Summary
This paper presents a protocol for ocular micro-dissection in rodents. The process involves the enucleation of the eyeball along with the nictitating membrane (i.e., the third eyelid). This is then followed by the separation of the posterior and anterior eye cups.
Abstract
The ocular micro-dissection of the rodent eye involves the segmentation of the enucleated eyeball with the attached nictitating membrane, or third eyelid, to obtain the anterior and posterior eyecups. With this technique, the sub-parts of the eye, including the corneal tissue, neural tissue, retinal pigment epithelial (RPE) tissue, and lens, can be obtained for wholemounts, cryo-sectioning, and/or single-cell suspensions of a specific ocular tissue. The presence of the third eyelid presents unique and significant advantages, as it benefits the maintenance of the orientation of the eye, which is important for understanding eye physiology following any localized intervention or in studies involving ocular analysis relating to the eye's spatial topography.
In this method, we enucleated the eyeball at the socket along with the third eyelid by carefully and slowly cutting through the extraocular muscles and severing the optic nerve. The eyeball was pierced through the corneal limbus using a microblade. The incision was used as the point of entry, allowing for cutting along the corneal-scleral junction by inserting micro-scissors through the incision point. Small and continuous cuts along the circumference were made until the cups separated. These could be further dissected by gently peeling the translucent layer of the neural retina using Colibri suturing forceps to obtain the neural retina and RPE layers. Further, three/four equidistant cuts were made from the periphery perpendicularly to the optic center until the optic nerve was reached. This opened the hemispherical cups into a floret shape so that they fell flat and could be easily mounted. This technique has been used in our lab for corneal wholemounts and retinal sections. The presence of the third eyelid delineates the nasal-temporal orientation, which allows for the study of various cell therapy interventions post-transplantation and, thus, the targeted physiological validation vital for visualization and accurate representation in such studies.
Introduction
Ocular dissection is an important technique in ophthalmic research and has allowed investigators to access the segments of the eye for targeted studies. Previously, ocular researchers relied on the ocular tissue from diseased individuals for their studies. However, the progressively growing number of strains of ophthalmic rodent models1 over the years has diminished the need for human ocular tissue. These mouse strains have permitted a deeper understanding of ocular disease and interventions. Yet, they have also generated a need for innovative techniques of ocular micro-dissection. The small size and limited area of operation severely constrain effective access to the ocular sub-parts. Further, owing to the homogenous cellular assembly of the posterior and anterior eyecups, it is difficult to conduct targeted interventions post-dissection. The current micro-dissection techniques of laser2 and surgical microdissection3,4 are inadequate in meeting such requirements of ocular research. Laser micro-dissection is very effective in single-cell analysis, but the specific tissue needs to be micro-dissected before the laser procedure2. The technique can isolate small regions of interest from a pre-dissected tissue for molecular analysis. Thus, the technique is not suitable for preparing wholemounts or for the isolation of axially packed ocular layers for optimum visualization.
The surgical method is the most widely used technique; this method involves immobilizing the eye via the optic nerve5 and then performing the dissection. This practice is arduous and can damage any fragile tissue, as the spherical eye continues to move during dissection. Despite being beneficial for isolating the various sections of the retinal layers, the technique cannot demarcate the spatial orientation of the tissue upon dissection.
During dissection, maintaining the presence of the attached nictitating membrane or the third eyelid (Figure 1) presents unique and significant advantages. In this method, first, the eyeball is enucleated with the third eyelid. Then, the third eyelid is used to immobilize the eye6 (Figure 2A). This is followed by piercing the eyeball through the corneal limbus and using the incision as the point of entry (Figure 2B,C). Then, the eyecups are separated by cutting along the circumference anteriorly and posteriorly (Figure 2D–G). By dissecting the posterior eyecup further, the translucent layer of the neural retina can be identified and gently peeled off. Three or four equidistant cuts are then made in the obtained hemispherical anterior and posterior cups, which allow these flower-shaped cups to fall flat onto a slide (Figure 2H).
The third eyelid aids in easy and efficient handling during the dissection, thus ensuring minimal damage to the tissue while accessing the various ocular layers and when producing wholemounts. Further, the presence of the third eyelid helps to locate and examine localized interventions during visualization.
The procedure, in our lab, has been performed on a CBA/J or an rd1 mouse strain at P28 of any sex. The procedure can be performed on any strain, age, or sex of animal and has no bias according to these characteristics.
The animals were procured from commercial sources (see Table of Materials) and maintained at the Small Animal Facility (SAF) at the National Institute of Immunology (NII). They were kept in individual ventilated cages (IVC) and received ad libitum access to acidified autoclaved water and food. They were maintained at 21-23 °C and with a 14 h/10 h light/dark cycle.
Given below is a modified surgical method for the micro-dissection of a mouse eye.
Protocol
This procedure was approved by the Institutional Animal Ethics Committee of the National Institute of Immunology, New Delhi. The serial reference number of the approval is IAEC#480/18. The experiments were performed in accordance with the regulation guidelines of the Committee for Control and Supervision of Experiments on Animals, Ministry of Fisheries, Animal Husbandry and Dairying, Government of India, under the supervision of a professional Veterinarian at the SAF, NII.
1. Preparation
- Dilate the rodent eyes using a drop of 0.8% tropicamide and 5% phenylephrine ophthalmic solutions in each eye. Place the animal in a dark area for 30 min before the procedure.
NOTE: An analgesic is not required as the animal is euthanized immediately after completing the 30 min. The dark adaptation and dilating the animal's eyes allow the observer to check for ocular tears and damage before the procedure. Additionally, dilation is advantageous while working with the pigmented eyeball tissue. - Euthanize the animal by intraperitoneally administering a 5x dose of anesthesia. A typical dose is 200 µL of PBS containing 10 mg of ketamine and 1 mg of xylazine.
NOTE: The dose for euthanizing the animal is ketamine at 400-500 mg/kg body weight and xylazine at 50 mg/kg body weight given intraperitoneally. For a typical mouse weighing 20 g, the dose would be 200 µL of PBS containing 10 mg of ketamine (100 µL of a stock solution of 100 mg/mL ketamine) and 1 mg of xylazine (100 µL of a stock solution of 10 mg/mL xylazine). - Confirm the complete lack of response to a stimulus by examining the pedal reflex. The absence of a heartbeat and respiration are also important indicators.
- Place the mouse in lateral recumbency to allow complete access to the eye.
- Prepare Colibri suturing forceps, curved forceps, a micro-blade (15°, 3 mm in size), spring micro-scissors, and angled spring micro-scissors ready for the procedure.
2. Enucleation of the mouse eye along with the third eyelid
- Place the mouse in lateral recumbency to allow for complete access to the eye. Open the outer eyelids with the forceps to optimally visualize the third eyelid.
NOTE: The third eyelid, also known as the nictitating membrane, is located toward the upper corner of the nasal tangent (Figure 1A). - Identify the third eyelid as a minor translucent protuberance with a pigmented boundary (Figure 1B).
- Place curved forceps around the eyeball, and pinch to free the eye from extraneous attachments. This will minimize bleeding into the eye from the surrounding tissue.
- Replace the curved forceps with spring micro-scissors, and cut around the eyeball with the third eyelid attached to it. Use small continuous cuts to release the eyeball from the surrounding tissue attachments.
- Place curved spring micro-scissors under the eyeball to free the eye by cutting the optic nerve. Ensure that the eyeball is completely released from the eye socket.
3. Clearing the extraneous tissue
- Place the enucleated eye from the euthanized mouse in a Petri dish with phosphate-buffered saline (PBS) buffer at pH 7.4, with the Petri dish filled such that the eyeball is submerged in PBS.
- Immobilize the enucleated eyeball of the mouse with the attached third eyelid with one hand using forceps.
- Suspend the eyeball in liquid medium to permit more efficient handling. Some extraocular muscles or tissues will begin to float away from the eyeball. Remove them by cutting them off the eyeball using spring micro-scissors.
- Cut and remove the optic nerve from the base of the eyeball using micro-scissors to allow the better separation of the retina for whole-mount preparations.
NOTE: It is not necessary to remove the optic nerve for tissue sectioning. - Wash the eyeball with PBS, and transfer it into a 1.5 mL tube containing 1 mL of 4% paraformaldehyde (PFA) for a minimum of 2 h at 4 °C for tissue fixation.
NOTE: The procedure can be paused at this step by leaving the tissue in PFA for up to 12 h at 4 °C. However, for the isolation of live cells, the fixation steps should not be performed, and the entire procedure needs to be performed without pauses.
4. Dissection of the enucleated eye to obtain the anterior and posterior eyecups
- After the PFA fixation (if collecting live cells, proceed without the fixation of the eyeball), transfer the eyeball into a Petri dish containing PBS, and place it under a dissecting microscope to perform the subsequent procedure.
- Immobilize the eyeball by holding down the third eyelid firmly with Colibri suturing forceps.
- Make a small incision at the limbus by piercing it using a micro-blade. The limbus is the corneal-scleral junction. Make the incision preferably opposite the third eyelid, thereby making an access point for the spring micro-scissors.
NOTE: Using a microblade of 15° and 3 mm in size may provide better control over the depth of this incision. - Next, using this incision as a starting point, with a pair of micro-scissors, make small and continuous cuts along the circumference of the corneal-scleral limbus. First, complete a semicircle of cuts starting from the incision point to the third eyelid. Then, complete the remaining semicircle of cuts on the other side. Finally, make a cut around the third eyelid to separate the posterior cup and the anterior cup.
NOTE: Depending on the eyecup of interest, the nictitating membrane can be left attached to either the anterior or posterior eyecup. - Remove the lens from the posterior cup using forceps to obtain the posterior cup comprising the neural retina and retinal pigment epithelium (RPE) layers. The lens, posterior cup, and anterior cup of the eye are, thus, separated.
NOTE: If working with unfixed tissues, the anterior and posterior cups can now be enzymatically digested to obtain a corneal or retinal single-cell suspension. - For fixing the tissues, transfer these cups into a 1.5 mL tube containing 1 mL of 4% PFA for 12 h at 4 °C.
NOTE: Post-fixation, the tissue can be embedded in an appropriate medium, and cryo-sectioning or paraffin sectioning can be done to obtain corneal or retinal sections.
5. Isolation of the neural retina and RPE layers
- Reintroduce the posterior cup into the Petri dish containing PBS buffer to submerge the tissue.
- Check for the presence of the neural retina. This can be visualized as an opaque layer on the pigmented RPE layer, which is attached at the peripheral circumference of the cup.
- Hold the third eyelid with forceps to immobilize the posterior cup, and peel off the neural retina starting from the periphery using a second pair of forceps.
NOTE: Very gentle and slight movements of the hand are highly recommended in order to not damage the tissue. At the optic nerve exit, the curved spring scissors can be inserted under the neural retina, and a cut can be made to completely free the layers if the neural retina remains attached as depicted in Figure 3. Optionally, free or detach the retina with gentle strokes using a soft brush. However, it would not be appropriate to do so if collecting the retina for histology.
NOTE: The neural retina and pigmented RPE layer with the third eyelid are, thus, obtained.
6. Segmentation of retinal and RPE layers for wholemounts
- Reintroduce the anterior and posterior cups into the Petri dish containing PBS buffer, and place the dish under the dissecting microscope.
- Immobilize the anterior cup by holding down the third eyelid.
- Use angled spring micro-scissors to make a cut of around 3/4 of the diameter of the eyecup next to the third eyelid, cutting from the periphery perpendicularly toward the optic center.
- Maintain a grip on the third eyelid, and make a cut next to the first cut.
- Make a similar cut between the second cut and the third eyelid.
NOTE: Three or four such equidistant cuts open the hemispherical cups into a flower shape, which falls flat and can be easily mounted. - Immobilize the posterior cup by holding down the third eyelid.
- Follow the same steps as given for making the cuts (steps 6.3-6.5) on the anterior cup to make three or four equidistant cuts on the posterior cup.
NOTE: For the histology of the neural retina isolated in step 5, make similar cuts to ensure it falls flat on a surface. Do not make very deep cuts to the center of the cup, as this may make the leaf sections fall apart or separate easily. - Perform the staining, and whole-mount the tissues for effective visualization7,8.
NOTE: The presence of the nictitating membrane acts as a constant physical indicator of the nasal side of the eyecup in wholemounts. It helps to demarcate the dorsal/ventral side of the eyecup, as the third eyelid of the eyecup indicates the nasal/ventral orientation.
Representative Results
A wholemount of rd1 mouse eye/corneal tissue was prepared to study potential lymph-angiogenesis in the anterior/corneal tissue in a diseased state. The attached conjunctival tissue from the third eyelid acted as a positive control, since the cornea lacks lymphatic vessels. For the study, the corneal tissue was dissected with the conjunctiva and was fixed with 4% PFA, followed by permeabilization and blocking. The tissue was then stained with a primary antibody against the lymphatic endothelial marker (LYVE1)9. This was followed by incubation with a secondary antibody labeled with Alexa Fluor 488 (green). Representative images (Figure 4) were captured using a fluorescence microscope at 4x and 10x.
A wholemount of the neural retina from the posterior eyecup was prepared to visualize the retinal vasculature for retinal morphological, structural, and functional studies7. The neural retina was carefully peeled from the choroid-RPE layer, and cuts were made to lay it flat in a floret structure. To visualize the retinal vascular structure, the leaflet was subjected to staining with Isolectin IB4-Alexa Fluor 488 for 1 h at room temperature and visualized at 2x and 20x magnification (Figure 5).
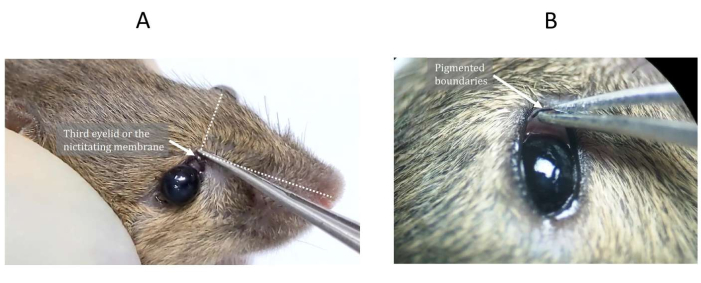
Figure 1: The third eyelid or nictitating membrane of the mouse. (A) The third eyelid is located toward the upper corner of the nasal tangent and (B) often has a pigmented boundary. Please click here to view a larger version of this figure.
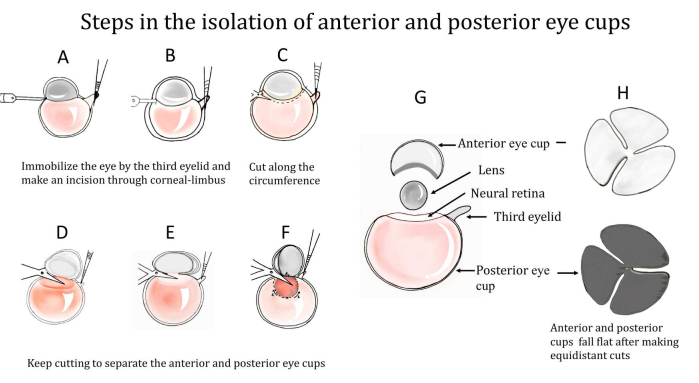
Figure 2: Steps in the isolation of the anterior and posterior eyecups from an enucleated eyeball. (A) The third eyelid is used to immobilize the eye, and (B) an incision is made through the corneal limbus. (C) An entry is made after forming the incision and cutting along the circumference, as shown in (D–F). (G) The anterior and posterior eyecups are, thus, separated. A translucent layer of the neural retina is present on the posterior eye cup, which is peeled off. (H) Three equidistant cuts are then made in the cups to make them fall flat. Please click here to view a larger version of this figure.
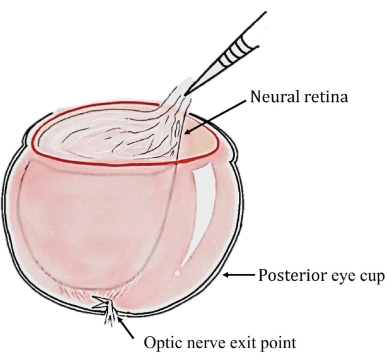
Figure 3: Schematic diagram showing how curved spring scissors can be inserted under the neural retina to free the layers. Please click here to view a larger version of this figure.
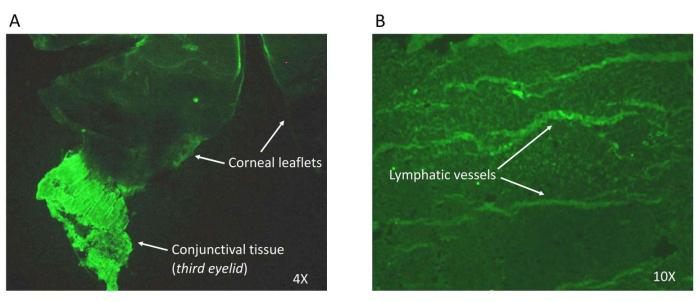
Figure 4: Wholemount of the anterior cup. The corneal tissue was microdissected as described to reveal the lymphatic vessels. The tissue was stained with the lymphatic endothelial marker (LYVE1) and an Alexa Fluor 488 (green)-labeled secondary antibody.(A) Images at 4x and (B) at 10x magnification. Please click here to view a larger version of this figure.
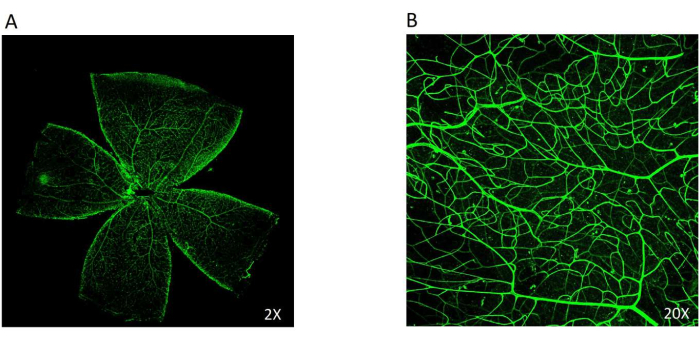
Figure 5: The wholemount of the neural retina. The Isolectin IB4 stains the retinal vasculature, which can be easily viewed in green. (A) The retinal flat mount obtained from the posterior eyecup was stained with Isolectin IB4 tagged with Alexa Fluor 488 (2x). (B) A 20x magnification of the retinal vasculature showing the intermediate vascular plexus in the retina. Please click here to view a larger version of this figure.
Discussion
Ocular microdissection has been found to be a difficult task owing to the small size and spherical shape of the rodent eye, and the rodent eye requires innovative techniques for efficient handling8.
In the current demonstrated method, the enucleated mouse eyeball is obtained with the third eyelid attached for effective and easy handling. Using the third eyelid, the eyeball can be immobilized completely, which allows the dissection to proceed with ease and with minimal errors. Further, the presence of the third eyelid delineates the orientation of the sub-parts of the eye. The eyeball is, thus, dissected with the third eyelid to obtain the anterior and posterior eyecups. The use of a slit for the entry of the scissors to allow for even and clean cuts makes it easier to work with the spherical nature of the eye. Besides, the posterior eyecup can be further dissected to obtain the neural retina and RPE layers for tissue-specific studies by gently peeling the translucent layer of the neural retina.
Furthermore, in this protocol, three/four equidistant cuts are made from the periphery perpendicularly to the optic center to reach the optic nerve in order to open the hemispherical cups into a floret shape, which falls flat and can be easily mounted.
Hence, this method is advantageous for ocular tissue-specific sectioning, single-cell analysis, and wholemounts. However, the need for repetitive tissue fixation renders the tissue unviable for cell culture or experiments that require live cells. This technique has been effectively used in our lab for corneal wholemounts, retinal sections, and single-cell studies of cellular interventions post-transplantation10. The presence of the third eyelid helps identify the orientation, which supports targeted approaches and acts as a guide during retinal visualization.
Divulgaciones
The authors have nothing to disclose.
Acknowledgements
Dr. Alaknanda Mishra, Department of Cell Biology and Human Anatomy, University of California Davis, USA, trained us in this method at the National Institute of Immunology, New Delhi. This work was supported by the core grant received from the Department of Biotechnology, Government of India to the National Institute of Immunology, New Delhi. P.S. was granted a research fellowship by the Department of Biotechnology.
Materials
| Acetaminophen (Biocetamol) | EG Pharmaceuticals | No specific Catalog Number (Local Procurement) | |
| Alkaline Phosphatase Kit (DEA) | Coral Clinical System, India | No specific Catalog Number (Local Procurement) | |
| Automated analyser | Tulip, Alto Santracruz, India | Screen Maaster 3000 | Biochemical analyser for liver functional test |
| Betadine (Povidon-Iodine Solution) | Win-Medicare; India | No specific Catalog Number (Local Procurement) | |
| Biological safety cabinet ( Class I) | Kartos international; India | No specific Catalog Number (Local Procurement) | |
| Bright Field Microscope | Olympus, Japan | LX51 | |
| CBA/J inbred mice | The Jackson Laboratory | Stock No. 000654 | |
| Cefotaxime (Taxim) | AlKem ; India | cefotaxime sodium injection, No specific Catalog Number (Local Procurement) | |
| Cell Strainer | Sigma ; US | CLS431752 | |
| Collagenase Type I | Gibco by Life Technologies | 17100-017 | |
| Cotton Buds | Pure Swabs Pvt Ltd ; India | No specific Catalog Number (Local Procurement) | |
| DPX Mountant | Sigma ; US | 6522 | |
| Drape Sheet | JSD Surgicals, Delhi, India | No specific Catalog Number (Local Procurement) | |
| Eosin Y solution, alcoholic | Sigma ; US | HT110132 | |
| Forceps | Major Surgicals; India | No specific Catalog Number (Local Procurement) | |
| Gas Anesthesia System | Ugo Basile; Italy | 211000 | |
| Glucose | Himedia, India | GRM077 | |
| Hair removing cream (Veet) | Reckitt Benckiser , India | No specific Catalog Number (Local Procurement) | |
| Hematoxylin Solution, Mayer's | Sigma ; US | MHS16 | |
| Heparin sodium salt | Himedia; India | RM554 | |
| Hyaluronidase From Sheep Testes | Sigma ; US | H6254 | |
| I.V. Cannula (Plusflon) | Mediplus, India | Ref 1732411420 | |
| Insulin Syringes | BD ; US | REF 303060 | |
| Isoflurane ( Forane) | Asecia Queenborough | No B506 | Inhalation Anaesthetic |
| Ketamine (Ketamax) | Troikaa Pharmaceuticals Ltd. | Ketamine hydrochloride IP, No specific Catalog Number (Local Procurement) | |
| Meloxicam (Melonex) | Intas Pharmaceuticals Ltd; India | No specific Catalog Number (Local Procurement) | |
| Micro needle holders straight & curved | Mercian ; England | BS-13-8 | |
| Micro needle holders straight & curved |
Mercian ; England | BS-13-8 | |
| Microtome | Histo-Line Laboratories, Italy | MRS3500 | |
| Nylon Thread | Mighty ; India | No specific Catalog Number (Local Procurement) | |
| Paraformaldehyde | Himedia; India | GRM 3660 | |
| Percoll | GE Healthcare | 17-0891-01 | |
| Refresh Tears/Eyemist Gel | Allergan India Private Limited/Sun Pharma, India | P3060 | No specific Catalog Number |
| RPMI | Himedia; India | No specific Catalog Number (Local Procurement) | |
| Scalpel | Major Surgicals; India | No specific Catalog Number (Local Procurement) | |
| Scissors | Major Surgicals; India | No specific Catalog Number (Local Procurement) | |
| SGOT (ASAT) KIT | Coral Clinical System, India | No specific Catalog Number (Local Procurement) | |
| SGPT (ALAT) KIT | Coral Clinical System, India | No specific Catalog Number (Local Procurement) | |
| Shandon Cryotome E Cryostat | Thermo Electron Corporation ; US | No specific Catalog Number | |
| Sucrose | Sigma ; US | S0389 | |
| Surgical Blade No. 22 | La Medcare, India | No specific Catalog Number (Local Procurement) | |
| Surgical Board | Locally made | No specific Catalog Number (Local Procurement) | |
| Surgical White Tape | 3M India ; India | 1530-1 | Micropore Surgical Tape |
| Sutures | Ethicon, Johnson & Johnson, India | NW 5047 | |
| Syringes (1ml, 26 G) | Dispo Van; India | No specific Catalog Number (Local Procurement) | |
| Trimmer (Clipper) | Philips | NL9206AD-4 DRACHTEN QT9005 | |
| Weighing Machine | Braun | No specific Catalog Number (Local Procurement) | |
| William's E Media | Himedia; India | AT125 | |
| Xylazine (Xylaxin) | Indian Immunologicals Limited | Sedative, Pre-Anaesthetic, Analgesic and muscle relaxant |
Referencias
- Choi, Y., et al. Studying cancer immunotherapy using patient-derived xenografts (PDXs) in humanized mice. Experimental and Molecular Medicine. 50 (8), 1-9 (2018).
- Sutherland, C., et al. Laser Capture Microdissection of Highly Pure Trabecular Meshwork from Mouse Eyes for Gene Expression Analysis. J Vis Exp. (136), e57576 (2018).
- Fernandez-Godino, R., Garland, D. L., Pierce, E. A. Isolation, culture and characterization of primary mouse RPE cells. Nature Protocols. 11 (7), 1206-1218 (2016).
- Shang, P., Stepicheva, N. A., Hose, S., Zigler, J. S., Sinha, D. Primary cell cultures from the mouse retinal pigment epithelium. Journal of Visualized Experiments. (133), e56997 (2018).
- Claybon, A., Bishop, A. J. Dissection of a mouse eye for a whole mount of the retinal pigment epithelium. Journal of Visualized Experiments. (48), e2563 (2011).
- Steven, P., et al. Experimental induction and three-dimensional two-photon imaging of conjunctiva-associated lymphoid tissue. Investigative Ophthalmology and Visual Science. 49 (4), 1512-1517 (2008).
- Tual-Chalot, S., Allinson, K. R., Fruttiger, M., Arthur, H. M. Whole mount immunofluorescent staining of the neonatal mouse retina to investigate angiogenesis in vivo. Journal of Visualized Experiments. (77), e50546 (2013).
- Ullmann, J. F., Moore, B. A., Temple, S. E., Fernandez-Juricic, E., Collin, S. P. The retinal wholemount technique: A window to understanding the brain and behaviour. Brain, Behavior and Evolution. 79 (1), 26-44 (2012).
- Nakao, S., Hafezi-Moghadam, A., Ishibashi, T. Lymphatics and lymphangiogenesis in the eye. Journal of Ophthalmology. 2012, 783163 (2012).
- Mishra, A., et al. Peripheral blood-derived monocytes show neuronal properties and integration in immune-deficient rd1 mouse model upon phenotypic differentiation and induction with retinal growth factors. Stem Cell Research and Therapy. 11 (1), 412 (2020).

