Generation of Human Patient iPSC-derived Retinal Organoids to Model Retinitis Pigmentosa
Summary
In this protocol, retinitis pigmentosa patient induced pluripotent stem cell (iPSC)-derived 3D retinal organoids were generated. Those organoids successfully recapitulated some clinical phenotypes of the retinitis pigmentosa disease.
Abstract
Retinitis pigmentosa (RP) is a rare and inherited retinal degenerative disease with a prevalence of approximately 1/4,000 people worldwide. The majority of RP patients have progressive photoreceptor degeneration leading to peripheral vision loss, night blindness, and finally, total blindness. To date, thousands of mutations in more than 90 genes have been reported to be associated with RP. Currently, there are few animal models available for all the affected genes and different types of mutations, which largely hampers the deciphering of the mechanisms underlying the gene/mutation pathology and limits treatment and drug development. Patient induced pluripotent stem cell (iPSC)-derived 3D retinal organoids (ROs) have provided a better system to model the human early-onset disease than cells and animals. In order to study RP, those patient-derived 3D retinal organoids were utilized to recapitulate the clinical phenotypes of RP. In the RP patient-derived ROs, Rhodopsin mislocalization was clearly displayed. Compared with other animal models, patient iPSC-derived retinal organoid models more closely recapitulated RP features and represent an ideal approach for investigating the disease pathogenesis and for drug development.
Introduction
Human retinal diseases, such as retinitis pigmentosa and age-related macular degeneration, are poorly understood due to the lack of appropriate experimental models1,2. Although the mouse retina is very similar to the human retina and is a powerful tool for studying the etiology of retinal degeneration, there are huge species differences between mice and humans3,4. For instance, the nuclear architecture of the photoreceptor cells in mice and humans is different, and the mouse retina does not possess a macula5,6. Induced pluripotent stem cell (iPSC) technology enables us to return the specialized cells of organisms to the initial pluripotent state through the "reprogramming" processes by combinations of transcription factors and/or compounds7,8,9,10. Those iPSCs have nearly unlimited division and proliferation ability and could develop into various types of cells. Recently, iPSC-derived 3D retinal organoids have been developed to model the early events of human retinal development and to delineate the pathophysiology of human retinal diseases11,12,13,14,15. Retinal organoids have many advantages: (1) they can be used to recapitulate in vivo retinal development and disease pathogenesis; (2) they can be used for high-throughput drug screening and preclinical trials of gene therapy; and (3) they can be used as preclinical evaluations of treatment options for retinal degenerative diseases16,17.
One objective of this project was to study the pathogenesis of retinal pigmentosa (RP), a disease remaining incurable because of its extreme heterogeneity18. To date, over 90 genes have been identified to be associated with RP19,20. The RPGR gene, which is considered one of the most prevalent causative genes of RP15, accounts for approximately 16% of all RP4,21,22. iPSCs carrying a frameshift mutation in the RPGR gene have been successfully generated and differentiated into organized and stratified 3D retinal organoids14. By utilizing these organoids, abnormal photoreceptor layer morphology and the dislocation of opsins in photoreceptors were observed.
Altogether, a step-by-step and approachable protocol is described in detail here on how to generate patient-derived 3D retinal organoids23,24. Those organoids successfully recapitulated some clinical phenotypes of the disease. This provides an encouraging model to study retinal development and disease mechanisms, for therapeutic screening, and to evaluate future preclinical gene therapy.
Protocol
The protocol follows the guidelines of Capital Medical University's human research ethics committee.
1. Cell culture and generation of iPSCs
- Choose RPGR patients for this study. Here, three patients, one familial carrier and three healthy controls, were used. Patient 1 possessed a mutation c.1685_1686delAT in exon 14 of the RPGR gene, patient 2 harbored a mutation c.2234_2235delGA in exon 15 of the RPGR gene, and patient 3 had a mutation c.2403_2404delAG in exon 15 of the RPGR gene. Choose three healthy volunteers as controls14. Collect peripheral whole blood samples from the patients and healthy controls with venipuncture, and put them in blood collection tubes (Table of Materials).
- Isolate peripheral blood mononuclear cells (PBMCs) by density gradient centrifugation with a density gradient medium25. Expand CD34+ PBMCs activated with cytokine in 8 mL of growth enhancement medium (Table 1) in a Petri dish.
- After 1 week, electroporate the CD34-positive peripheral blood mononuclear cells with a transfection system (Table of Materials) and choose CD34-positive cells and the inbuilt T-01 Program without any modification. Transfect a cocktail of reprogramming plasmids (2 µg) that could produce human OCT4, SOX2, NANOG, LIN28, c-MYC, and KLF4 proteins.
- Seed the transfected cells in vitronectin human recombinant protein-coated six-well plates in 2 mL of DMEM/ F12 supplemented with B-27 (1x), N-2 (1x), PD0325901 (0.5 µM), bFGF (100 ng/mL), CHIR99021 (3 µM), HA-100 (10 µM) , A-83-01 (0.5 µM), and hLIF (1000 U/mL) to maintain the growth of the iPSCs.
- 3 weeks after transduction, pick the colonies of iPSCs manually, subsequently transfer them into six-well plates, and maintain them in reagent B (Table of Materials) for expansion.
NOTE: The six-well plates should be coated with vitronectin human recombinant protein. Dilute vitronectin solution in DPBS to reach a final concentration of 10 µg/mL. Add the diluted vitronectin to tissue culture-treated six-well plates. Swirl the cultureware to spread the vitronectin solution evenly across the surface of the six-well plates. Incubate the plates at 37 °C for at least 1 h. - Ensure that cells are disaggregated with 0.5 mM EDTA when the iPSCs reach around 80%-90% confluence and that the splitting ratio is 1:8 or 1:10. On the day of passage, add blebbistatin (2.5 µM) to reagent B (Table 1).
NOTE: Blebbistatin was used to promote cell adhesion and stem cell viability and prevent stem cell apoptosis. 18 h later, the medium should be refreshed with reagent B.
2. Generation of human ROs
NOTE: The iPSCs must be dissociated when the iPSCs reach around 80%-90% confluence.
- Dissociate the human iPSC (hiPSC) colonies using 0.5 mM EDTA at 37 ◦C for 5 min.
- Segregate the hiPSC clumps into single cells by gentle pipetting. Count the cells for maintenance and grow them in reagent B (Table 1).
- On day 0, seed 12,000 cells/well in 96 V-bottomed conical wells for cell reaggregation in 100 µL of differentiation medium (Table 1).
- On day 6, carefully aspirate the cell culture medium and add 100 µL of the differentiation medium containing 20 μM Y 27632 and 1.5 nM (55 ng/mL) hBMP4.
- On day 9, replace half of the differentiation medium to maintain the hBMP4 concentration at around 0.75 nM.
- Aspire 60 µL of the differentiation medium from each well and add 60 µL of the fresh differentiation medium containing 20 μM Y-27632 to each well.
- On day 12, replace half of the differentiation medium with fresh differentiation medium to achieve 0.375 nM hBMP4.
- On day 18, transfer the aggregates into a new Petri dish with 10 mL tips and cut each aggregate into 2-4 pieces with a microsurgical knife under an inverted microscope to generate more organoids.
- Transfer the aggregates from the same group into one 15 mL centrifuge tube. When the aggregates settle down to the bottom of the tubes, aspirate the supernatant.
- Resuspend the aggregates with 1 mL of neural retina medium (DMEM/F12, 10% fetal bovine serum, 1% N-2 supplement, 0.5 µM retinoic acid, 0.1 mM taurine, 1% penicillin-streptomycin), and transfer them into non-stick Petri dishes with 15 mL of neural retina medium in it. Put 10-15 retinal organoids in one Petri dish.
- Refresh the culture medium every 5 days. Keep the culture in the dark since retinoic acid is sensitive to light (Figure 1) and check the formation of the self-organizing human retinal tissue with an inverted microscope26,27,28,29.
3. Analysis of retinal organoids
- Immunofluorescence staining of hiPSC-derived 3D retinae
- Fix the 3D retinae in 1 mL of fresh 4% paraformaldehyde in DPBS for 20 min at 4 °C. Then, rinse the organoids with 1 mL of DPBS buffer.
- Measure 0.5 g of agarose (Table of Materials), and mix the agarose powder with 100 mL of DPBS in a microwavable flask. Use a microwave to dissolve the agarose for 1-3 min until it is completely dissolved. Let the agarose solution cool down to about 40 °C.
- Embed them in 2% in DPBS.
- Slice the samples into sections with a thickness of 50 µm using a vibratome. Place the sliced sections on adhesion microscope slides (Table of Materials) and store them at −80 °C.
- Thaw the slices for 15 min at room temperature (RT).
- Wash the slices with DPBS for 5 min, and repeat 3x.
- Use 0.5% Triton X-100 in DPBS solution to permeabilize the slices for 15 min at RT.
- Remove the permeabilization solution, wash the slices with DPBS for 5 min, and repeat 3x.
- Incubate the slices with 1% BSA and 0.5% Triton X-100 in DPBS for 30 min at RT.
- Incubate the slices with anti-Rhodopsin antibody (1:1000) and anti-L/M opsin antibody (1:1000) diluted in 1% BSA and 0.1% Triton X-100 for 16 h at 4 °C.
- Wash the slices with DPBS for 5 min, and repeat 3x.
- Incubate the slices with donkey anti-mouse and rabbit secondary antibody (1:500) diluted in 1% BSA and 0.1% Triton X-100 for 1 h at RT.
- Add 30 nM 4',6-diamidino-2-phenylindole (DAPI) in DPBS for 15 min at RT for nuclear staining.
- Wash the slices with DPBS for 5 min, and repeat 5x.
- Mount the slices with embedding medium (Table of Materials) and cover the slides (Table of Materials). Keep in a dark box and store at 4 °C for up to 4 weeks.
- RNA extraction and RNA sequencing
- Collect 1-3 (according to sizes) 3D retinae derived from each control and patient at the same differentiation time points (day 0, day 47, day 91, day 121, and day 151) in 1 mL of RNA extraction reagent (Table of Materials) on ice.
- Use the homogenizer (Table of Materials) to disrupt the samples, 1 min ON, 30 s OFF, for three cycles, and check the sample for disruption.
- Stop the homogenization when the samples are totally homogenized, and vortex the tube vigorously for 30 s.
- Incubate the samples on ice for 15 min.
- Centrifuge the samples at 13,800 x g for 15 min at 4 °C and transfer the supernatant to a new 2 mL RNase-free tube.
- Add 0.4 mL of chloroform to the tube, vortex, and centrifuge at 13,800 x g for 10 min at 4 °C.
- Transfer a portion (about 2/3) of the colorless upper phase to a new RNase-free tube carefully. Do not aspirate or disturb the white interface containing DNA.
- Add 0.5 mL of cold isopropanol, vortex for 30 s, and centrifuge at 13,800 x g for 15 min at 4 °C. A pellet forms on the side of the tube bottom.
- Carefully aspirate the supernatant with a pipette tip and keep the pellet.
NOTE: The RNA pellet is jelly-like and transparent when the purity is very high. - Add 1 mL of cold 70% ethanol, vortex the samples vigorously for 30 s, and centrifuge at 13,800 x g for 10 min at 4 °C.
- Carefully aspirate the supernatant with a pipette tip and air-dry the pellet in the hood at RT for 5-10 min. Do not overdry the pellet.
- Add 20-50 µL of RNase-free water and resuspend the pellet carefully by pipetting up and down.
- Apply 1 µL of RNA on a spectrophotometer (Table of Materials). A total of around 10 µg of RNA from controls and patients respectively were used for Illumina library preparation30.
- Store the RNA at −80 °C.
Representative Results
The schematic illustration describes the differentiation procedures to generate healthy and patient iPSC-derived retinal organoids (Figure 1). From iPSC to ROs, variations can be produced owing to several factors. The status of the iPSC is the determinant step of the RO generation. In addition, it is highly recommended that researchers should record every step, catalog, and lot number of all media so that the entire experiments are trackable. In Figure 2A, the immunostaining of the rod marker Rhodopsin (red) and the L/M-cone marker L/M-opsin (green) in healthy and patient iPSC-derived retinal organoids at day 130 are presented. In patient iPSC-derived ROs, the cell morphology of ONL was disrupted, consistent with the clinical phenotype of RP. RNA-seq analysis was used to investigate the differences between the transcription factors of photoreceptors of the healthy and patient iPSC-derived retinal organoids at day 0, day 47, day 91, day 121, and day 151. The results showed that the expression of some of the transcription factors and maturation regulation genes of photoreceptors was much lower in the patient iPSCs-derived ROs than in control iPSCs-derived ROs. Of note, the expression of CRX, OPN1SW, OPN1MW, and Rhodopsin were higher in healthy retinae on day 91, day 121, and day 151 than the patient retinae (Figure 2B)14.
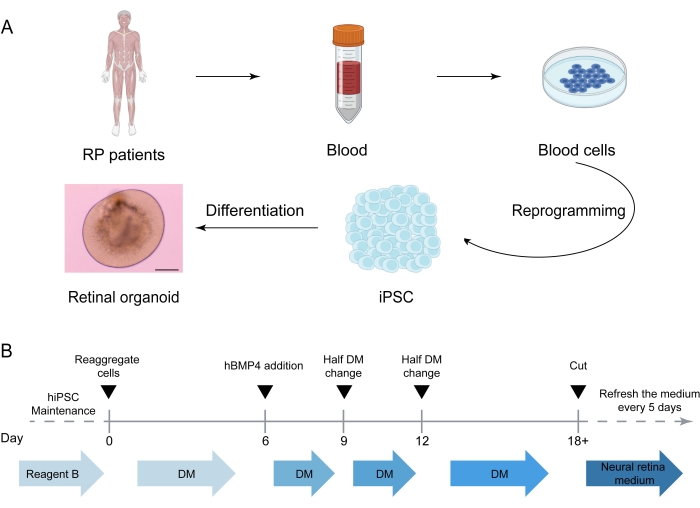
Figure 1: Generation of retinal organoids derived from healthy and patient iPSCs. (A) Generation of iPSC-derived human retinal organoids (Figures created in BioRender.com). Step 1: Choose RPGR patients and controls for this study. Step 2: Isolate peripheral blood mononuclear cells. Step 3: Reprogram the peripheral blood mononuclear cells to iPSCs. Step 4: Start retinal organoid differentiation. (B) Timeline for stepwise treatment for retinal organoid differentiation from iPSCs. iPSC, induced pluripotent stem cell; DM, differentiation medium. Scale bar = 400 µm. Please click here to view a larger version of this figure.
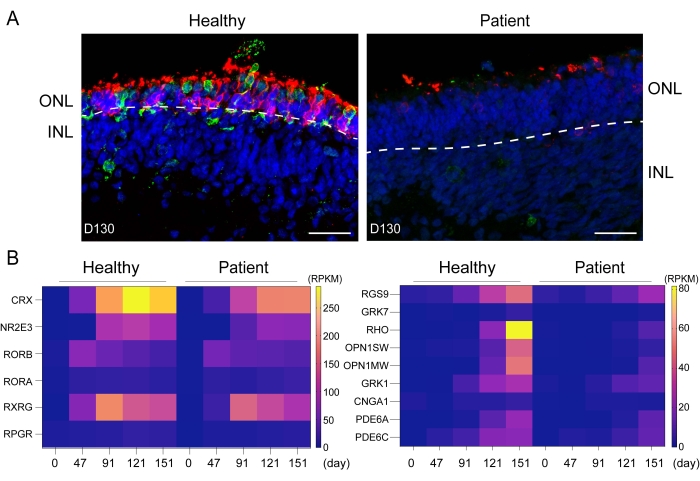
Figure 2: Generation of retinal organoids derived from healthy and patient iPSCs to model retinitis pigmentosa. (A) Immunostaining of the rod marker Rhodopsin (red) and the L/M-cone marker L/M-opsin (green) in healthy and patient iPSC-derived retinal organoids at day 130; (B) Heat maps displayed differences in the expression of key photoreceptor cell fate-determining transcription factors between the healthy and patient iPSC-derived retinal organoids at day 0, day 47, day 91, day 121, and day 151. Scale bars = 5 µm. ONL, outer nuclear layer; INL, inner nuclear layer; CRX, cone-rod homeobox; NR2E3, nuclear receptor subfamily 2 group E member 3; RORB, RAR-related orphan receptor B; RORB, RAR-related orphan receptor B; RORA, RAR-related orphan receptor A; RXRG, retinoid X receptor gamma; RPGR, retinitis pigmentosa GTPase regulator; RGS9, regulator of G protein signaling 9; GRK7, G protein-coupled receptor kinase 7; RHO, Rhodopsin; OPN1SW, opsin 1, short wave sensitive; OPN1MW, opsin 1, medium wave sensitive; GRK1, G protein-coupled receptor kinase 1; CNGA1, cyclic nucleotide gated channel subunit alpha 1; PDE6A, phosphodiesterase 6A; PDE6C, phosphodiesterase 6C. Please click here to view a larger version of this figure.
| Name | Composition | Concentration |
| Growth enhancement medium | Growth enhancement media supplement | |
| L-glutamine supplement | 2 mM | |
| Stem cell factor | 250 ng/mL | |
| FLT3L | 250 ng/mL | |
| Thrombopoietin | 100 ng/mL | |
| Interleukin (IL)-3 | 20 ng/mL | |
| IL-6 | 50 ng/mL | |
| Reagent B | Basal hpsc Medium | 99.2% |
| 125x Supplement | 0.8% | |
| Differentiation medium | Iscove’s Modified Dulbecco Medium | 44% |
| F12 | 44% | |
| Serum replacement media | 10% | |
| L-glutamine supplement | 1% | |
| Monothioglycerol | 450 μM | |
| Penicillin-streptomycin | 1% |
Table 1: Media composition. The table lists the constituents and their concentrations required to prepare growth enhancement medium, reagent B, and differentiation medium
Supplementary File 1: Troubleshooting. This part discusses common problems that can occur in the experiment, possible reasons, and the potential solutions. Please click here to download this File.
Discussion
Retinal organoids are 3D, laminated structures derived from hiPSCs or embryonic stem cells (ESCs) and feature as a very promising model to mimic the spatial and temporal patterns of human retinal development31,32. The ROs consist of various types of retinal cells, including photoreceptors, bipolar cells, ganglion cells, amacrine cells, horizontal cells, and Müller glia33. 2D culture cannot precisely mimic the orientation and development of the outer segment of photoreceptor cells. Although generating ROs is a lengthy process, which often takes more than 6 months of labor-intensive work, these 3D human retinal organoids are a good system since I) this in vitro approach has different neurodevelopment differentiation stages, and they are similar to their in vivo counterparts; importantly, II) they form well-structured and organized tissues containing various types of retinal cell types; and III) they make possible the genetic manipulations of a range of patient samples with various genetic backgrounds34,35.
RP is an extremely heterogeneous group of incurable retinal degenerative disorders with a prevalence of approximately 1/4,000 people36. The majority of RP patients are characterized by progressive rod photoreceptor degeneration and death, which lead to peripheral vision loss and night blindness37,38,39. With further disease advancement, the loss of cone photoreceptors results in blindness37,38,39. Thousands of mutations in more than 90 genes have been reported to be linked with RP19,20,40. Currently, there are no animal models available for all the affected genes and different types of mutations. This largely hampers the deciphering of the mechanisms underlying the gene/mutation pathology and limits treatment and drug development. In 2016 and 2017, Leber congenital amaurosis (LCA) models were developed by making use of patient iPSC-derived optic cups, which originated from fibroblasts41,42. In the current protocol, human iPSC-derived 3D retinal organoids were employed to recapitulate the clinical phenotype of RP. These organoids contain a well-structured photoreceptor cell layer and an outer segment-like structure. This model allows us to recapitulate the clinical phenotypes from RPGR mutations. Studies have already indicated that RPGR regulates the transportation of Rhodopsin22,43,44. In this protocol, Rhodopsin mislocalization was detected in the patient iPSC-derived ROs. Compared with animal models, the patient iPSC-derived retina models more closely recapitulated the RP features and performed as an ideal approach for understanding the disease pathogenesis and for drug screening45,46.
It should be noted that hiPSC passage number, hiPSC differentiation after colony isolation, experimental procedures, and the culture environment may result in low efficiency of the differentiation of the retinal organoids from iPSCs. The retinal organoids generated in this protocol do not have a vascular system. Therefore, for future applications, protocols should focus on how to improve the differentiation efficiency (Supplementary File 1) and generate a vascularized retinal organoid. Those might be of interest for future studies to investigate retinal disease.
In summary, some clinical RP phenotypes were successfully recapitulated by using RPGR mutation patient iPSC-derived retinae in a dish. The phenotypes in patient iPSC-derived 3D retinal organoids displayed a high level of consistency with the clinical phenotypes. This protocol may give support to the development of novel treatment strategies for RP and to the establishment of a pipeline for personalized medicine in RP.
Divulgaciones
The authors have nothing to disclose.
Acknowledgements
We thank M.S. Yan-ping Li and Zhuo-lin Liu for their technical support and helpful comments regarding the manuscript. This work was partly supported by the National Natural Science Foundation of China (82171470, 31871497, 81970838, Z20J00122), Beijing Municipal Natural Science Foundation (Z200014, 82125007), and National Key R&D Program of China (2017YFA0105300).
Materials
| 96 V-bottomed conical wells | Sumitomo Bakelite | MS-9096VZ | |
| A-83–01 | R&D Systems | 2939/10 | |
| Adhesion microscope slides | CITOtest | 188105 | |
| Agarose | Gene Tech | 111760 | |
| Amaxa Nucleofector 2b Device | Lonza | AAB-1001 | Transfection system |
| B-27 | Thermo Fisher Scientific | 17504044 | |
| bFGF | R&D Systems | 3718-FB | |
| Blebbistatin | Nuwacell Biotechnologies | RP01008 | |
| Blood collection tube | BD Vacutainer EDTA | 366643 | |
| CHIR99021 | TOCRIS | 4423/10 | |
| Cover slides | CITOGLAS | 10212440C | |
| cTarget hPSC Medium | Nuwacell Biotechnologies | RP01020 | |
| DAPI | Invitrogen | D-1306 | |
| DMEM/Ham’s F12 | Gibco | 10565-042 | |
| Donkey anti-mouse 488 | Invitrogen | A-21202 | |
| Donkey anti-rabbit 594 | Invitrogen | A-21207 | |
| EDTA | Nuwacell Biotechnologies | RP01007 | |
| Embedding medium | FluorSaveTM Reagent | 345789 | |
| EX-CYTE growth enhancement medium | Sigma | 811292 | Growth enhancement medium |
| Fetal bovine serum | Gibco | 04-002-1A | |
| Ficoll | Sigma-Aldrich | 26873-85-8 | Density gradient medium |
| FLT3L | Peprotech | 300-19 | |
| GlutaMAX | Life Technologies | 35050-061 | L-glutamine supplement |
| HA-100 | STEMCELL Technologies | 72482 | |
| Ham’s F12 | Gibco | 11765-054 | |
| hLIF | Thermo Fisher Scientific | AF-250-NA | |
| Homogenizer | EDEN lab | D-130 | |
| IL-3 | Peprotech | 213-13 | |
| IL-6 | Peprotech | 200-06 | |
| Iscove’s Modified Dulbecco Medium | Gibco | 12440053 | |
| KnockOut Serum Replacement – Multi-Species | Gibco | A3181502 | Serum replacement media |
| L/M-opsin | Millipore | ab5405 | |
| Monothioglycerol | Sigma | M6145 | |
| N-2 supplement | Thermo Fisher Scientific | 17502048 | |
| Nanodrop Spectrophotometer | Thermo Fisher Scientific | ND2000 | Spectrophotometer |
| ncEpic 125x Supplement | Nuwacell Biotechnologies | RP01001-02 | 125x Supplement |
| ncEpic Basal Medium | Nuwacell Biotechnologies | RP01001-01 | Basal hpsc medium |
| ncLaminin511 human recombinant protein | Nuwacell Biotechnologies | RP01025 | |
| PD0325901 | STEMCELL Technologies | 72182 | |
| Penicillin-streptomycin | Gibco | 15140-122 | |
| Recombinant human BMP4 | R&D Systems | 314-BP | |
| Retinoic acid | Sigma | R2625 | |
| Rhodopsin | Sigma | O4886 | |
| RNeasy Mini Kit | Qiagen | 74104 | |
| RNeasy Mini Kit | Qiagen | 74104 | |
| sIL6-R | Thermo Fisher Scientific | RP-75602 | |
| StemSpan SFEM medium | STEMCELL Technologies | 09600 | |
| Taurine | Sigma | T8691 | |
| Trizol reagent | Invitrogen | 15596026 | |
| Vitronectin | Nuwacell Biotechnologies | RP01002 | |
| V-Lance knife | Alcon Surgical | 8065912001 |
Referencias
- Jin, Z. B., et al. Stemming retinal regeneration with pluripotent stem cells. Progress in Retinal and Eye Research. 69, 38-56 (2019).
- Lin, Q., et al. Generation of nonhuman primate model of cone dysfunction through in situ AAV-mediated CNGB3 ablation. Molecular Therapy. Methods & Clinical Development. 18, 869-879 (2020).
- Zhou, M., Liu, Y., Ma, C. Distinct nuclear architecture of photoreceptors and light-induced behaviors in different strains of mice. Translational Vision Science & Technology. 10 (2), 37 (2021).
- Zito, I., et al. RPGR mutation associated with retinitis pigmentosa, impaired hearing, and sinorespiratory infections. Journal of Medical Genetics. 40 (8), 609-615 (2003).
- Solovei, I., et al. Nuclear architecture of rod photoreceptor cells adapts to vision in mammalian evolution. Cell. 137 (2), 356-368 (2009).
- Volland, S., Esteve-Rudd, J., Hoo, J., Yee, C., Williams, D. S. A Comparison of some organizational characteristics of the mouse central retina and the human macula. PLoS One. 10 (4), 0125631 (2015).
- Takahashi, K., Yamanaka, S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 126 (4), 663-676 (2006).
- Han, J. W., Yoon, Y. S. Induced pluripotent stem cells: Emerging techniques for nuclear reprogramming. Antioxidants & Redox Signaling. 15 (7), 1799-1820 (2011).
- Kim, J. S., Choi, H. W., Choi, S., Do, J. T. Reprogrammed pluripotent stem cells from somatic cells. International Journal of Stem Cells. 4 (1), 1-8 (2011).
- Li, Y. P., Liu, H., Jin, Z. B. Generation of three human iPSC lines from a retinitis pigmentosa family with SLC7A14 mutation. Stem Cell Research. 49, 102075 (2020).
- Capowski, E. E., et al. Reproducibility and staging of 3D human retinal organoids across multiple pluripotent stem cell lines. Development. 146 (1), 171686 (2019).
- Pan, D., et al. COCO enhances the efficiency of photoreceptor precursor differentiation in early human embryonic stem cell-derived retinal organoids. Stem Cell Research & Therapy. 11 (1), 366 (2020).
- Liu, H., et al. Human embryonic stem cell-derived organoid retinoblastoma reveals a cancerous origin. Proceedings of the National Academy of Sciences of the United States of America. 117 (52), 33628-33638 (2020).
- Deng, W. L., et al. Gene correction reverses ciliopathy and photoreceptor loss in iPSC-derived retinal organoids from retinitis pigmentosa patients. Stem Cell Reports. 10 (4), 1267-1281 (2018).
- Gao, M. L., et al. Patient-specific retinal organoids recapitulate disease features of late-onset retinitis pigmentosa. Frontiers in Cell and Developmental Biology. 8, 128 (2020).
- Del Amo, E. M., et al. Pharmacokinetic aspects of retinal drug delivery. Progress in Retinal and Eye Research. 57, 134-185 (2017).
- Aasen, D. M., Vergara, M. N. New drug discovery paradigms for retinal diseases: A focus on retinal organoids. Journal of Ocular Pharmacology and Therapeutics. 36 (1), 18-24 (2020).
- Hartong, D. T., Berson, E. L., Dryja, T. P. Retinitis pigmentosa. Lancet. 368 (9549), 1795-1809 (2006).
- Anasagasti, A., Irigoyen, C., Barandika, O., Lopez de Munain, A., Ruiz-Ederra, J. Current mutation discovery approaches in Retinitis Pigmentosa. Vision Research. 75, 117-129 (2012).
- Daiger, S. P., Bowne, S. J., Sullivan, L. S. Genes and mutations causing autosomal dominant retinitis pigmentosa. Cold Spring Harbor Perspectives in Medicine. 5 (10), 017129 (2014).
- Kortum, F., et al. X-linked retinitis pigmentosa caused by non-canonical splice site variants in RPGR. International Journal of Molecular Sciences. 22 (2), 850 (2021).
- Megaw, R. D., Soares, D. C., Wright, A. F. RPGR: Its role in photoreceptor physiology, human disease, and future therapies. Experimental Eye Research. 138, 32-41 (2015).
- Li, Y. P., Deng, W. L., Jin, Z. B. Modeling retinitis pigmentosa through patient-derived retinal organoids. STAR Protocols. 2 (2), 100438 (2021).
- Liu, H., Hua, Z. Q., Jin, Z. B. Modeling human retinoblastoma using embryonic stem cell-derived retinal organoids. STAR Protocols. 2 (2), 100444 (2021).
- Zhang, X. H., Xie, Y., Xu, K., Li, Y. Generation of an induced pluripotent stem cell line BIOi002-A from a patient with autosomal dominant optic atrophy. Stem Cell Research. 53, 102278 (2021).
- Nakano, T., et al. Self-formation of optic cups and storable stratified neural retina from human ESCs. Cell Stem Cell. 10 (6), 771-785 (2012).
- Kuwahara, A., et al. Generation of a ciliary margin-like stem cell niche from self-organizing human retinal tissue. Nature Communication. 6, 6286 (2015).
- Cowan, C. S., et al. Cell types of the human retina and its organoids at single-cell resolution. Cell. 182 (6), 1623-1640 (2020).
- Eldred, K. C., et al. Thyroid hormone signaling specifies cone subtypes in human retinal organoids. Science. 362 (6411), (2018).
- Kumar, R., et al. A high-throughput method for illumina RNA-seq library preparation. Frontiers in Plant Science. 3, 202 (2012).
- Fligor, C. M., et al. Three-dimensional retinal organoids facilitate the investigation of retinal ganglion cell development, organization and neurite outgrowth from human pluripotent stem cells. Scientific Reports. 8 (1), 14520 (2018).
- Morizur, L., Herardot, E., Monville, C., Ben M’Barek, K. Human pluripotent stem cells: A toolbox to understand and treat retinal degeneration. Molecular and Cellular Neurosciences. 107, 103523 (2020).
- Bhatt, L., Groeger, G., McDermott, K., Cotter, T. G. Rod and cone photoreceptor cells produce ROS in response to stress in a live retinal explant system. Molecular Vision. 16, 283-293 (2010).
- O’Hara-Wright, M., Gonzalez-Cordero, A. Retinal organoids: A window into human retinal development. Development. 147 (24), (2020).
- Xue, Y., et al. Retinal organoids long-term functional characterization using two-photon fluorescence lifetime and hyperspectral microscopy. Frontiers in Cellular Neurosciences. 15, 796903 (2021).
- Colombo, L., et al. Comparison of 5-year progression of retinitis pigmentosa involving the posterior pole among siblings by means of SD-OCT: A retrospective study. BMC Ophthalmology. 18 (1), 153 (2018).
- Ferrari, S., et al. Retinitis pigmentosa: Genes and disease mechanisms. Current Genomics. 12 (4), 238-249 (2011).
- Shintani, K., Shechtman, D. L., Gurwood, A. S. Review and update: Current treatment trends for patients with retinitis pigmentosa. Optometry. 80 (7), 384-401 (2009).
- Wang, A. L., Knight, D. K., Vu, T. T., Mehta, M. C. Retinitis pigmentosa: Review of current treatment. International Ophthalmology Clinics. 59 (1), 263-280 (2019).
- Daiger, S. P., Sullivan, L. S., Bowne, S. J. Genes and mutations causing retinitis pigmentosa. Clinical Genetics. 84 (2), 132-141 (2013).
- Parfitt, D. A., et al. Identification and correction of mechanisms underlying inherited blindness in human iPSC-derived optic cups. Cell Stem Cell. 18 (6), 769-781 (2016).
- Shimada, H., et al. In vitro modeling using ciliopathy-patient-derived cells reveals distinct cilia dysfunctions caused by CEP290 mutations. Cell Reports. 20 (2), 384-396 (2017).
- Deretic, D., Wang, J. Molecular assemblies that control rhodopsin transport to the cilia. Vision Research. 75, 5-10 (2012).
- Rao, K. N., Li, L., Anand, M., Khanna, H. Ablation of retinal ciliopathy protein RPGR results in altered photoreceptor ciliary composition. Scientific Reports. 5, 11137 (2015).
- Zhang, X., Wang, W., Jin, Z. B. Retinal organoids as models for development and diseases. Cell Regeneration. 10 (1), 33 (2021).
- Zhang, X. H., Jin, Z. B. Patient iPSC-derived retinal organoids: Observable retinal diseases in-a-dish. Histology and Histopathology. 36 (7), 705-710 (2021).

.
