive blood pressure measurement in patients with shock: a prospective observational study in the emergency department. Journal of Clinical Monitoring and Computing. 35 (2), 387-393 (2021).
- Cralley, A. L., et al. Predicting success of resuscitative endovascular occlusion of the aorta: Timing supersedes variable techniques in predicting patient survival. Journal of Trauma and Acute Care Surgery. 91 (3), 473-479 (2021).
- Holtestaul, T., et al. REBOA management guided by a novel handheld pressure transducer. Journal of Trauma and Acute Care Surgery. 92 (4), 729-734 (2021).

