Summary
We introduce a method for quantifying Stentor habituation using a microcontroller board-linked apparatus that can deliver mechanical pulses at a specified force and frequency. We also include methods for assembling the apparatus and setting up the experiment in a way that minimizes external perturbations.
Abstract
Learning is usually associated with a complex nervous system, but there is increasing evidence that life at all levels, down to single cells, can display intelligent behaviors. In both natural and artificial systems, learning is the adaptive updating of system parameters based on new information, and intelligence is a measure of the computational process that facilitates learning. Stentor coeruleus is a unicellular pond-dwelling organism that exhibits habituation, a form of learning in which a behavioral response decreases following a repeated stimulus. Stentor contracts in response to mechanical stimulation, which is an apparent escape response from aquatic predators. However, repeated low-force perturbations induce habituation, demonstrated by a progressive reduction in contraction probability. Here, we introduce a method for quantifying Stentor habituation using a microcontroller board-linked apparatus that can deliver mechanical pulses at a specified force and frequency, including methods for building the apparatus and setting up the experiment in a way that minimizes external perturbations. In contrast to the previously described approaches for mechanically stimulating Stentor, this device allows the force of stimulation to be varied under computer control during the course of a single experiment, thus greatly increasing the variety of input sequences that can be applied. Understanding habituation at the level of a single cell will help characterize learning paradigms that are independent of complex circuitry.
Introduction
Learning is usually associated with a complex nervous system, but there is increasing evidence that life at all levels, down to single cells, can display intelligent behaviors. In both natural and artificial systems, learning is the adaptive updating of system parameters based on new information1, and intelligence is a measure of the computational process that facilitates learning2.
Stentor coeruleus is a unicellular pond-dwelling organism that exhibits habituation, a form of learning in which a behavioral response decreases following a repeated stimulus3. Stentor contracts in response to mechanical stimulation3, which is an apparent escape response from aquatic predators. However, repeated low-force perturbations induce habituation, demonstrated by a progressive reduction in contraction probability3. The habituated Stentor still contracts after receiving high-force mechanical stimulation4 or photic stimulation5. These observations, which align with Thompson and Spencer's classic criteria for habituation in animals6, strongly suggest that the original contractile response decrement is due to learning rather than fatigue or ATP depletion. As a free-living cell, Stentor can be studied without much interference from surrounding cells, as would be the case in a multicellular tissue. Several additional features make Stentor a tractable system for studying learning: its large size (1 mm), its quantifiable habituation response3, the ease of injection and micromanipulation7, the fully sequenced genome8, and the availability of RNA interference (RNAi) tools9. Using this model organism to explore cell learning without a brain or nervous system requires a reproducible procedure for stimulating Stentor cells and measuring the response.
Here, we introduce a method for quantifying Stentor habituation using a microcontroller board-linked apparatus that can deliver mechanical pulses at a specified force and frequency, including methods for building the apparatus and setting up the experiment in a way that minimizes external perturbations (Figure 1). Understanding habituation at the level of a single cell will help characterize learning paradigms that are independent of complex circuitry.
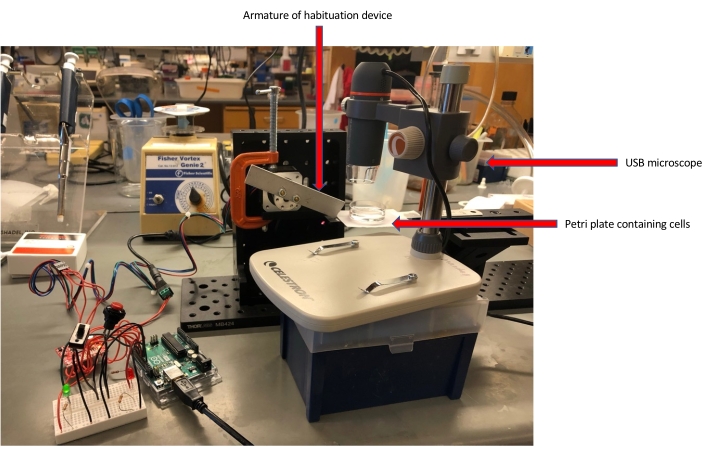
Figure 1: Habituation experiment setup. The Petri plate containing Stentor is placed atop the flexible metal ruler of the habituation device. The armature of the habituation device then hits the metal ruler at a specified force and frequency, producing a stimulus wave across the field of cells. The USB microscope camera records the responses of the Stentor to the stimulation. Please click here to view a larger version of this figure.
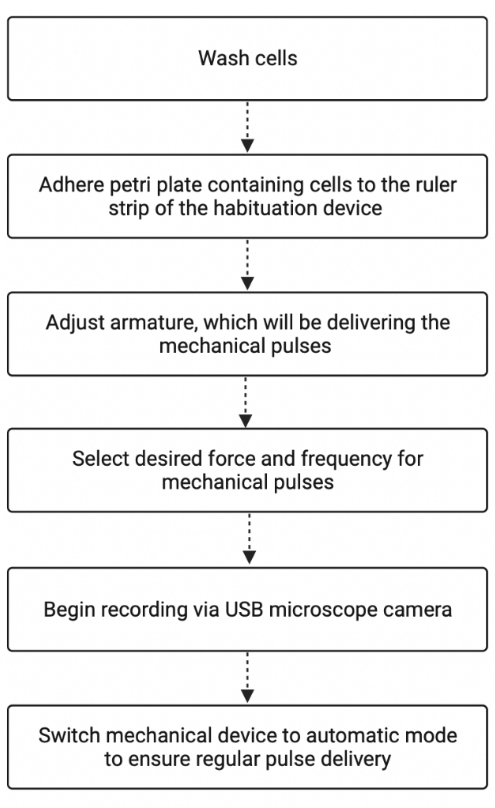
Figure 2: Summary of the habituation experiment workflow. The figure shows the basic steps involved in studying Stentor using the habituation device. The figure was created with BioRender.com. Adapted from "Process Flowchart", by BioRender.com (2022). Retrieved from https://app.biorender.com/biorender-templates. Please click here to view a larger version of this figure.
Protocol
NOTE: A summary of the habituation experiment workflow is shown in Figure 2.
1. Assembling the habituation device
- Hook the motor driver to the motor (see Figure 3).
- Connect the two wires labeled A from the driver board to the blue and red wires on the motor. Connect the two wires labeled B from the driver board to the green and black wires on the motor.
NOTE: Looking down on the driver board from above with the motor wires at the top, the four input wires should connect to the motor leads in this order: blue, red, black, and green.
- Connect the two wires labeled A from the driver board to the blue and red wires on the motor. Connect the two wires labeled B from the driver board to the green and black wires on the motor.
- Build the breadboard circuit shown in Figure 4, with special care to connect the LEDs in the correct polarity.
- Connect the Vcc (+5 V) from the driver board to the top rail of the white breadboard and the Gnd from the driver board to the bottom rail of the breadboard.
- Connect the ground of the breadboard to the ground pin of the microcontroller board. Connect the green LED, red LED, switch, and button wires, respectively, to the microcontroller board digital pins 8, 9, 10, and 11.
- Connect the microcontroller board digital pins 2 and 3 to the driver board wires Step and Dir.
- Connect the microcontroller board digital pins 4, 5, 6, and 7 to the driver board wires.
- Connect Pin 4 to MS1, connect Pin 5 to MS2, connect Pin 6 to MS3, and connect Pin 7 to Enable.
- Power the driver board with a 12 V power supply. Plug the 12 V supply into the black/green adaptor plug attached by two red wires to the motor driver board.
NOTE: Do not plug the 12 V supply into the microcontroller board plug. - Download the control program (https://github.com/WallaceMarshallUCSF/StentorHabituation/blob/main/stentor_habituator_stepper_v7.ino) onto the microcontroller board.
- Use a USB cable to attach the microcontroller board to a computer, which will also serve as the power source for the microcontroller board.
- Check that user controls are working.
- Confirm that the slide switch turns the automatic mode on and off. In automatic mode, the system will take a step at regular intervals specified by the user (see below).
- Check that the green LED turns on when the automatic mode is on.
- Check that the red LED flashes 1 s before the motor applies a pulse. The red LED is a warning light that indicates when the system is about to deliver a mechanical pulse.
- Test the red button, which triggers a 1/16 micro step every time the button is pushed, regardless of whether the system is in automatic mode.
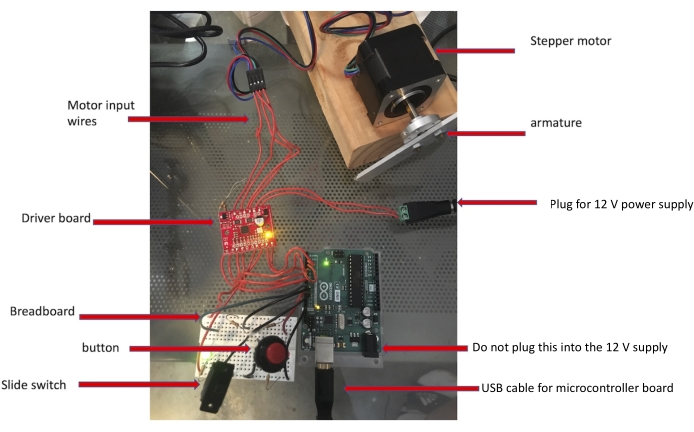
Figure 3: Components of the habituation device. All the labeled electronics are required to assemble the machine. Please click here to view a larger version of this figure.
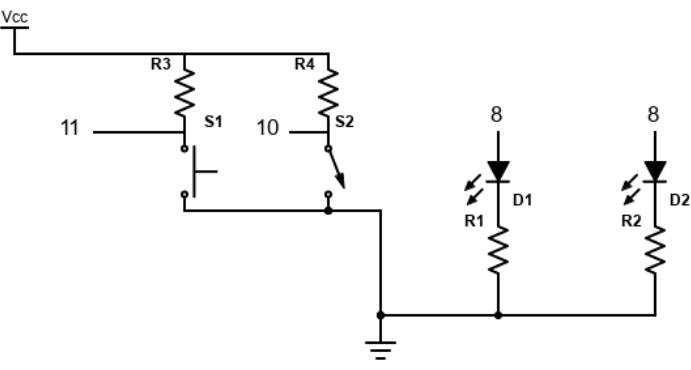
Figure 4: Electronics schematic. This is the circuit on the breadboard. The wires connecting to the microcontroller board are numbered as described in the protocol. D1 and D2 are the red and green LEDs, respectively, and are connected to ground through 330 Ω resistors. The two switches are pulled up with 10 KΩ resistors. Please click here to view a larger version of this figure.
2. Setting up the habituation experiment
- Obtain Stentor.
- Coat a 35 mm plate with 0.01% poly-ornithine solution.
- Add 3 mL of the 0.01% poly-ornithine solution to the plate and leave overnight.
- Wash the plate twice with ultrapure water and once with pasteurized spring water (PSW) (Table of Materials).
- Add 3.5 mL of PSW to the 35 mm plate.
- Wash the Stentor in a 6-well plate (Table of Materials).
- Add 3 mL of PSW to the first well and 5 mL of PSW to the second and third wells. Use a P1,000 pipette to add 2 mL of Stentor from a culture dish to the first well of the 6-well plate.
- Identify individual Stentor with a stereo microscope (Table of Materials) and then use a P20 pipette to transfer 100 Stentor from the first well to the second well.
- Identify individual Stentor with a stereo microscope and then use a P20 pipette to transfer 100 Stentor from the second well to the third well.
- Use a P200 pipette to transfer 100 Stentor in a total volume of 500 µL from the third well of the 6-well plate into the 35 mm plate such that the final volume in the 35 mm plate is 4 mL.
- Tape a piece (7 cm x 7 cm) of white paper to the metal ruler on the habituation device. Ensure that the left edge of the paper is 2 cm from the end of the ruler closest to the armature.
- Use double-sided tape to adhere the bottom of the 35 mm plate to the center of the 2 in x 2 in paper atop the ruler on the habituation device.
- Leave the 35 mm plate on the habituation device for at least 2 h (this can be extended to overnight) with the lid closed. Throughout this acclimatization period, keep the plate in ambient light conditions that match the experimental light conditions (i.e., do not subject the cells to light/dark fluctuations). Furthermore, ensure that the plate does not experience any mechanical perturbations from accidental jostling.
- Center the USB microscope camera (Table of Materials) directly above the 35 mm plate of Stentor. If necessary, place a prop such as a pipette tip box underneath the universal serial bus (USB) microscope camera to adjust the height. Alternatively, a ring stand can be used to adjust the height.
- Install the Webcam recorder application on a laptop (Table of Materials) and use it to visualize the cells via the microscope input.
- Open the Webcam recorder app and select the USB microscope from the dropdown menu. Adjust the focus on the USB microscope camera so that the cells are clearly in view.
- Adjust the position of the USB microscope camera to maximize the number of cells in the field of view.
- Open the microcontroller board serial monitor: select No Line Ending and set it to 9,600 baud.
- Use the l command on the microcontroller board program to lower the armature until it barely touches the ruler. Use the r command to raise the arm if necessary to adjust the exact position.
NOTE: If the armature is a significant distance away from the ruler, type in the d command to disable the motor coil current so that the arm can be moved manually toward the ruler. After moving the arm manually, use the e command to enable the motor coil current and keep the arm locked in position. When properly lowered prior to the start of an experiment, the bottom tip of the armature should be 1 cm away from the left edge of the ruler. The armature will deliver the mechanical pulse by hitting the ruler. - Use the i command to initialize the automatic mode on the habituation device.
- Enter the step size in the command line. Level 5 is the smallest step, and Level 1 is the largest step. Level 4 is the step size used for baseline habituation experiments.
NOTE: A Level 5 stimulus results in a downward displacement of the ruler by ~0.5 mm; Level 4 results in downward displacement by ~1 mm; Level 3 results in downward displacement by ~2 mm; Level 2 results in downward displacement by ~3-4 mm; and Level 1 results in downward displacement by ~8 mm. A Level 5 stimulus results in a downward peak force of the armature against the ruler of ~0.122 N; Level 4 results in a downward peak force of ~0.288 N; and Level 3 results in a downward peak force of ~0.557 N. The downward forces generated by Level 1 and Level 2 are more difficult to empirically quantify with a dynamometer due to the significant ruler oscillations that occur after the armature makes contact. - Enter the time between pulses in minutes. The interval used for baseline habituation experiments is 1 min.
- Start taking a video using the Webcam recorder app by pressing the red record button. Then, flip the switch on the habituation apparatus to begin the experiment with the first automated mechanical pulse delivery.
3. Analyzing the experiment video
- Immediately before the first mechanical pulse appears on the video, pause and count the number of Stentor that are both anchored to the bottom of the 35 mm plate and extended in an elongated, trumpet-like shape (Figure 5A, Video 1).
- Immediately after the first pulse, count the number of Stentor that are both anchored to the bottom of the plate and contracted into a ball-like shape (Figure 5B, Video 1).
NOTE: Contracted cells are easily discernable from elongated cells because Stentor shorten their body length by over 50% within 10 ms during a contraction event3. - Divide the second count by the first count to determine the fraction of Stentor that contracted in response to the mechanical stimulus.
- Repeat steps 3.1-3.3 for all the mechanical pulses in the experiment video.
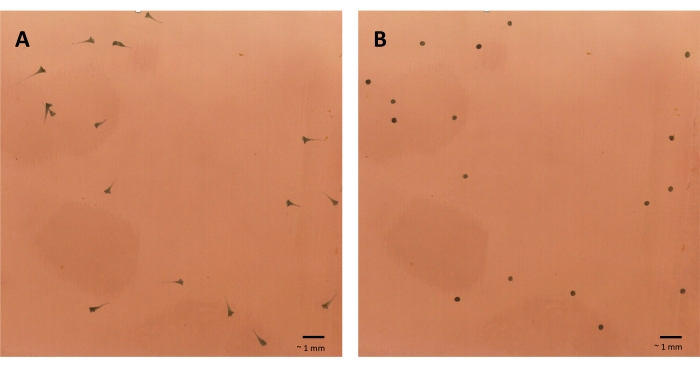
Figure 5: Stentor contracting after receiving a mechanical stimulus. (A) The Stentor are in their elongated state and anchored to the bottom of the Petri plate. (B) The Stentor have contracted after receiving a Level 4 mechanical stimulation from the habituation device. The images were taken with a USB microscope. Please click here to view a larger version of this figure.
Video 1: Video of Stentor contracting. The Stentor receive a Level 4 mechanical stimulus from the habituation device every minute. These cells have not yet habituated, so they contract after receiving the pulse. The cells are in the Petri plate placed atop the habituation device. Please click here to download this Video.
Representative Results
The method described above, using the Level 4 mechanical pulse at a frequency of 1 tap/min, should result in a progressive reduction in the contraction probability of the Stentor within 1 h. This is indicative of habituation (see Figure 6, Video 2).
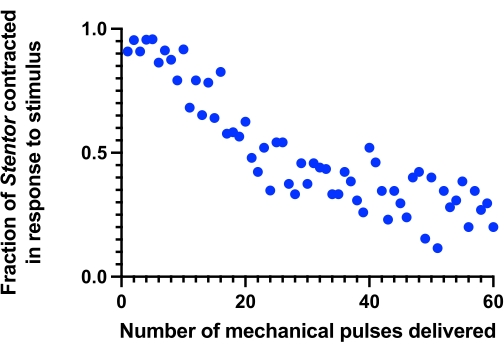
Figure 6: Baseline habituation. The contraction probability of Stentor progressively declines over the course of 1 h after receiving Level 4 mechanical pulses at a frequency of 1 tap/min (n = 22-27). Please click here to view a larger version of this figure.
Video 2. Video of habituated Stentor. The cells receive a Level 4 mechanical stimulus after 1 h of receiving mechanical pulses of the same force at a frequency of 1 tap/min. Most of the cells have habituated to the stimuli during the hour and, thus, do not contract. Please click here to download this Video.
Altering the force and/or frequency of the mechanical pulse delivery can change the Stentor habituation dynamics. For example, using the Level 2 pulse at a frequency of 1 tap/min precludes habituation over the course of 1 h (see Figure 7). A Level 5 pulse should elicit contractions in few to zero Stentor.
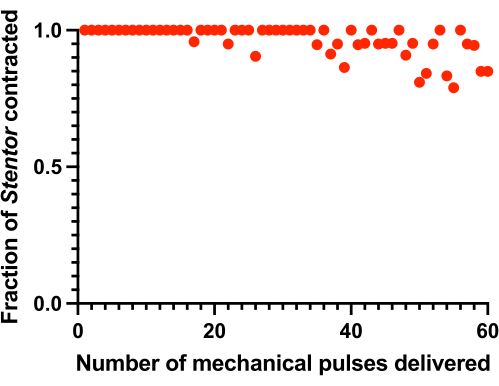
Figure 7: Lack of habituation within 1 h for stronger forces. The contraction probability of Stentor does not appreciably decline over the course of 1 h after receiving Level 2 mechanical pulses at a frequency of 1 tap/min (n = 7-33). Please click here to view a larger version of this figure.
Discussion
The most critical steps in the protocol relate to ensuring that the Stentor remain in optimal conditions for contractions to occur. The contraction response in the habituation assay requires that Stentors are anchored to a surface using their sticky holdfast since they rarely contract when they are freely swimming. However, the bottom surface of the 35 mm Petri plate used for habituation experiments is not typically conducive for anchoring unless coated with poly-ornithine. Furthermore, the Stentor cannot be exposed to any mechanical perturbation for a minimum of 2 h before the start of the habituation experiment because the Stentor forgetting timescale is 2-6 h3. If Stentor receive mechanical stimulation within 2 h of the habituation experiment start time, there is a possibility that this prior stimulation will induce a slight level of habituation in advance of the experiment, thereby reducing the contraction probability after the habituation device delivers the first mechanical pulse. Finally, during the analysis stage, it is important to only count the number of Stentor that contract after a pulse – rather than any incidental spontaneous contractions that occur prior to the pulse delivery – to obtain an accurate readout of the fraction of cells that contracted in response to the mechanical stimulation.
The protocol can be readily modified to study different types of habituation dynamics by changing the force and frequency of the mechanical pulses delivered by the habituation device. This also provides an opportunity to explore other types of learning, such as sensitization, that might occur in Stentor. The microcontroller board program code itself can also be adjusted to deliver different patterns of mechanical taps to the Stentor.
One potential issue to troubleshoot with this protocol is the low frequency of Stentor anchoring, which could constrain the number of Stentor that can be observed in the habituation experiment. Anchoring frequency is sometimes reduced in Stentor cultures that have not recently been fed or are contaminated. To address this problem, one should wash a fresh batch of Stentor to start a new culture and feed them regularly according to the protocol described in Lin et al.10.
This protocol is limited in that only a single plate of Stentor can be tested at a time, resulting in relatively low-throughput measurements. Furthermore, current software does not allow for the automation of single-cell image analysis. Most data acquired are, therefore, on a population level. Future models of the habituation device and image analysis tools may facilitate high-throughput single-cell experiments.
Habituation in Stentor has been previously studied using methods described by Wood3, but this new protocol allows experiments to be automated. Automation not only allows the researcher to reproducibly deliver mechanical pulses of a specified force and frequency but also facilitates long-term habituation experiments since the device can be left running without supervision for days. Furthermore, using a stepper motor rather than the solenoid employed in Wood's experiments3 reduces the risk of demagnetization over time and also allows the strength of the stimulus to be varied during the course of a single experiment.
Studying cellular habituation may reveal clinical insights for conditions such as attention-deficit/ hyperactivity disorder (ADHD) and Tourette's syndrome in which habituation is impaired11. Stentor habituation mechanisms may also unveil new non-synaptic learning paradigms independent of complex cellular circuitry. Finally, insights about single-cell learning could inspire methods for reprogramming cells within multicellular tissues – another potential avenue to fight disease.
Divulgaciones
The authors have nothing to disclose.
Acknowledgements
We thank Tatyana Makushok for innumerable discussions about Stentor learning. This work was funded by NSF grant MCB- 2012647 and by NIH grant R35 GM130327, as well as by the I2CELL award from the Foundation Fourmentin-Guilbert.
Materials
| 0.01% Poly-ornithine | Millipore Sigma | P4957 | Used to coat Petri plate |
| 35-mm Petri plate | Benz Microscope Optics Center Inc. | L331 | Contains Stentor during experiments |
| 6-well plate | StemCell Technologies | 38016 | Used to wash Stentor |
| Aluminum breadboard, 4" x 24" x 1/2" (x1) | Thorlabs | MB424 | Used to construct habituation device |
| Big easy driver stepper motor driver board (x1) | Sparkfun | ROB-12859 | Used to construct habituation device |
| Construction rail, 1" x 5'' (x2) | Newport | Newport CR-1 | Used to construct habituation device |
| Laptop | Apple Store | https://www.apple.com/macbook-air-m1/ | Connect laptop to USB microscope to visualize experiments |
| Large right-angle bracket (x1) | Thorlabs | AP90RL | Used to construct habituation device |
| Microcontroller board | Arduino | A000066 | Used to control habituation device |
| Nema 17 Stepper Motor Bipolar 59Ncm 2A 84oz.in 48mm 4-Lead | Stepperonline.com | 5-17HS19-2004S1 | Used to construct habituation device |
| Pasteurized spring water | Carolina | 132458 | Media for Stentor experiments |
| Right-angle bracket (x3) | Thorlabs | AP90 | Used to construct habituation device |
| Stemi 2000 stereo microscope | Zeiss | Used to visualize Stentor during wash steps | |
| Stentor coeruleus | Carolina | 131598 | These are the cells used for habituation experiments |
| USB microscope | Celestron | 44308 | Used to visualize and record experiments |
| Webcam recorder | Apple Store | https://apps.apple.com/us/app/webcam-recorder/id1508067444?mt=12 | Install this application to take videos of experiments |
Referencias
- Dussutour, A. Learning in single cell organisms. Biochemical and Biophysical Research Communications. 564, 92-102 (2021).
- Sternberg, R. J. Intelligence. Dialogues in Clinical Neuroscience. 14 (1), 19-27 (2012).
- Wood, D. C. Parametric studies of the response decrement produced by mechanical stimuli in the protozoan, Stentor coeruleus. Journal of Neurobiology. 1 (3), 345-360 (1969).
- Tang, S. K. Y., Marshall, W. F. Cell learning. Current Biology. 28 (20), 1180-1184 (2018).
- Wood, D. C. Stimulus specific habituation in a protozoan. Physiology and Behavior. 11 (3), 349-354 (1973).
- Thompson, R. F., Spencer, W. A. Habituation: A model phenomenon for the study of neuronal substrates of behavior. Psychological Review. 73 (1), 16-43 (1966).
- Slabodnick, M. M., Marshall, W. M. Stentor coeruleus. Current Biology. 24 (17), 783-784 (2014).
- Slabodnick, M. M., et al. The macronuclear genome of Stentor coeruleus reveals tiny introns in a giant cell. Current Biology. 27 (4), 569-575 (2017).
- Slabodnick, M. M., et al. The kinase regulator Mob1 acts as a patterning protein for Stentor morphogenesis. PLoS Biology. 12 (5), 1001861 (2014).
- Lin, A., Makushok, T., Diaz, U., Marshall, W. F. Methods for the study of regeneration in Stentor. Journal of Visualized Experiments. (136), e57759 (2018).
- McDiarmid, T. A., Bernardos, A. C., Rankin, C. H. Habituation is altered in neuropsychiatric disorders-A comprehensive review with recommendations for experimental design and analysis. Neuroscience and Biobehavioral Reviews. 80, 286-305 (2017).

