Flotation-Based T Cell Isolation, Activation, and Expansion from Human Peripheral Blood Mononuclear Cell Samples Using Microbubbles
Summary
The objective of this study is to demonstrate the feasibility of flotation-based separation to isolate, activate, and expand primary human T cells.
Abstract
The process of isolating T cells from peripheral blood mononuclear cells (PBMCs) to establish ex vivo cultures is crucial for research, clinical testing, and cell-based therapies. In this study, a simple, novel protocol to isolate, activate, and expand T cells from PBMCs ex vivo is presented. This study utilizes functionalized buoyancy-activated cell sorting (BACS) technology to isolate and activate T cells. Briefly, the protocol involves the positive selection of CD3+ cells from leukopak-derived PBMCs, followed by a 48 h co-stimulation with pre-conjugated anti-CD28-bound streptavidin microbubbles (SAMBs) prior to transduction in 24-well plates. Functionalized microbubbles offer a unique opportunity to buoyantly activate cells, leading to proliferative phenotypes that allow for expansion with minimal exhaustion. This technique offers reduced exhaustion because the co-stimulatory microbubbles remain buoyant and return to the top of the culture medium, thus reducing the amount of time that the expanding cells are in contact with the co-stimulatory factors. The results indicate that the isolated and cultured T cells receive enough stimulation to activate and proliferate but not to an extent that leads to overactivation, which then leads to exhaustion, as demonstrated by the presence of excessive PD-1.
Introduction
More than 500 chimeric antigen receptor (CAR)-T cell therapy clinical trials are currently being conducted across the world, and four CAR-T cell therapy products are available on the market1. However, numerous CAR-T cell research and manufacturing needs still exist that must be addressed to improve the efficacy, scalability, and long-term success of these potentially curative therapies2,3,4,5. Adoptive CAR-T cell clinical research and manufacturing begins with T cell isolation from a peripheral blood sample and the subsequent stimulation, transduction, and expansion of the isolated cells. Parameters such as T cell recovery, purity, and activation/exhaustion signals require careful consideration when choosing the cell isolation and stimulation techniques for CAR-T cell research and manufacturing3,4,6. Importantly, improvement in the therapeutic persistence of CAR-T cell therapies by minimizing the biological impediments that result from the current manufacturing processes, such as T cell exhaustion, is needed to enhance the therapeutic efficacy6,7.
As an alternative to traditional cell isolation methods such as fluorescence-activated cell sorting (FACS) and magnetic-activated cell sorting (MACS), here, buoyancy-activated cell sorting (BACS) with microbubbles for T cell isolation is demonstrated. Microbubble separation uses buoyant, hollow microspheres (microbubbles) to bind the targets and float them to the surface of fluid samples8,9. By functionalizing microbubbles with antibodies (i.e., anti-CD3), the desired T cell populations can be positively selected from peripheral blood samples. Subsequently, the use of a different population of antibody-functionalized microbubbles (i.e., anti-CD28) to co-stimulate and activate positively selected T cells in suspension is demonstrated in this work. Microbubbles offer a simple and highly tunable isolation and activation workflow that generates T cells ready for suspended cell culture and downstream applications such as genetic modification and expansion. Critically, buoyant cell activation with microbubbles promotes restrained cell stimulation to prevent excessive T cell exhaustion7.
For this study, flow cytometry was the primary tool used to analyze the isolation, activation, and transduction success of the functionalized microbubbles, as well as to provide detailed information about the specific subpopulations present during the growth and expansion phases post-transduction. In addition to flow cytometry, brightfield and fluorescence microscopy were used to confirm the cell health, morphology, and transduction success. Based on these results, the microbubble technology and protocol provide a more tunable and gentler alternative to the traditional isolation and activation methods currently in use today; in particular, microbubble-activated cells show notably lower expression of T cell exhaustion markers than that typically observed with industry-standard tools and kits.
Protocol
1. Isolation of T cells with microbubbles using positive selection
NOTE: This protocol details a small-scale CD3+ positive selection approach using SAMBs.
- Incubate 3 x 108 commercially obtained PBMCs in 2.5 mL of separation buffer with biotinylated anti-CD3 (OKT3) antibody at a concentration of 25 ng of antibody per 1 million cells (25 ng/M). Gently mix by pipetting up and down, and incubate at room temperature for 10 min.
- Add streptavidin microbubbles (SAMBs) at a ratio of 0.5 (SAMB quantity):1 (cell quantity) according to the manufacturer's reported SAMB concentration.
- Mix using a commercial end-over-end (EOE) rotator at 20 rpm for 10-15 min at room temperature. Centrifuge for 5 min at 400 x g at room temperature.
- After centrifugation, the positively selected cells will be at the top of the suspension with the SAMBs. The remaining non-selected cells will be in the cell pellet at the bottom of the tube. Using a 9 in glass pipette, insert the tip below the bubble-cell layer to the bottom of the tube, aspirate the cell pellet and subnatant with an electronic pipette and transfer them to a new tube.
2. Co-stimulation (activation) of the positively selected T cells
- Resuspend the bubble-cell layer remaining in the original tube in 1 mL of complete T cell medium (or another desired medium).
- Count the cells in the subnatant with brightfield microscopy using an automated cell counter, and subtract this value from the starting cell number to determine the number of cells captured in the bubble-cell layer.
- Prior to this step, mix the biotinylated anti-CD28 antibody with commercial SAMBs for a minimum of 2 h to create the conjugated anti-CD28 SAMBs. Contact the SAMBs manufacturer to determine the amount of anti-CD28 antibody needed for conjugation. Add the anti-CD28 conjugated SAMBs to the bubble-cell suspension resulting from step 2.1 using a ratio of 1.5 (anti-CD28 SAMBs):1 (cells).
- Mix using EOE rotation for 15 min, and then adjust the total volume to 2 million cells per mL with complete T cell medium or another desired medium according to the cell number obtained in step 2.2.
3. Expansion of the co-stimulated cells in cell culture medium
- Distribute 1 mL of activated cells from step 2.4 at a concentration of 2 M/mL per well in a 24-well plate. Incubate in a humidified 5% CO2 incubator at 37 °C.
- After 24 h, add 50 U/mL of IL-2 and 25 ng/M of soluble anti-CD3 (OKT3) to further encourage expansion, as calculated using the initial number of cells plated on day 0. Place the cell plate back into the humidified CO2 incubator, and let it incubate overnight at 37 °C.
4. Optional: Transduction of activated T cells with lentivirus
NOTE: The approach used here is adapted from Prommersberger et al.10.
- Thaw the lentivirus at room temperature, and briefly mix by pipetting.
- Gently remove the mid-natant, 600 µL from each well, without touching the daughter cells that are now at the bottom of the well or the bubble layer that has remained at the surface of the solution.
- Add 5 µg/mL hexadimethrine bromide per well to enhance viral transduction. Add the lentiviral particles at a multiplicity of infection (MOI) of 3 (lentiviral particles per cell).
- Centrifuge the plate for 45 min at 800 x g and 32 °C using slow acceleration and no break for deceleration. Incubate the cells for 4 h in a humidified CO2 incubator at 37 °C.
- After 4 h, add 600 µL of commercially available, fresh complete T cell medium and 50 U/mL of IL-2 to each well, and place the cell plate back into the humidified CO2 incubator at 37 °C for T cell expansion.
5. Expansion of the T cells (with or without prior transduction)
- Every 2 days, remove half of the medium from the mid-natant, replace it with fresh, complete T cell medium, and add IL-2 at a concentration of 50 U/mL.
- Count the T cells twice per week to assess the cell density. When the cell density exceeds 2 x 106-2.5 x 106 cells/mL, transfer the cells into a bigger vessel, diluting them to 5 x 105 cells/mL.
6. Harvesting the T cells and flow cytometry
- Gently mix the contents of each well by pipetting up and down. Remove the entire contents of the well, including the microbubbles, and transfer them into a 1.5 mL tube.
- Wash each well with 400 µL of calcium-free and magnesium-free DPBS (−/−), and transfer the solution into a 1.5 mL tube. Centrifuge at 400 x g for 5 min at room temperature.
- Aspirate the supernatant, and resuspend the cell pellet in 50 µL of separation buffer.
- Stain with an activation and exhaustion antibody/staining cocktail, and incubate for 10 min at room temperature in the dark. Prepare the staining cocktails as follows- activation cocktail: AF700-CD3, PE/Dazzle-CD4, PE/Cy7-CD8, BV510-CD25, PE-CD69; exhaustion cocktail: AF700-CD3, PE/Dazzle-CD4, PE/Cy7-CD8, PE-PD-1.
- Add 1 mL of separation buffer, and gently mix. Centrifuge at 400 x g for 5 min at room temperature to wash out excess antibodies. Aspirate the supernatant completely.
- Resuspend the cell pellet in 1 mL of separation buffer, and transfer to an appropriate vessel (e.g., a FACS tube, a 96-well plate, etc.) for the flow cytometry analysis. The recommended flow cytometry analysis gating scheme is detailed in Figure 1.
Representative Results
T cells were isolated from purchased PBMCs and plated for activation as described in the protocol. The negative control samples (purchased PBMCs) were not activated. These control samples were included to demonstrate the effect that the microbubble activation process had on the experimental samples as compared to the untouched and unstimulated T cell controls, ensuring that the activation markers observed were the result of the added activation factors and were not inherent to the T cells themselves. As per the experimental outline in Figure 2, the cells were seeded at 2 x 106 cells/mL in T cell medium and were either untouched/unstimulated or co-stimulated with anti-CD3 (clone OKT3) and anti-CD28 (clone 28.2) SAMBs. After 48 h of stimulation in culture, the cells were transduced with the lentiviral particle encoding for zsGreen. At 4 days and 6 days post-transduction, the cells were imaged, harvested, and surface-stained using AF700-CD3, PE/Dazzle-CD4, PE/Cy7-CD8, BV510-CD25, PE-PD-1 (or PE-CD69 depending on if the exhaustion or activation panels were used, respectively), and 7-AAD. The zsGreen transgene was detectable in the FITC channel. The flow cytometry gating approach is detailed in Figure 1. Increases in viable T cell numbers and transgene-positive T cells were observed between the control sample and the cells that received microbubble co-stimulation (Figure 3). Increased effector cell populations were also observed in the microbubble samples (Figure 4). T cells expressing increased activation and exhaustion markers were observed among the cell samples that received microbubble co-stimulation (Figure 5 and Figure 6). Cell expansion was observed between the day 4 and day 6 time points of the co-stimulated samples, indicating that the cells were active, proliferative, and passing the transgene as they expanded.
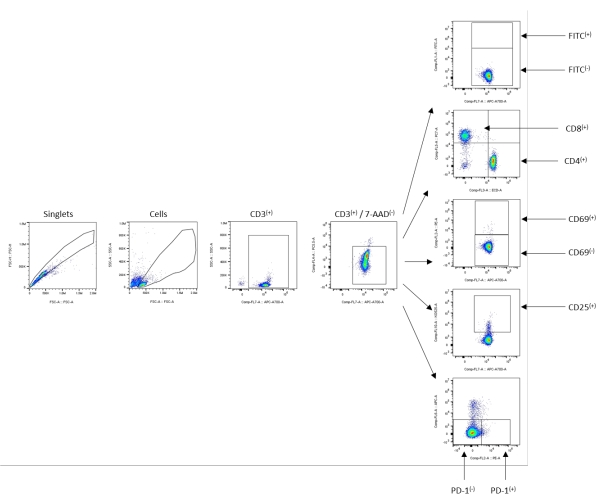
Figure 1: Example gating scheme-untouched/negative control sample. Starting from the singlets, the population cells were gated next using SSC-A/FSC-A. The total CD3+ cells were gated out, followed by viable CD3+ gating using 7-AAD to determine the viability of the population. All the subsequent populations and calculations were determined from the viable 7-AAD(−)/CD3+(+) population, as shown using the arrows indicating the subpopulation gates. Please click here to view a larger version of this figure.
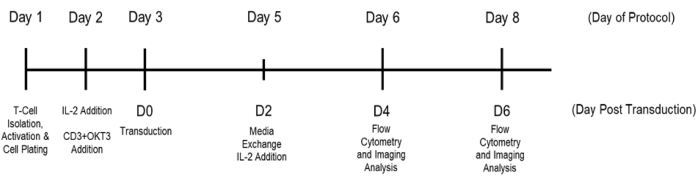
Figure 2: Experimental timeline overview. The days of the protocol are noted above, and the corresponding days post-transduction (D0-D6) are used in the figures below. The cells were plated immediately following selection and activation. The control wells were generated using a microbubble negative selection protocol. The control T cells did not receive co-stimulation agents and did not undergo transduction, although they did receive IL-2 to ensure the cells were kept healthy enough to maintain reasonable viability throughout the experiment. Please click here to view a larger version of this figure.
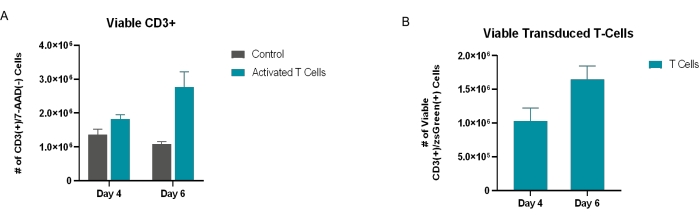
Figure 3: Assessment of viable and successfully transduced cells post-transduction. (A) Viable CD3+ cells 4 days and 6 days post-transduction. Viability was determined through flow cytometry analysis, in which the population was quantified by gating on 7-AAD(−)/CD3+(+) cells. (B) The number of cells successfully transduced with rLV.EF1.zsGreen was determined through flow cytometry, in which the viable 7-AAD(−)/CD3+(+) population was further gated into zsGreen(+) cells. All the conditions were performed in triplicate (n = 3). The data represent mean ± SD. Please click here to view a larger version of this figure.
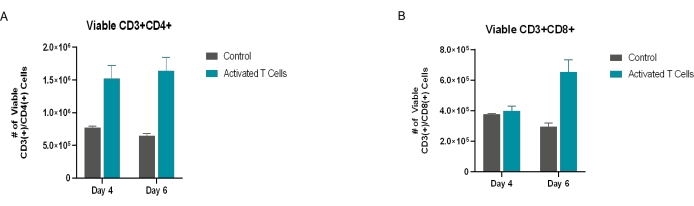
Figure 4: Viable CD4+ and CD8+ T cells. The CD4+ and CD8+ T cell subpopulations were quantified by gating on the viable CD3+ population (CD3+ [+]/7-AAD[−]) and measuring the expression of (A) CD4+ and (B) CD8+. All the conditions were performed in triplicate (n = 3). The data represent mean ± SD. Please click here to view a larger version of this figure.
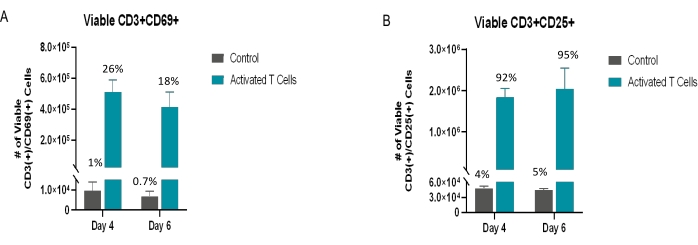
Figure 5: Viable activated T cells. The viable CD3+ population was also analyzed for specific activation markers as denoted in the figures above. (A) CD69 is an early marker of activation; (B) CD25 is a middle-to-late activation marker. The percentages above the error bars represent the percentage of viable CD3+ cells expressing the respective marker. All the conditions were performed in triplicate (n = 3). The data represent mean ± SD. Please click here to view a larger version of this figure.
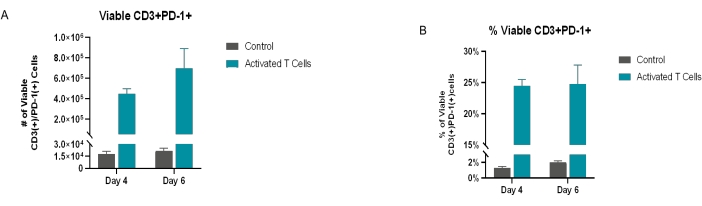
Figure 6: Exhausted T cells. The viable CD3+ population was also analyzed for exhaustion (PD-1) markers. (A) The total number of 7-AAD(−)/CD3+(+)/PD-1+(+) cells at day 4 and day 6 post-transduction. (B) The percentage of PD-1+ cells. On day 4 and day 6, the activated/transduced sample population had ~25% viable CD3+/PD-1+ cells, whereas the control sample population had ~2% viable CD3+/PD-1+ cells. Of note, the starting/isolated material had <~15% viable CD3+/PD-1+ cells (post-isolation/pre-culture data not shown). All conditions were performed in triplicate (n = 3). The data represent mean ± SD. Please click here to view a larger version of this figure.
Discussion
The described protocol allows for the isolation of T cells from PBMC samples and the activation of suspended T cells in culture media with microbubbles. This method relies on functionalized microbubbles whose inherent buoyancy offers a unique opportunity to introduce co-stimulatory signals to cells and activate them while they are suspended in a culture medium, thereby reducing the exposure of the expanding cells to prolonged stimulation; such overstimulation can result in the increased expression of T cell exhaustion markers and reduced therapeutic efficacy11. Stimulated T cells that are buoyantly attached to functionalized microbubbles produce untouched daughter cells that drop to the bottom of the cell culture plate for expansion, allowing a period of growth away from the buoyant stimulation factors. It has been detailed in the literature how the prolonged exposure of isolated T cells to stimulation factors-such as magnetic bead-based protocols12-can adversely impact the expansion and therapeutic efficacy 6,7,11.
As this reported protocol relies on the positive selection of CD3+ cells, it is critical to remove the subnatant below the bubble-cell layer carefully but thoroughly during the isolation phase of this protocol. This ensures that only the positively selected T cell population is further stimulated and plated. This is also an important step for determining the number of cells selected from the starting PBMC sample, which is necessary for accurate co-stimulation and plating calculations.
These microbubble protocol development activities for T cell activation and expansion leveraged a wide array of markers during flow cytometry analysis, allowing for the thorough characterization of the isolated and stimulated T cell population to assess critical T cell parameters, including activation, exhaustion, and clonal expansion. When compared to commonly used T cell isolation and stimulation technologies, such as magnetic bead-based protocols, this microbubble protocol aims to minimize the overstimulation of cells without sacrificing the expansion and corresponding effector function abilities of the isolated T cells. Future applications of this microbubble technique will include various protocols for T cell positive selection, co-stimulation, and subsequent microbubble cell cultures to meet a variety of workflow needs for cell therapy research and manufacturing communities.
Divulgaciones
The authors have nothing to disclose.
Acknowledgements
None.
Materials
| 2-Mercaptoethanol | Gibco | 21985-023 | CAS: 60-24-2 |
| Biologix Multi-Well Culture Plates 24-well plates | VWR | 76081-560 | |
| Biotin anti-human CD28 (28.2) Antibody | Biolegend | 302904 | |
| Biotin anti-human CD3 (OKT3) Antibody | Biolegend | 317320 | |
| DPBS, no calcium, no magnesium | Gibco | 14190-136 | |
| GlutaMAX Supplement | Thermofisher | 35050061 | |
| Human Recombinant IL2 | BioVision (vwr) | 10006-122 | |
| Lentiviral Particle rLV.EF1.zsGreen1-9 | Takara Bio | 0038VCT | |
| Leukopak | BioIVT | HUMANLMX100-0001129 | |
| Normal Human PBMCs | BioIVT | HUMANHLPB-0002562 | |
| Penicillin/Streptomycin 100X for tissue culture | VWR | 97063-708 | CAS: 8025-06-7 |
| Polybrene Infection/Transfection Reagent | Millipore Sigma | TR-1003-G | CAS:28728-55-4 |
| Pooled Human AB Serum Plasma Derived Heat Inactivated | Innovative Research | ISERABHI100mL | |
| RPMI 1640 Medium, GlutaMAX Supplement, HEPES | Gibco | 72400047 | |
| Streptavidin Microbubble Kit (includes Akadeum's separation buffer) | Akadeum | 11110-000 |
Referencias
- Albinger, N., Hartmann, J., Ullrich, E. Current status and perspective of CAR-T and CAR-NK cell therapy trials in Germany. Gene Therapy. 28 (9), 513-527 (2021).
- Tyagarajan, S., Spencer, T., Smith, J. Optimizing CAR-T cell manufacturing processes during pivotal clinical trials. Molecular Therapy. Methods & Clinical Development. 16, 136-144 (2019).
- Stock, S., Schmitt, M., Sellner, L. Optimizing manufacturing protocols of chimeric antigen receptor T cells for improved anticancer immunotherapy. International Journal of Molecular Sciences. 20 (24), 6223 (2019).
- Rohaan, M. W., Wilgenhof, S., Haanen, J. B. A. G. Adoptive cellular therapies: The current landscape. Virchows Archiv. 474 (4), 449-461 (2019).
- Abou-El-Enein, M., et al. Scalable manufacturing of CAR T cells for cancer immunotherapy. Blood Cancer Discovery. 2 (5), 408-422 (2021).
- Poltorak, M. P., et al. Expamers: A new technology to control T cell activation. Scientific Reports. 10, 17832 (2020).
- Kagoya, Y., et al. Transient stimulation expands superior antitumor T cells for adoptive therapy. JCI Insight. 2 (2), 89580 (2017).
- Snow, T., Roussey, J., Wegner, C., McNaughton, B. Application No. 63/326,446. US Patent. , (2022).
- McNaughton, B., et al. Application No. 16/004,874. US Patent. , (2018).
- Prommersberger, S., Hudecek, M., Nerreter, T. Antibody-based CAR T cells produced by lentiviral transduction. Current Protocols in Immunology. 128 (1), 93 (2020).
- Wijewarnasuriya, D., Bebernitz, C., Lopez, A. V., Rafiq, S., Brentjens, R. J. Excessive costimulation leads to dysfunction of adoptively transferred T cells. Cancer Immunology Research. 8 (6), 732-742 (2020).
- Li, Y., Kurlander, R. J. Comparison of anti-CD3 and anti-CD28-coated beads with soluble anti-CD3 for expanding human T cells: Differing impact on CD8 T cell phenotype and responsiveness to restimulation. Journal of Translational Medicine. 8, 104 (2010).

