Measurement of Heart Contractility in Isolated Adult Human Primary Cardiomyocytes
Summary
This protocol describes how to measure contractility in adult human primary cardiomyocytes from donor hearts with the MyoBLAZER system, a reliable platform for assessing drug-induced changes in contractility during preclinical development.
Abstract
The evaluation of changes in heart contractility is essential during preclinical development for new cardiac- and non-cardiac-targeted compounds. This paper describes a protocol for assessing changes in contractility in adult human primary ventricular cardiomyocytes utilizing the MyoBLAZER, a non-invasive optical method that preserves the normal physiology and pharmacology of the cells. This optical recording method continuously measures contractility transients from multiple cells in parallel, providing both medium-throughput and valuable information for each individual cell in the field of view, enabling the real-time tracking of drug effects. The cardiomyocyte contractions are induced by paced electrical field stimulation, and the acquired bright field images are fed to an image-processing software that measures the sarcomere shortening across multiple cardiomyocytes. This method rapidly generates different endpoints related to the kinetics of contraction and relaxation phases, and the resulting data can then be interpreted in relation to different concentrations of a test article. This method is also employed in the late stages of preclinical development to perform follow-up mechanistic studies to support ongoing clinical studies. Thus, the adult human primary cardiomyocyte-based model combined with the optical system for continuous contractility monitoring has the potential to contribute to a new era of in vitro cardiac data translatability in preclinical medical therapy development.
Introduction
Myocardial contractility (inotropy), which represents the natural capacity of the heart muscle to contract, is a key property of cardiac function and depends on the dynamics of the electro-mechanical coupling. Drug-induced changes in myocardial contractility are desired to treat heart disease (e.g., heart failure) and unsought in the context of cardiotoxicity (e.g., reduction in left ventricular ejection fraction). Therefore, preclinical contractility models must be associated with accurate predictivity to make sure novel drugs can succeed during clinical development. However, current preclinical strategies, which rely on reductionist artificial cellular models (e.g., genetically engineered immortalized cell lines that overexpress specific cardiac targets of interest) and non-human animal models, have shown significant limitations and have been found to be associated with high drug attrition rates (i.e., a high rate of false signals)1,2,3,4. Accordingly, it is imperative to establish new and reliable human cellular heart contractility models that are associated with high power (i.e., high rate of true signals) to predict drug outcomes in humans and, hence, help to accelerate the launch of new therapies5.
The groundbreaking methods recently established for the recovery of human donor hearts for research6,7,8,9,10 and in cardiomyocyte isolation techniques11,12,13,14,15 have provided a unique opportunity for conducting human-based studies during preclinical development. To this end, adult human primary cardiomyocytes have already shown utility in assessing drug-induced changes in human heart contractility11,12,13,14. The current article details the protocol for investigating the contractility effects of novel compounds in adult human cardiomyocytes.
Protocol
All methods were carried out in accordance with relevant guidelines and regulations. All human hearts used for the studies were non-transplantable and ethically obtained by informed legal consent (first person or next-of-kin) from cadaveric organ donors in the United States (US). The recovery protocols and in vitro experimentation were pre-approved by Institutional Review Boards (IRBs ) at transplant centers within the US Organ Procurement Transplant Network (OPTN). Furthermore, all transfers of the donor hearts are fully traceable and periodically reviewed by US federal authorities.
NOTE: Apply all necessary safety procedures during the execution of this protocol, including wearing appropriate personal protective equipment (e.g., laboratory coats, safety glasses, gloves).
1. Isolation of cardiomyocytes (1 day before measuring contractility)
- Re-perfuse donor hearts immediately on arrival at the laboratory with ice-cold proprietary cardioplegic solution6 and isolate enzymatically adult human primary myocytes from the ventricles11,12,13,14,15.
- Then, maintain the cardiomyocytes in suspension and store them with 10 mL of solution A (110 mM sucrose, 0.005 mM CaCl2, 3 mM MgCl2, 70 mM KOH, 60 mM lactobionic acid, 10 mM KH2PO4, 20 mM taurine, 20 mM L-histidine, 20 mM HEPES, 2 mM L-glutamic acid, 2 mM L-(-)-malic acid, pH 7.4 with KOH) in 20 mL Wheaton vials (Table of Materials) at a refrigerated temperature until they are experimentally interrogated.
NOTE: Store 200,000 cells per vial.
2. Laminin coating preparation (1 day before measuring contractility)
- Place a single glass coverslip (25 mm x 25 mm square, Table of Materials) in each well of an eight-well culture plate (Table of Materials).
- Then, coat the coverslips with laminin at a 5 µg/mL concentration. To do so, add 800 µL of the human recombinant Laminin 521 stock (Table of Materials, stored at −20 °C) to 7.2 mL of solution B (145 mM NaCl, 4 mM KCl, 2.5 mM CaCl2, 1 mM MgCl2, 11.1 mM glucose, and 10 mM HEPES, pH 7.4 with NaOH) and mix well.
- Drop 200 µL of the diluted laminin solution into the center of each coverslip. Then, cover the plate and stack it in a 4 °C fridge.
NOTE: Prepare multiple eight-well culture plates to ensure there are enough laminin-coated coverslips.
3. Preparation of Ca2+-tolerant cardiomyocytes (on the day of measuring contractility)
- Remove a vial of cells from the fridge, aspirate solution A from the vial and add a refrigerated 10 mL of solution B.
NOTE: Ensure the cells are not suctioned and disperse solution B into the vial by placing the pipette on the inside of the top of the vial wall. - Cap the vial of cells and then gently swirl to ensure the cells are dispersed throughout solution B. Finally, allow the cells to settle for 1 h at room temperature (RT).
4. Preparation of the recording system (on the day of measuring contractility)
NOTE: The recording system includes a temperature control box, stimulator, computer-controlled pressure-driven perfusion, pressure-driven perfusion bottles, in-house acquisition software permitting the selection of regions of interest (ROIs) and display of contractility transients, inverted microscope, cell chamber, and camera (Figure 1).
- Turn on the suction vacuum system. Then, remove the microscope cover, turn it on, connect the camera (Table of Materials, Figure 1), and finally, confirm the fan is turned on to ensure the camera does not overheat.
- Then, turn on the microscope heating plate (Table of Materials) and temperature control box (Table of Materials, Figure 1), and set them to the proper operating temperature. Next, turn on the field stimulator (Table of Materials, Figure 1) and the pressure system. Finally, connect the solution's tubing (Table of Materials) to the recording chamber (Table of Materials, Figure 1).
5. Preparation of test compounds (on the day of measuring contractility)
- Dilute dimethyl sulfoxide (DMSO; Table of Materials) 1,000-fold in solution C (145 mM NaCl, 4 mM KCl, 1.8 mM CaCl2, 1 mM MgCl2, 11.1 mM glucose, and 10 mM HEPES, pH 7.4 with NaOH) to make a 0.1% DMSO vehicle solution.
- To test a compound at 1-fold, 10-fold, 100-fold, and 1000-fold of its therapeutic exposure of 0.001 µM, dissolve the test compound in DMSO at a concentration of 1 mM.
- Dilute this solution serially with DMSO to produce three further DMSO stocks (e.g., 0.001 mM, 0.01 mM, and 0.1 mM). Finally, dilute each test compound stock 1,000-fold in 100 mL of solution C to obtain final test concentrations (0.001 µM, 0.01 µM, 0.1 µM, and 1 µM).
- Add the vehicle solution and solutions of final micromolar concentrations of the compound to 100 mL glass bottles (Table of Materials, Figure 1) and then connect the bottles to the pressure-driven perfusion system (Table of Materials, Figure 1).
- Then, prime the perfusion system with a computer-driven program (Table of Materials) using a pressure of 200-300 mbar to deliver a constant perfusion flow at a rate of 1.8 mL/min.
NOTE: Do not conduct the experiment if the test compound is found to be associated with a solubility issue. Also, formulate the compound test solutions from stock solutions within 30 min prior to experimental application to the cells.
6. Plating of cardiomyocytes (on the day of measuring contractility)
- Take an eight-well plate containing laminin-coated coverslips out of the 4 °C fridge and carefully place one coverslip in a very well-cleaned recording chamber (Figure 1). Then, return the eight-well plate to the 4 °C fridge until the next plating.
NOTE: Use a lint-free wipe (Table of Materials) to wipe up the coated coverslip while keeping the coverslip coated in a thin layer of laminin. Also, ensure to dispose of used coverslips in a sharps container. - Then, take an escalated vial of cells (step 3.2) and aspirate solution B from the vial to reach as small a volume as possible without losing cells (~200 µL). Next, dispense the 200 µL of cells into the recording microscope chamber mounted on the stage of an inverted microscope (Table of Materials).
- Let the cells settle on the coverslip for 5 min. While waiting for the cells to fully settle, open the field of view on the microscope and determine if the cell density is adequate (~70%) for the experimental run to begin.
NOTE: Ensure the suction does not aspirate all the cells away if more than 200 µL are dispensed.
7. Recording of cardiomyocyte contractility
- After plating is completed, equilibrate the cells for 5 min by continuously perfusing them with solution C using the pressure-driven perfusion system (Table of Materials). Adjust the suction correctly, turn on both the temperature control box and heating plate, and set them to deliver ~35 °C.
- Then, stimulate the cells with supra-threshold voltage at a 1 Hz pacing frequency (bipolar pulse of 3 ms duration) on a field stimulator with a pair of platinum wires placed on opposite sides of the chamber connected to a field stimulator.
- Set the amplitude of the stimulating pulse at 1 V, and then increase it until the cardiomyocytes start generating contraction-relaxation cycles. Use a value of 1.5x threshold throughout the experiment.
NOTE: Select healthy cells with rod-shaped morphology and clear striations. Then, adjust the field of view and focus on bringing as many contracting cells as possible into view (Figure 2A). - Next, display the digitized images of the cells within the acquisition software of the optical contractility recording system. This software utilizes a high-resolution, high-frame rate camera with a rate of 150 FPS (Table of Materials, Figure 1) to record a video stream containing multiple myocytes in the same frame.
NOTE: This provides sufficient temporal and spatial resolution to capture and measure the sarcomere dynamics of several healthy myocytes simultaneously. Modern high-core-count processors are leveraged to enable the parallel calculation of changes in sarcomere length from the video stream in real time (optical density data are collected from user-defined regions of interest [ROI] placed over the images of healthy cardiomyocytes and parallel to the axis of contraction, Figure 2B). The optical intensity data show bright and dark bands corresponding to the Z-lines of the cardiomyocytes (Figure 1, Figure 2C). Finally, these data are displayed to the user for monitoring purposes and saved to a disk for later analysis (Figure 2C).- Avoid areas of the cells that are not in focus. Also, do not position the ROIs close to the edge of the cells, as the changes in the cells' contraction during the application of drugs can cause the ROIs to be unable to read the cells' contraction.
- When the selection of ROIs is completed and the contractions meet the necessary standards for use (e.g., sarcomere shortening >1%; rhythmic contraction upon application of a 1 Hz pacing frequency, absence of arrhythmic events), start the experiment to assess the effects of a compound. The stimulation protocol and the sequence of the compound's application of test concentrations are shown in Table 1. The acquisition software will manage, in an automated fashion, the acquisition and display of data and labeling of test concentrations and treatment time.
NOTE: Apply test concentrations if the contraction remains at a stable amplitude throughout the entirety of the baseline vehicle period. Disqualify cells displaying either a run-up or run-down. Also, ensure perfusion is switched to the different test concentrations throughout the experiment. - Upon completion of the experiment and data storage on the server, switch off the perfusion and heater, stop the stimulation, clean the microscope chamber with distilled water, then remove the glass coverslip, and dry the chamber thoroughly.
NOTE: Clean the chamber thoroughly as this is critical to avoid exposing the next set of cells to any compound prematurely, thus invalidating any data collected. - Next, perform a new plating of cardiomyocytes and perform an additional experiment to obtain enough data for completing the testing of the compound or testing a new compound.
8. Turning off the optical contractility recording system
- When daily testing of the compound(s) is completed, first switch off all equipment that is not necessary to clean the system and copy the data for offline analysis.
- Next, remove the 100 mL glass bottles (Table of Materials) containing solutions of final test concentrations and replace them with glass bottles halfway filled with RBS 25 Concentrate solution (Table of Materials). Perfuse RBS solution through the microscope chamber for 5 min, then disconnect the perfusion tubing from the chamber, clean it with distilled water, and finally, dry it thoroughly.
NOTE: Ensure the vacuum is working well to prevent any possible flooding. - Next, connect the perfusion tubing to the drainage tubing. Using the software of the pressure-driven perfusion system (Table of Materials), run the RBS solution for 10 min while ensuring the pressure and flow rate are adequately set. Then, perfuse 1% DMSO for 5 min and then distilled water for 15-20 min to complete the cleaning of the perfusion system.
- Turn off the microscope light and put its cover. Then, unplug the camera wires from the box on the side of the microscope and turn off the fan. Ensure that all used bottles are sent for cleaning and autoclave. Finally, ensure all drainage buckets are 1/4 full or less.
NOTE: Make sure there are enough laminin-coated coverslips for use the following day. - Finally, copy the files in the acquisition folder of all the experiments done on each recording system and paste them into a secure backup server for additional offline analysis. Next, delete all the data files from the acquisition folder.
9. Analysis of contractility data
- Perform offline analysis using the analysis software and a custom-made macro to average the data. The analysis software calculates and reports various metrics from the sarcomere dynamics data produced by the acquisition software. A detailed list of these parameters is provided in Figure 2D.
NOTE: The application of low-level software techniques allows many runs of recordings to be analyzed in 5 s. The principal parameter to assess drug-induced changes in contractility is the contractility amplitude (sarcomere shortening % = change in sarcomere length). It is calculated as the sarcomere length at peak contraction/resting sarcomere length and averaged over the last 20 contractility transients for each test concentration. - Quantify the test compound effects on the averaged contractility amplitude relative to each cardiomyocyte's specific baseline vehicle control condition.
- Express the average results as mean ± SEM and produce a graph plotting the concentration effect of the test compound on the contractility amplitude. Next, fit the concentration-response curve to the Hill equation using analysis software (Table of Materials) to derive IC50 (concentration producing a 50% inhibition of contractility amplitude) and EC50 (concentration producing a 50% increase in contractility amplitude) values.
- In addition to the contractility amplitude, you can also calculate other parameters:
- Aftercontraction: Identify aftercontraction as a spontaneous secondary contraction transient of the cardiomyocyte that occurs before the next regular contraction and produces an abnormal and unsynchronized contraction.
- Contraction failure: Identify contraction failure as the inability of the electrical stimulus to induce a contraction.
- Short-term variability (STV) and alternans: Visualize these two parameters in Poincaré plots of contraction amplitude variability.
- Calculate the STV (∑|CAn + 1 − CAn| (20 × √2)−1) with the last 20 transients of each control and test article concentration period. Identify alternans as repetitive alternating short and long contractility amplitude transients.
- To calculate the incidence of pro-arrhythmia, normalize the STV values to the vehicle control value of each cell, plot aftercontraction, contraction failure, STV, and alternans, and express them as % of the incidence of cells exhibiting each of the signals.
- Complete the multiparametric mechanistic profiling with the calculation of time Ato peak, decay to 30% relaxation (Decay 30), decay to 90% relaxation (Decay 90), time to 90% relaxation (TR90) (Figure 2D), baseline sarcomere length, time to 50% peak, peak height, sarcomere length at peak contractility, maximum contraction velocity, and maximum relaxation velocity12. Like the contractility amplitude, express these parameters relative to each cardiomyocyte's specific baseline control condition and graph them in concentration-response plots.
Representative Results
Described in this protocol is a procedure for measuring contractility in isolated adult human primary cardiomyocytes from organ donors and evaluating acute changes in contractility parameters induced by a test compound. The measurement of the contractility transient is accomplished using a video-based cell geometry system, the MyoBLAZER, that measures sarcomere dynamics, and the adult heart condition is emulated by inducing contractility with electrical stimulation at the physiological pacing frequency (1 Hz). The functional viability of the cardiomyocytes is confirmed by assessing their excitation-contraction coupling (i.e., cellular processing linking electrical excitation [the sarcolemmal action potential] with contraction). There are no spontaneous contractility transients in human cardiomyocytes, and the cardiomyocytes respond to external electrical stimulation with contractility/relaxation cycles (Figure 2C, E), as well as to isoproterenol, a β-adrenergic agonist (Figure 2F,G). Isoproterenol causes a concentration-dependent increase in contractility (Figure 2G), and its effects on the kinetics of contractility transient can also be characterized (Figure 2D)12.
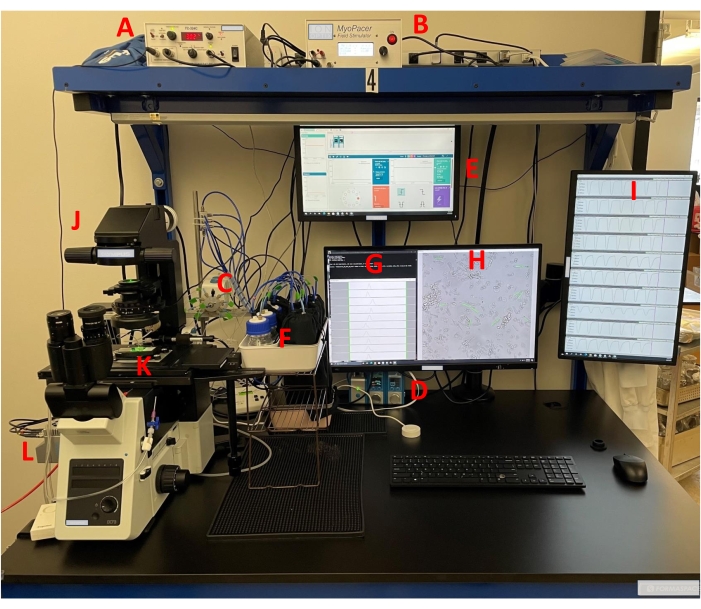
Figure 1: Experimental setup of the optical contractility recording system. Bright-field contractility recordings are made from adult human primary cardiomyocytes as previously described11,12,13,14. The setup includes a (A) temperature control box, (B) field stimulator, (C,D) pressure-driven perfusion system, (E) computer pressure-driven program, (F) pressure plus bottles, (G) acquisition software, (H) selection of ROIs with acquisition software, (I) display of contractility transients with acquisition software, (J) inverted microscope, (K) microscope chamber, and (L) camera. All features of the equipment are given in the Table of Materials. Please click here to view a larger version of this figure.
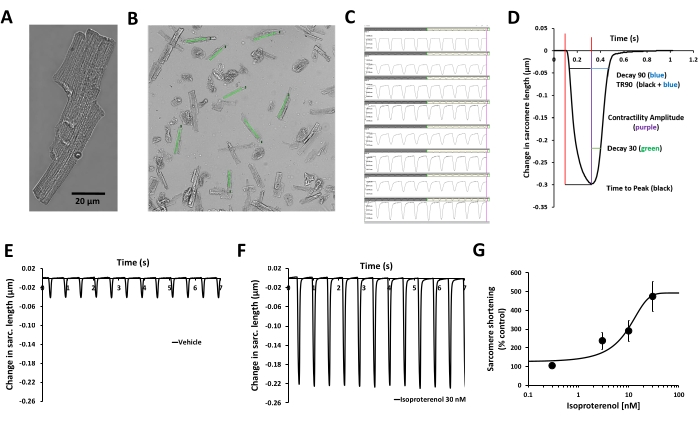
Figure 2: Validation of human cardiomyocyte contractility measurement and the effect of β-adrenergic stimulation. (A) Phase contrast microscopy image of a representative adult human primary cardiomyocyte, (B) user-defined regions of interest (ROI) placed over the images of healthy cardiomyocytes and parallel to the axis of contraction, (C) display of contractility transients obtained from the same ROIs in (B) with the acquisition software, (D) parameters related to the contractility transient measured with the acquisition software: contractility amplitude, time to peak, Decay 30, Decay 90, TR90. Additional parameters can also be measured: baseline sarcomere length, time to 50% peak, peak height, sarcomere length at peak contractility, maximum contraction velocity, and maximum relaxation velocity. (E) Typical contractility transients recorded from an adult human primary ventricular myocyte at a pacing frequency of 1 Hz in the presence of vehicle control (F) and after exposure to 30 nM isoproterenol, a non-selective β-adrenergic agonist. The contractility transients demonstrated in panels E and F were collected from the same cardiomyocyte. Isoproterenol from human cardiomyocytes paced at 1 Hz pacing frequency was used to generate the potency information. (G) Typical cumulative concentration-response curve generated by human cardiomyocyte contractility measurement with isoproterenol (n = 8 cells). Please click here to view a larger version of this figure.
| Perfusion Sequence | Vehicle solution | Concentration 1 (µM) | Concentration 2 (µM) | Concentration 3 (µM) | Concentration 4 (µM) | Wash |
| Treatment Time | 120 s | 300 s | 300 s | 300 s | 300 s | 300 s |
| Stimulation Frequency | 1 Hz | 1 Hz | 1 Hz | 1 Hz | 1 Hz | 1 Hz |
Table 1: Stimulation protocol and test compound application sequence. The stability of contractility transients is assessed by continuous recording for 120 s in solution C, establishing the vehicle control (typically in 0.1% DMSO). Subsequently, the test article concentration is applied for a minimum of 300 s or until a steady-state effect is attained. Four ascending test article concentrations are used, providing cumulative concentration-effect (C-E) curves. At the end of the cumulative additions of the test article, a 300-s washout period can be implemented.
Discussion
This manuscript provides a detailed protocol for the adult human cardiomyocyte contractility-based optical system for a simplified medium-throughput method that enables the testing of the acute efficacy and cardiotoxicity of novel compounds. This optical contractility recording system is easy to use, allows recordings from multiple cells in parallel, enables the simultaneous assessment of cell health, physiology, and pharmacology, comes with automated and rapid data analysis (a run of multiple cells is analyzed in 5 s), and permits rapid data collection (concentration-response curve every 30 min/compound/device). Taking into consideration these attributes, the recording system can be used not only to detect the effects of drugs on cardiomyocyte contractility but also to provide structure-activity relationship data for supporting medicinal chemistry efforts during the early phases of drug discovery16. Since tens of millions of cells can be obtained from a cardiomyocyte isolation protocol, the application of the optical contractility recording system-cardiomyocyte platform is currently being explored to achieve increased testing capacity (with the use of well-based plates) with reduced cost. Moreover, the assessment of drug effects on the systolic and relaxation parameters measured with the recording system can provide multiparametric mechanistic profiling of inotropic drugs12. Additionally, cardiomyocyte contractility data can be used to rank novel drugs from most to least cardiotoxic (e.g., safety margin) and from least to most efficacious (e.g., potency margin). Follow-up cardiomyocyte contractility studies can also be conducted to support development programs that have been associated with a clinical decrease in myocardial contractility12.
Another significant advantage of using the human cardiomyocyte contractility optical recording system is its alignment with the 3Rs concept (replacement, reduction, and refinement)17 since it can be considered as an alternative method that avoids or replaces the use of animals for data generation within the pharmaceutical industry. This 3Rs benefit can also be extended to academic cardiac research. The entirety of current knowledge of cardiomyocyte physiology and pharmacology comes from academic research studies conducted with cells isolated from animal hearts18. Thus, the human cardiomyocyte optical contractility model opens the possibility for critical translational studies to be performed. To perform these studies, protocols for the preservation and shipment of human adult cardiomyocytes must be developed (currently under evaluation in AnaBios' laboratory), and the contractility system must have the ability to record changes in sarcomere length from non-human cardiomyocytes (this is the case with the optical contractility recording system since sarcomeres are well conserved among species).
The human cardiomyocyte contractility system can emulate several physiological conditions (e.g., electromechanical coupling, pacing frequency mimicking heart rate, body temperature, the integration of all human cardiac targets) and has demonstrated translational value as a key component in drug discovery11,12,13,14, although it cannot mimic the changes in mechanical load and shear stress seen during the cardiac contractile cycle. The structure and function of cardiac extracellular matrices are now better understood19, the development of such matrices can potentially help overcome the mechanical load limitation, and matrices with different heart-like stiffnesses are currently being evaluated in AnaBios' laboratory. Another limitation of the human cardiomyocyte optical contractility system is the absence of the network of nerves that supplies the heart (e.g., sympathetic and parasympathetic fibers)20. This neuro-cardiac contact can be re-established with the co-application of neurotransmitters (e.g., isoproterenol, an agonist of β-adrenoceptor receptors; acetylcholine, an agonist of M2 muscarinic receptors), with the compound being assessed for its potential effects on cardiomyocyte contractility. Furthermore, the contractility transients are recorded with no simultaneous measurements of action potentials and Ca2+ transients, which are also essential when evaluating drug effects on the electrocardiogram and Ca2+ handling. Although this omission can be considered a limitation of the system, it is not too critical to have since the recordings of action potential signals (with the current-clamp method or voltage-sensitive dyes) and Ca2+ transients (with Ca2+ indicators/dyes) can be associated with cytotoxicity. Such cytotoxic effects can impact the assessment of novel drugs to modulate heart contractility. On the contrary, the use of a non-invasive optical method that preserves the health, physiology, and pharmacology of the cardiomyocytes, like the recording system described in this protocol, would not only ensure that the highest quality of contractility data is obtained but also provide data that can predict well the contractile effects of novel drugs in humans.
Divulgaciones
The authors have nothing to disclose.
Acknowledgements
This work was supported by the AnaBios Corporation and an NIH Small Business Innovation Research (SBIR) grant (1R44TR003162-01).
Materials
| 100–1000 µL Filtered, Wide Orifice, Sterile tips | Pipette | UF-1000W | |
| 100 mL, Duran pressure plus bottles | DWK Life Sciences | 218102406 | |
| 1 L, 0.22 µm Vacuum Filter system | VWR | 567-0020 | |
| 290 mmol/kg Osmolarity Standard | Wescor | OA-029 | |
| Benchtop pH Meter | Mettler Toledo | https://www.mt.com/us/en/home/products/Laboratory_Analytics_Browse/pH-meter/pH-meters.html | |
| Calcium Chloride dihydrate (CaCl2) | Sigma-Aldrich | C3881 | |
| Camera | Optronis GmbH | Cyclone-25-150-M | https://optronis.com/en/products/cyclone-25-150/ |
| Corning 25 mm x25 mm Square #1 Cover Glass | Corning | 2845-25 | |
| Cyclone-25-150 | Optronis | https://optronis.com/en/products/cyclone-25-150/ | |
| D-(+)-Glucose | Sigma-Aldrich | G8270 | |
| Digital Timer/Stopwatch | Fisher Scientific | 14-649-17 | |
| Dimethyl sulfoxide (DMSO) | Sigma-Aldrich | D8418 | |
| Eight-well rectangular polystyrene sterile culture plate | Thermo Fisher Scientific | 73521-426 | https://us.vwr.com/store/product/4679368/nunclontm-delta-rectangular-dishes-polystyrene-sterile-thermo-scientific |
| FHD Microscope Chamber System | IonOptix | ||
| Flow EZ, Modular pressure-based flow controller with a computer driven program version 1.1.0.0. | Fluigent OxyGEN | ||
| Heavy Duty Vacuum Bottles | VWR | 16211-080 | |
| HEPES | Sigma-Aldrich | H3375 | |
| Human Recombinant Laminin 521 | BioLamina | LM521-05 | |
| Idex Chromatography Tubing, Natural FEP, 1/16" OD x 0.030" ID | Cole-Palmer | 1520L | |
| Kimberly-Clark Professional Kimtech Science Kimwipes | Fisher Scientific | 06-666 | |
| L-(-)-Malic acid | Sigma-Aldrich | 112577 | |
| Lactobionic acid | Sigma-Aldrich | 153516 | |
| L-Glutamic acid | Sigma-Aldrich | 49449 | |
| L-Histidine | Sigma-Aldrich | H8000 | |
| Magnesium Chloride hexahydrate (MgCl2) | Sigma-Aldrich | M9272 | |
| Microscope Temperature Control Stage Warmer | AmScope | TCS-100 | |
| MyoPacer Field Stimulator | IonOptix | ||
| Nunc Rectangular Dishes | Thermo Scientific | 267062 | |
| Olympus IX83P1ZF Ixplore Standard microscope | Olympus | https://www.olympus-lifescience.com/en/microscopes/inverted/ixplore-standard/?campaignid=657680540&adgroupid =116963199831&keyword=ix73%20 microscope&gclid=EAIaIQobChMIl qjyiMWP-AIVVx-tBh2JoQ85EAA YASAAEgLp3fD_BwE |
|
| pH 4.01, 7.00, and 10.01 Standards | Oakton | WD-05942-10 | |
| Potassium Chloride (KCl) | Sigma-Aldrich | 746436 | |
| Potassium Hydroxide (KOH) | Sigma-Aldrich | P4494 | |
| Potassium phosphate monobasic (KH2PO4) | Sigma-Aldrich | 795488 | |
| Prism Software | GraphPad Software – Dotmatics | https://www.graphpad.com/ | |
| RBS 25 Liquid Detergent | Sigma-Aldrich | 83460 | |
| Sharps Container | Uline | S-15307 | |
| SigmaPlot analysis software | Systat Software Inc. | https://systatsoftware.com/ | |
| Sodium Chloride (NaCl) | Sigma-Aldrich | S3014 | |
| Sodium Hydroxide (NaOH) | Sigma-Aldrich | 221465 | |
| Student Dumont #5 Forceps | Fine Science Tools | 91150-20 | |
| Sucrose | Sigma-Aldrich | S7903 | |
| Taurine | Sigma-Aldrich | T0625 | |
| Temperature Control Box | Warner Insturments | TC-324C | |
| Vapor Pressure Osmometer | ELITechGroup | Model 5600 | |
| Wheaton 20 mL Vials | DWK Life Sciences | 225288 |
Referencias
- Abi-Gerges, N., Miller, P. E., Ghetti, A. Human heart cardiomyocytes in drug discovery and research: new opportunities in translational sciences. Current Pharmaceutical Biotechnology. 21 (9), 787806 (2020).
- Cook, D., et al. Lessons learned from the fate of AstraZeneca’s drug pipeline: A five dimensional framework. Nature Reviews Drug Discovery. 13 (6), 419-431 (2014).
- Van Meer, B. J., Tertoolen, L. G. J., Mummery, C. L. Measuring physiological responses of human pluripotent stem cell derived cardiomyocytes to drugs and disease. Stem Cell. 34 (8), 2008-2015 (2016).
- Piccini, J. P., et al. Current challenges in the evaluation of cardiac safety during drug development: translational medicine meets the critical path initiative. American Heart Journal. 158 (3), 317-326 (2009).
- Pang, L., et al. Workshop report: FDA workshop on improving cardiotoxicity assessment with human-relevant platforms. Circulation Research. 125 (9), 855-867 (2019).
- Page, G., et al. Human ex-vivo action potential model for pro-arrhythmia risk assessment. Journal of Pharmacological and Toxicological Methods. 81, 183-195 (2016).
- Britton, O. J., et al. Quantitative comparison of effects of dofetilide, sotalol, quinidine, and verapamil between human ex vivo trabeculae and in silico ventricular models incorporating inter-individual action potential variability. Frontiers in Physiology. 8, 597 (2017).
- Qu, Y., et al. Action potential recording and pro-arrhythmia risk analysis in human ventricular trabeculae. Frontiers in Physiology. 8, 1109 (2017).
- Trovato, C., et al. Human Purkinje in silico model enables mechanistic investigations into automaticity and pro-arrhythmic abnormalities. Journal of Molecular and Cellular Cardiology. 142, 24-38 (2020).
- Otsomaa, L., et al. Discovery and characterization of ORM-11372, a novel inhibitor of the sodium-calcium exchanger with positive inotropic activity. British Journal of Pharmacology. 177 (24), 5534-5554 (2020).
- Nguyen, N., et al. Adult human primary cardiomyocyte-based model for the simultaneous prediction of drug-induced inotropic and pro-arrhythmia risk. Frontiers in Physiology. 8, 1073 (2017).
- Abi-Gerges, N., et al. Multiparametric mechanistic profiling of inotropic drugs in adult human primary cardiomyocytes. Scientific Reports. 10, 7692 (2020).
- Jordaan, P., et al. Cardiotoxic potential of hydroxychloroquine, chloroquine and azithromycin in adult human primary cardiomyocytes. Toxicological Sciences. 180 (2), 356-368 (2021).
- Ton, A. T., et al. Arrhythmogenic and antiarrhythmic actions of late sustained sodium current in the adult human heart. Scientific Reports. 11, 12014 (2021).
- Schmid, C., Abi-Gerges, N., Leither, M. G., Zellner, D., Rast, G. Ion channel expression and electrophysiology of singular human (primary and induced pluripotent stem cell-derived) cardiomyocytes. Cells. 10 (12), 3370 (2021).
- Guha, R. On exploring structure activity relationships. Methods in Molecular Biology. 993, 81-94 (2013).
- Tannenbaum, J., Bennett, B. T. Russell and Burch’s 3Ra then and now: The need for clarity in definition and purpose. Journal of the American Association for Laboratory Animal Science. 54 (2), 120-132 (2015).
- Ruiz-Meana, M., Martinson, E. A., Garcia-Dorado, D., Piper, H. M. Animal ethics in cardiovascular research. Cardiovascular Research. 93 (1), 1-3 (2012).
- Rienks, M., Papageorgiou, A. P., Frangogiannis, N. G., Heymans, S. Myocardial extracellular matrix. Circulation Research. 114 (5), 872-888 (2014).
- Zaglia, T., Mongillo, M. Cardiac sympathetic innervation, from a different point of (re)view. Journal of Physiology. 595 (12), 3919-3930 (2017).

