Description of a Swine Infant Model of Volume-Controlled Hemorrhagic Shock
Summary
This article aims to provide researchers with a detailed and accessible guide to set up an infant porcine model of hemorrhagic shock.
Abstract
Hemorrhagic shock is a leading cause of morbidity and mortality in pediatric patients. Interpretation of the clinical indicators validated in adults to guide resuscitation and comparison between different therapies is difficult in children due to the inherent heterogeneity of this population. As a result, compared to adults, appropriate management of pediatric hemorrhagic shock is still not well established. In addition, the scarcity of pediatric patients with hemorrhagic shock precludes the development of clinically relevant studies. For this reason, an experimental pediatric animal model is necessary to study the effects of hemorrhage in children as well as their response to different therapies. We present an infant animal model of volume-controlled hemorrhagic shock in anesthetized young pigs. Hemorrhage is induced by withdrawing a previously calculated blood volume, and the pig is subsequently monitored and resuscitated with different therapies. Here, we describe a precise and highly reproducible model of hemorrhagic shock in immature swine. The model yields hemodynamic data that characterizes compensatory mechanisms that are activated in response to severe hemorrhage.
Introduction
Life-threatening hemorrhage due to trauma, although uncommon, is the leading cause of death in pediatric patients1,2. Additional causes of hemorrhagic shock include hemorrhagic fever, gastrointestinal bleeding, liver surgery, and cardiac surgery, especially when cardiopulmonary bypass is used3.
Contrary to the adult population, there is insufficient data on the management of pediatric hemorrhagic shock, which is largely based on expert opinions or directly translated from adult practice2,4. However, the translation of management strategies from adults may not be appropriate. For example, clinical indicators validated in adults are difficult to extrapolate to pediatric patients due to the physiologic heterogeneity present across groups of different ages and the different injury patterns predominant in the pediatric population. Consequently, specific endpoints that would trigger intervention in the pediatric patient are not well defined. Moreover, there is not enough evidence on the deleterious effects that therapies currently implemented in adults may have on children2,4,5.
In view of all this, further investigation is needed to establish specific resuscitation thresholds to prompt intervention, as well as to better determine which are the most appropriate therapies for pediatric hemorrhagic shock. However, the development of quality and clinically relevant studies of life-threatening hemorrhage in children is difficult, due to the paucity of patients and the already mentioned heterogeneity in the pediatric population from the neonatal period through adolescence.
The clinical relevance of hemorrhagic shock, in addition to the difficulties in performing clinical studies on pediatric patients, emphasize the need for preclinical evaluations on animal models to study pathophysiology following hemorrhagic shock in children, as well as to compare different therapies.Several animal models have been widely used in research to study hemorrhagic shock6,7,8,9. Due to their anatomical and physiological similarities with humans, pigs are highly valued in biomedical research. Regarding the advantages of using specific infant models, evidence shows that immature swine hemodynamics, as well as respiratory, hematologic, and metabolic systems, are highly comparable to those in young humans9. This confers a unique opportunity to simulate a clinical scenario of hemorrhagic shock in children.
In this model, hemorrhage is induced by withdrawing a previously calculated blood volume. Subsequently, the pig is monitored, and different resuscitation fluids are administered.
Here, we describe a precise and highly reproducible model of hemorrhagic shock in immature swine. The model yields hemodynamic data that characterizes compensatory mechanisms activated in response to severe hemorrhage.
Protocol
The experiments in this protocol were approved by the Institutional Ethics Committee for Animal Research of the Gregorio Marañón University Hospital, Madrid, Spain, and the Agriculture and the Environment Council of the Madrid Autonomous Government (permit number: 12/0013). European and Spanish guidelines for ethical care and the use of experimental animals were applied throughout the study. The experiments were performed in the Department of Experimental Medicine and Surgery, Gregorio Marañón University Hospital, Madrid, Spain.
NOTE: The animal model chosen consisted of healthy 2-3-month-old (8-12 kg) minipigs (Sus scrofa domestica). Minipigs are the result of a crossing of three different breeds that make them suitable for biomedical research. The animals are near-identical lines and are provided by a specifically authorized breeding facility in Madrid (IMIDRA), which keeps the maintenance of three homozygous genetic lines in purity. Male and female animals were used interchangeably. Animals were fed a standard swine diet and observed for a minimum of 2 days to ensure good health. Food, but not water, was withdrawn the night before the procedures to reduce the risk of aspiration. A typical experiment requires approximately 6 h to complete, including 30 min for anesthesia induction and surgical preparation, 60 min for instrumentation, 30 min for recovery, 60 min for hemorrhage induction and posterior stabilization, 30 min for resuscitation, and 120 min for follow-up.
1. Anesthesia, intubation, and mechanical ventilation
- Premedicate the pig with an intramuscular injection of ketamine (10 mg/kg) and atropine (0.02 mg/kg) in the lateral region of the neck, behind the ear, or in the posterior femoral region.
NOTE: Anticholinergic drugs, such as atropine, are useful as swine may salivate excessively under anesthesia10. In our experience, this dose of ketamine is enough to reduce stress and induces adequate sedation and analgesia in pigs without adverse effects. However, if the animal is not properly sedated or if the distance from the housing to the operating room is long, another dose of ketamine (10 mg/kg) can be administered safely.
CAUTION: Gloves are necessary when handling animals. - Transport the sedated animal to the operating room and place it on a surgical table provided with a warming blanket.
- Measure peripheral oxygen saturation (Sp02) with a sensor clipped to the pig's ear and initiate continuous three-lead electrocardiographic (EKG) monitoring.
- Disinfect the skin with at least 3 alternating rounds of povidone-iodine or chlorhexidine scrub and alcohol. Insert a peripheral vein catheter (22-24 G) into the ear vein. Disinfect the skin priorly with an antiseptic solution.
- Induce anesthesia by an intravenous injection of fentanyl (5 µg/kg), propofol (4 mg/kg), and atracurium (0.5 mg/kg). Once spontaneous breathing disappears and the absence of reflexes is confirmed, place the animal in the dorsal recumbency position and immediately initiate hand-bag mask ventilation with a dog mask with fraction of inspired oxygen (Fi02) set at 100%.
NOTE: In order to reduce the risk of accidental awareness related to the use of neuromuscular blockers, anesthetic agents with known effectivity in swine and with doses on the higher limit should be used to ensure an adequate level of anesthesia. Additionally, continuously monitor cardiovascular signs such as heart rate, blood pressure, and body temperature, and administer neuromuscular blockers only when withdrawal reflexes are absent (pedal withdrawal, palpebral reflexes, and jaw tone) and muscle tone is relaxed. - Perform endotracheal intubation. At least two operators are needed for this procedure.
- Ensure that the basic equipment and surgical tools needed for endotracheal intubation are ready: tie gauze to open the mouth and secure the tube, veterinary laryngoscope with a straight blade between 17 and 25 cm long, a common endotracheal tube (ID 4-5), stylet, syringe with air, and adhesive tape.
- Pull out the tongue slightly and hold the jaw open using tie gauze placed behind the upper and lower canine teeth.
- Perform a laryngoscopy and, once the epiglottis is visible, use the tip of the laryngoscope to press the epiglottis upward toward the base of the tongue.
NOTE: If the epiglottis is stuck to the soft palate, it can be displaced dorsally with the tip of the tube. Operator 1 performs step 1.6.2 while operator 2 performs step 1.6.3. - Once the vocal cords are visualized, gently advance the tube with slight rotation into the trachea.
NOTE: The narrowest point of the trachea is on the subglottic level. If tube insertion is difficult, try slight rotation or a smaller tube. - Remove the stylet and use a 5 mL syringe to inflate the cuff.
- Ensure placement of the endotracheal tube by observing symmetrical chest rise, adequate oxygen saturation (95%-100%), and a proper waveform and end-tidal CO2 (EtCO2) reading.
CAUTION: Pigs are very susceptible to laryngospasm and edema of the larynx mucosa, and laryngeal perforation may even occur after several attempts of intubation or if sedation is inadequate10.
- After confirming intubation, initiate mechanical ventilation using a mechanical ventilator with a respiratory rate of 20 breaths per minute, tidal volume of 8 mL/kg, FiO2 of 40%, and positive end-expiratory pressure of 4 cm H2O. Adjust ventilation to achieve a partial pressure of carbon dioxide (PaCO2) between 35 and 45 mmHg.
- Maintain deep anesthesia throughout the experiment via a continuous infusion of fentanyl (10 µg/kg/h), propofol (10 mg/kg/h), and atracurium (2 mg/kg/h).
2. Instrumentation
- Prepare the femoral area for vessel catheterization. Use bandages to pull the legs back and disinfect the inguinal area with at least 3 alternating rounds of povidone-iodine or chlorhexidine scrub and alcohol.
- Assess the femoral vessels with an ultrasound and use the Doppler technique to distinguish between the artery and the vein. Depending on the size of the vein, insert a 5.5-7.5 French (F) central venous catheter with three ports in one of the femoral veins under continuous ultrasound view and using the Seldinger technique11,12.
- Immediately after central venous catheter placement, connect a transducer system to measure the central venous pressure.
- Ensure that an electrolyte with glucose infusion (20 mL/h) is connected to one of the central line ports and that a maintenance saline infusion (5 mL/h) is infused via the remaining port to prevent occlusion of the catheter.
- Use the same technique to cannulate the opposite femoral artery with a 4 F arterial catheter specifically designed for cardiac output monitoring. Perform a blood gas test to establish the correct position of the catheter if ultrasound confirmation is not possible.
NOTE: In case of significant spasm or hematoma, cross over to the contralateral femoral artery. - Once the arterial catheter is inserted, connect the arterial wire of the cardiac output monitor system and the arterial transducer directly to the monitor port. Simultaneously connect the venous measuring unit of the monitor to the central venous transducer.
NOTE: The cardiac output monitor used in this experiment is specified in the Table of Materials. For setup, calibration, and measures, see the manufacturer instructions13. - Ensure both the venous and arterial transducers are calibrated to zero.
- Expose the left internal carotid artery and left external jugular vein via the cut-down technique.
- Ensure the necessary equipment and surgical tools are available: scalpel, blunt-tip surgical scissors, tissue forceps, small self-retaining tissue retractor, needle holder, surgical swaps, suture with needle, one 18 G IV cannula, one 5 F catheter sheath with an introducer, and one Seldinger guide wire.
- With the animal in the dorsal recumbency position, disinfect the neck skin with an antiseptic solution.
- Use a scalpel to make an ~10 cm left paratracheal incision, bisecting a line between the manubrium and the angle of the jaw.
- To expose the external jugular vein, dissect the tissue lateral to the SCM and isolate the vein from the surrounding fascia.
- After being isolated, use two non-absorbable silk sutures (USP-0) looped around the vein to fixate the vessel prior to the puncture.
- Incise the vein with a Venflon needle (18 G). Once inside the vein, retract the needle and insert the guide wire through the Venflon tube.
- Remove the Venflon tube and insert the sheath with the introducer (5 F) over the wire. After insertion, remove both the introducer and the wire.
- Immediately after insertion, rinse the sheaths with 0.9% NaCl (5 mL/h) to prevent thrombus formation.
- Tie up the proximal silk suture around the sheath to fix it. After that, secure the handle of the sheath to the SCM and close the skin with staples.
- Following surgical preparation, allow the animals to stabilize for 30 min before obtaining the baseline monitoring values and blood samples.
- Maintain the blood temperature at 37-39 ˚C using a thermal blanket and an overhead warmer throughout the experiment.
NOTE: Temperature is measured with a thermistor located at the thermodilution arterial catheter tip.
3. Hemodynamic and perfusion monitoring
- Monitor the EKG, peripheral oxygen saturation, respiratory volumes and pressures, and Fi02.
- Connect a spirometer between the endotracheal tube and a multiparameter monitor to measure qualitative and quantitative EtC02.
NOTE: For more information about the multiparameter monitor, see the Table of Materials. - Use near-infrared spectroscopy (NIRS) to monitor the brain tissue oxygenation index (bTOI) and splanchnic tissue oxygenation index (aTOI). Place the sensors on the skin of the forehead and the anterior abdominal wall (subhepatic region).
NOTE: Do not place the brain sensor in the midline, as it may be contaminated with the superior sagittal sinus venous blood14. - Connect the blood flow probe attached to the internal carotid artery to a flow monitor to measure the carotid blood flow (CaBF).
- Place a laser Doppler sensor over the skin of the anterior abdominal wall for continuous measurement of the cutaneous tissular blood flow (CuTBF).
NOTE: For more information about the carotid and cutaneous tissular blow flow sensors, see the Table of Materials. - Record the following parameters at baseline and every 30 min: blood temperature, inspiratory tidal volume, EtCO2, cardiac rhythm, heart rate (HR), systolic and diastolic blood pressures, mean arterial blood pressure (MAP), shock index (HR/systolic blood pressure)15, central venous pressure, cardiac index (CI), global end diastolic volume index (GEDVI), stroke volume index (SVI), left ventricular contractility (Dt/Dpmax), systemic vascular resistance index (SVRI), extravascular lung water index (ELWI), pressure pulse variation (PPV), peripheral hemoglobin saturation, central venous saturation (ScvO2), cerebral (bTOI) and splanchnic (aTOI) tissue oxygenation index by NIRS, CaBF, and CuTBF.
- To obtain CI values, infuse 5 mL boluses of 0.9% normal saline at a temperature below 8 °C through the central venous catheter. Record the average of two consecutive measures.
- Determine the arterial and venous blood gas profiles and lactate concentration every 30 min by drawing 0.3 mL of blood from the femoral vessels. Perform standard complete blood counts, coagulation studies, and biochemistry at baseline, after hemorrhage induction, and at the end of the experiment.
- After each blood draw, flush the lines with 0.5 mL of 100 IU/mL heparin.
4. Hemorrhagic shock induction
- Once a steady state is achieved after instrumentation and baseline data has been gathered, induce hypovolemic shock by withdrawing 30 mL/kg of blood from the jugular vein over 30 min.
- Allow a 30 min period for stabilization. Do not make resuscitation efforts during this period to emulate the delay in arrival of emergency medical teams.
5. Infusion and follow-up
- After the stabilization period, infuse a bolus of a volume expander or a vasoactive agent over a 30 min period.
NOTE: Examples of volume expanders and vasoactive agents tested are normal saline, hypertonic albumin, angiotensin, and terlipressin. In this study, 30 mL/kg normal saline (NS) (n = 13), 15 mL/kg 5% albumin plus 3% hypertonic saline (AHS) (n = 13), or a single intravenous bolus of 15 µg/kg terlipressin plus 15 mL/kg 5% albumin plus 3% hypertonic saline (TAHS) (n = 13) were used. - After the infusion, follow up the animal for 120 min. Record the hemodynamic parameters and obtain blood samples every 30 min for arterial and venous blood gas profiles and lactate concentration determination. Do not make resuscitation efforts during this period.
6. End of the experiment and euthanasia
- Once the experiment is completed, use a sedative overdose (5 µg/kg fentanyl and 10 mg/kg propofol) and an intravenous injection of potassium chloride (2 mEq/kg) to sacrifice all successfully resuscitated animals.
- Confirm the absence of circulation by asystole or pulseless electrical activity on a continuous EKG display, the absence of pulsatile flow during invasive arterial pressure monitoring, and the absence of other vital signs.
- If, during the follow-up period, arterial blood pressure decreases below 25 mmHg, sacrifice the animal to avoid further suffering.
Representative Results
The presented model has been successfully used in several experiments to study macrocirculatory and microcirculatory changes following hemorrhagic shock and subsequent resuscitation, comparing different fluids and vasoactive drugs16,17,18,19.
Considering the response to shock, this model has consistently shown that a controlled hemorrhage produces marked changes in hemodynamic parameters, as well as in cerebral and tissue perfusion.
Following volume withdrawal, significant tachycardia and a decrease in MAP, CI, SVI, blood volume parameters (GEDVI and ITBI), and carotid arterial blood flow, along with an increase in systemic vascular resistance index, are detected (Figure 1 and Figure 2).
Regarding systemic perfusion parameters, lactate increases significantly, whereas ScvO2, CuTBF, and bTO decreases (Figure 3). Variations in central venous pressure, Dt/Dpmax, and ELWare not usually registered.
As for laboratory parameters, hemoglobin content and hematocrit do not decrease until after fluids have been administered. Albumin concentration decreases, and troponin levels increase significantly after controlled hemorrhage. Other parameters, including core temperature, PaO2, PaCO2, arterial oxygen saturation, EtCO2, electrolytes, and kidney and liver function parameters, usually remain stable.
Besides its utility in analyzing the cardiovascular and biochemical responses to shock, this model has been shown to successfullydiscriminate between different resuscitation fluids.
In previous studies, we have aimed to determine if, in an infant animal model of hemorrhagic shock, the use of a lower volume infusion of hypertonic fluids-alone or combined with different vasopressors- would improve global hemodynamic and perfusion parameters when compared to normal saline.
As reported previously, we have consistently observed that the infusion of hypertonic fluids produces a similar response to the infusion of twice the volume of isotonic fluid16,17,18.
More specifically, the use of albumin plus hypertonic saline produced a greater and longer volume expansion than normal saline or hypertonic saline alone, with significant differences in HR, SVI, and PPV, and the absence of a progressive fall after volume expansion in blood pressure and GEDVI, as observed in the other groups (Figure 1 and Figure 2). Furthermore, we have also observed a greater improvement of perfusion parameters with hypertonic albumin, represented as a greater increase of bTOI and CaBF, and a greater decrease of lactate levels than the other groups in comparison with the beginning of the fluid expansion (Figure 3). We believe that this difference might be secondary to the capacity of albumin to increase the blood volume and remain for a longer period of time within the intravascular compartment than normal saline.Interestingly, we have seen that the addition of a single bolus of terlipressin at the beginning of fluid resuscitation yielded similar results to those observed in the hypertonic albumin group, without any extra benefits in terms of hemodynamic or perfusion parameters17,18.
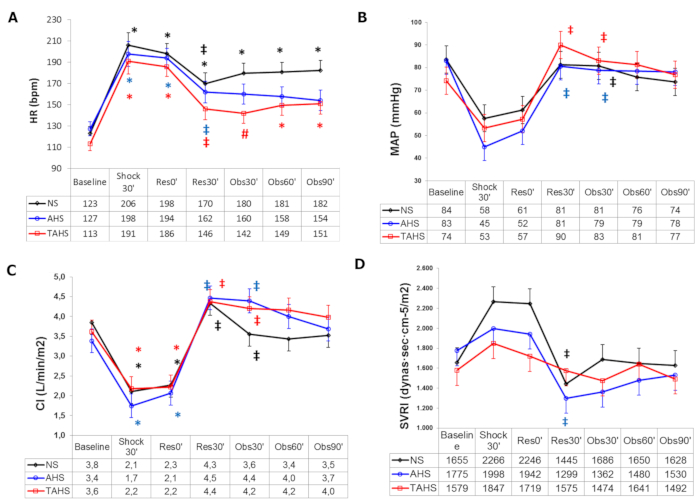
Figure 1: Hemodynamic parameters. (A) Evolution of heart rate, (B) mean arterial pressure, (C) cardiac index at baseline (t0'), and (D) systemic vascular resistance index at baseline (t0'). Throughout the course of the experiment: end of controlled bleeding (Shock30'); the beginning of infusion, 30 min after the end of controlled bleeding (Res0'); end of infusion (Res30'); follow-up 30 min after the end of infusion (Obs30'); follow-up 60 min after the end of infusion (Obs60'); follow-up 90 min after the end of infusion (Obs90'). (*) Significant difference (p < 0.05) from the baseline, same group. (‡) p < 0.05 from hemorrhage, same group. (#) p < 0.05 from group NS. Abbreviations: NS = normal saline; AHS = hypertonic saline albumin; TAHS = terlipressin plus hypertonic saline albumin. Data are presented as mean and standard deviation. This figure is adapted with permission from Urbano et al.17. Please click here to view a larger version of this figure.
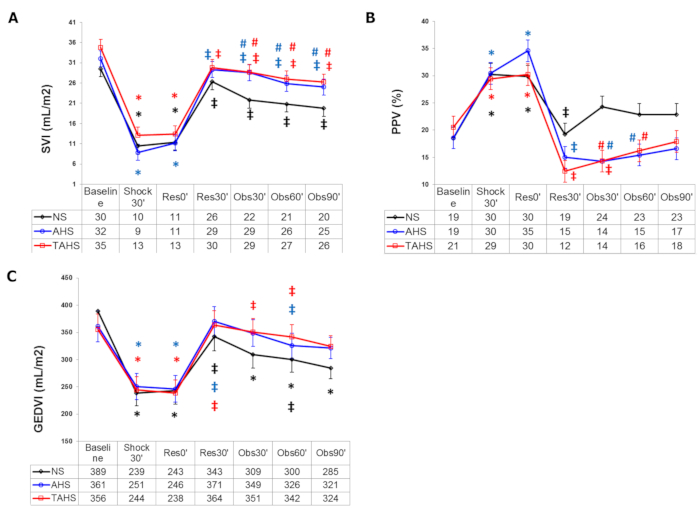
Figure 2: Blood volume parameters. (A) Evolution of stroke volume index, (B) pulse pressure variation, and (C) global end diastolic volume index at baseline (t0'). Throughout the course of the experiment: end of controlled bleeding (Shock30'); beginning of infusion, 30 min after the end of controlled bleeding (Res0'); end of infusion (Res30'); follow-up 30 min after the end of infusion (Obs30'); follow-up 60 min after the end of infusion (Obs60'); follow-up 90 min after the end of infusion (Obs90'). (*) Significant difference (p < 0.05) from baseline, same group. (‡) p < 0.05 from hemorrhage, same group. (#) p < 0.05 from group NS. Abbreviations: NS = normal saline; AHS = hypertonic saline albumin; TAHS = terlipressin plus hypertonic saline albumin. Data are presented as mean and standard deviation. This figure is adapted with permission from Urbano et al.17. Please click here to view a larger version of this figure.
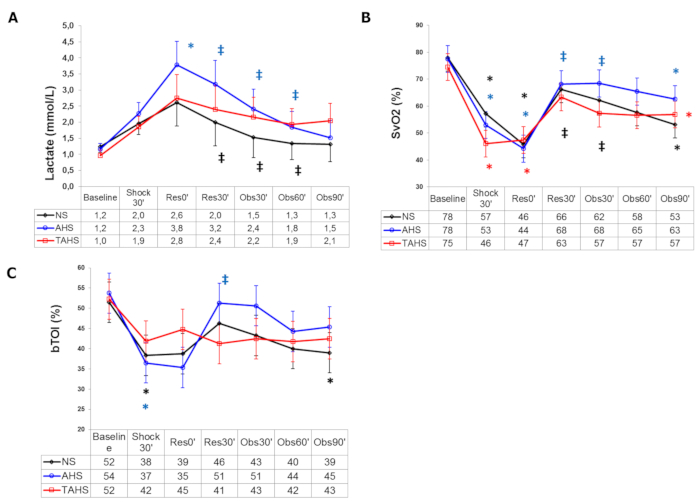
Figure 3: Systemic perfusion parameters. (A) Evolution of arterial blood lactate, (B) central venous blood oxygen saturation, and (C) brain tissue oxygenation index at baseline (t0'). Throughout the course of the experiment: end of controlled bleeding (Shock30'); beginning of infusion, 30 min after the end of controlled bleeding (Res0'); end of infusion (Res30'); follow-up 30 min after the end of infusion (Obs30'); follow-up 60 min after the end of infusion (Obs60'); follow-up 90 min after the end of infusion (Obs90'). (*) Significant difference (p < 0.05) from baseline, same group. (‡) p < 0.05 from hemorrhage, same group. (#) p < 0.05 from group NS. Data are presented as mean and standard deviation. This figure is adapted with permission from Urbano et al.17. Please click here to view a larger version of this figure.
Discussion
Performing procedures on young pigs can be complex and potentially life-threatening due to certain anatomical and physiological features of these animals. To achieve consistent results and reduce the loss of animals, there are some critical steps that should be carefully considered. First, achieving an adequate level of sedation is essential to minimize the animal stress response, which, if excessive, may alter the results due to endogenic catecholamine release. It is also important to avoid delays between the intramuscular injection and intubation, as animals can develop a severe stress response with tachycardia and irreversible metabolic acidosis that may precipitate the end of the experiment. Although other groups use inhaled anesthetics with good results20,21, we prefer intravenous medications, as inhaled sedatives do not allow the measurement of respiratory gas exchange with indirect calorimetry. In our experience, a combination of propofol and fentanyl is effective and has very few adverse effects. Careful temperature management throughout the experiment is another key aspect of the protocol, as rapid changes in temperature can significantly affect the animal's hemodynamic response to shock, falsifying the results or ultimately leading to the failure of the experiment.
Another crucial part of instrumentation is intubation, given the particularities of porcine anatomy and their susceptibility to laryngospasm. Therefore, the procedure should be performed by at least one operator with previous experience, and the use of a stylet and muscle relaxation is advisable10,22. Catheterization of vessels can also be challenging due to the small size of the animals. For femoral access, a sonography-guided puncture is preferable, as the vessels are located deep, usually have small diameters, and show different courses and positions22. For cervical access, we use surgical access to allow the placement of the carotid flow probe, but the ultrasound technique is also feasible23,24. Cannulation of the external jugular vein is usually preferred due to its wider diameter, its superficial location, and lower number of surrounding structures22. Catheters should be flushed immediately after insertion with saline solutions to prevent occlusion. We do not use heparin to avoid coagulation alterations. Also, initially, we avoided the administration of glucose infusions to prevent potential distortion of the hemodynamic response by the administration of extra fluids, but we found that animals developed severe and early hypoglycemia. Finally, even with anesthesia and the less invasive techniques used nowadays, instrumentation generates a significant stress response in animals, so it is desirable to leave enough time for recovery before initiating the removal of blood. Regarding induction of the hemorrhagic shock, we recommend the removal of 30 mL/kg, as this generates a significant pathophysiological response with excellent survival rates. In our experience, infant swine do not tolerate larger amounts of blood loss, and mortality is high. Gradual withdrawal of blood is also important, as rapid removal can result in severe hemodynamic instability and early death of the animal.
Although there is a wide variety of species and experimental models available to researchers, the ideal model of animal hemorrhagic shock-simple, easily reproducible, and accurate replication of the clinical situation-still represents a challenge. Small animal models-primarily mice and rats-are used to investigate the pathophysiological mechanisms of shock. However, their small size significantly complicates the performance of surgical and sampling procedures. Larger animals, such as dogs and pigs, are more expensive and complex to handle, but their size and physiological similarities with humans make them more suitable for preclinical evaluation of the treatment strategies. However, the use of dogs in the past and still nowadays is ethically questionable. They do not offer any advantage over pigs as experimental animal models, and their intelligence and the special bilateral relationship between humans and dogs situate them in a higher position in the phylogenetic scale6,7,8.
In view of all this, adult swine have been extensively used for cardiovascular research due to their similarities with adult human physiology, size, and anatomy, which is better than most species. However, as it has been well established in the literature, there are significant differences between human adult and pediatric patients in terms of the cardiovascular system, blood volume, temperature regulation, and response to shock2,3,4. At the same time, evidence shows that these differences also apply to swine, and piglets have been found to have cardiovascular, cerebrovascular, hematological, and electrolyte profiles very similar to those in pediatric human patients9,25. Finally, beyond these anatomic and physiologic differences between adults and infants in both species, the use of infant animal models, especially minipigs, provides the opportunity to test the same devices that are used in the real clinical setting for monitoring. In many cases, the reliability of these devices has been proven to be low because of a simple adaptation of the adult algorithms, sensors, or scales. All these aspects support the importance of developing specific pediatric animal models and their relevance in terms of translational utility to the pediatric clinical setting.
Besides the type of animal, there are three basic models generally used in the study of hemorrhagic shock: controlled hemorrhage-either by volume or pressure-and uncontrolled hemorrhage. The protocol presented in this article describes a fixed-volume hemorrhage model, in which a fixed blood volume, usually calculated by the percentage of body weight, is removed over a time period set by the observer. On the contrary, in fixed-pressure hemorrhage models, animals are bled to a predetermined MAP, which is then maintained with periodic bleeding or fluid infusions during a specified period, depending on the animal species and the degree or outcome of shock. Both fixed-volume and fixed-pressure hemorrhagic shock models allow the study of shock-induced pathophysiological changes under controlled conditions, offering a clear advantage in terms of reproducibility and standardization. However, their main limitation is that they do not allow the study of the effects of different resuscitation strategies on active bleeding, where aggressive fluid resuscitation before the surgical control of hemorrhage is known to increase bleeding and decrease survival, due to inhibition of the formation of the thrombus and the rise in the mean blood pressure. Uncontrolled hemorrhage models induced by a standardized vascular trauma-crush/laceration of liver and spleen, artery injury, or amputation of an appendage-have been suggested to better reflect the clinical situation, thereby allowing a better understanding of the effects of different fluid resuscitation strategies and other interventions, such as hypothermia and hemostatic products. However, despite being clinically most relevant, these uncontrolled hemorrhage models exert some clear disadvantages in terms of standardization and reproducibility. In view of all this, it seems that the ideal model does not exist, and therefore research in this field must balance clinical relevance with experimental standardization and reliability6,7,8,9,26.
The model described in this study may offer broad potential applications in cardiovascular research, such as the investigation of endothelial dysfunction and microcirculation alterations18 during shock, as well as the validation of different hemodynamic monitoring systems. Moreover, it can also be used in other research fields, allowing the study of endocrine or immune responses after severe hemorrhage as well as the determination of side effects of different fluids and vasopressors. However, regarding the research on different resuscitation strategies, it is advisable to study their effects in uncontrolled hemorrhage models before implementing changes in the clinical setting7,26.
Besides the difficulty of extrapolating the results to real life, this model has other limitations. To begin with, there are some confounding variables related to the experimental setup, such as the use of anesthetic agents or mechanical ventilation, that may attenuate the physiologic responses during shock and complicate the interpretation of results. Besides, instrumentation stress response on the animals and temperature control may affect macro- and microcirculation through different mechanisms. Another important limitation of this model-related to the experimental necessities and availability of resources-is the limited post-traumatic observation period, which further limits the study of the long-term consequences of hemorrhagic shock. In addition, despite the physiological similarities between humans and pigs, there are some differences between species that should be considered. The coagulation system, for example, appears to be more effective in swine27,28. Also, lactate and succinate plasma levels differ between species, and pigs have basal alkalosis, which may lead to an underestimation of the effects of hemorrhage on acid-base balance29. Finally, it is also well known that the inflammatory and immune responses, as well as some vasopressor receptors, are different in swine9. Specific animal differences also need to be considered as influencing factors. Several studies have indicated gender differences in terms of susceptibility to shock, with females having a significant survival advantage over males6,9. Nevertheless, in the experiments conducted in this study, we use animals from the same age group and with a similar genetical background to minimize the potential variability inherent to species.
In conclusion, this article provides a practical and step-by-step guide to setting up a swine model of pediatric hemorrhagic shock. Compared to other existing models, this is a reliable and easy-to-follow protocol with broad applicability in biomedical research, either for the investigation of pathophysiological responses after severe hemorrhage or for the evaluation of different resuscitation strategies.
Divulgaciones
The authors have nothing to disclose.
Acknowledgements
This study has been funded by the Instituto de Salud Carlos III (ISCIII) through the project "PI20/01706" and co-founded by the European Union. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. We would like to show out gratitude to all our colleagues from Gregorio Marañón Pediatric Intensive Care Unit and from the Gregorio Marañón Experimental Institute, as without their work this project would not have been possible.
Materials
| ADA swabs | Albino Dias de Andrade, S.A. | 300575750400 | Non-woven swabs |
| Alaris SE | Carefusion | N/A | Volumetric infusion pump |
| Atracurium | Aspen Pharma Trading Limited. Dublin, Ireland | N/A | Muscle relaxant |
| Atropine 1 mg/mL | B. Braun | 481377/1013 | |
| Barrier adhesive aperture drape | Mölnlycke | 63621 | |
| BD emerald syringe 5 mL, 10 mL, 20 mL | Becton Dickinson S.A | https://www.bd.com/en-eu/offerings/capabilities/syringes-and-needles/injection-syringes/bd-emerald-3-piece-syringe | various options available |
| BLF21A laser doppler monitor | Transonic Systems Inc. | BLF21A | Skin blood flow monitor |
| BlueSensor NF ECG electrodes | Ambu | NF-50-A/12 | |
| Check-Flo performer introducer set 5Fr | Cook Medical | G12018 | Vascular Sheath |
| Datex ohmeda S5 | GE Healthcare Finland Oy, Helsinki, Finland | M1162897 | Hemodynamic monitor |
| Fentanyl 0.05 mg/mL | Kern Pharma | N/A | Anesthesia |
| GE Vivid S5 | GE Healthcare | S series | Ultrasound machine |
| Introcan Safety 18 G, 22 G, 24 G | B. Braun | Introcan series | Safety intravenous catheter |
| INVOS cerebral/somatic oximetry adult sensors | Medtronic PLC, USA | https://www.medtronic.com/covidien/en-us/products/cerebral-somatic-oximetry/invos-cerebral-somatic-oximetry-adult-sensors.html | |
| INVOS OXIMETER cerebral/somatic | Somanetics | 08-10566 | Regional oxygenation monitor |
| Ketamin 50 mg/mL | Pfizer, S.L. | 47034 | Sedation |
| Leon plus | Heinen + Löwenstein | N/A | Ventilator |
| Life scope VS | Nihon Kohden | N/A | Bedside monitor |
| Miller laryngoscope blade 12″ | Jorgensen Labs, USA | J0449F | Laryngoscope |
| Multi-lumen central venous catheterization set 7 French, 3 lumen, 30 cm | Arrow | CS-14703 | Central venous catheter |
| Nellcor WarmTouch 5300A | Covidien | Thermal blancket | |
| Nitrile gloves | Medihands | KS-ST RT021 | Single use gloves |
| Pediatric SomaSensor INVOS cerebral/somatic | Covidien | https://www.medtronic.com/covidien/en-us/products/cerebral-somatic-oximetry.html | Disposable regional oxygen saturation sensor |
| PICCO monitoring kit | Pulsion Medical Systems | PV8215 | |
| PICCO thermodilution catheter 5F/20 cm | Pulsion Medical Systems | N/A | |
| Propofol Lipoven 10 mg/mL | Fresenius Kabi, Spain | N/A | Anesthesia |
| Pulse contour cardiac output (PiCCO2) | Pulsion Medical Systems | N/A | Hemodynamic monitor |
| Rüsch flexislip | Teleflex Medical | 503700 | Endotracheal tube stylet |
| Softa swabs | B. Braun | 19579 | Alcohol pads |
| Surgical silk sutures USP 0 | Aragó, Barcelona, Spain. | 6245 | |
| TruWave pressure monitoring set | Edwards | T001767A | Pressure monitoring set |
| Ultrasound transmission gel | Ultragel Hungary 2000 Kft. | UC260 |
Referencias
- Disease Control and Prevention (CDC). Vital signs: Unintentional injury deaths among persons aged 0-19 years-United States, 2000-2009. MMWR. Morbidity and Mortality Weekly Report. 61, 270-276 (2012).
- Russell, R. T., et al. Pediatric traumatic hemorrhagic shock consensus conference recommendations. The Journal of Trauma and Acute Care Surgery. 94, S2-S10 (2023).
- Leonard, J. C., et al. Life-threatening bleeding in children: A prospective observational study. Critical Care Medicine. 49 (11), 1943-1954 (2021).
- Cannon, J. W. Hemorrhagic shock. The New England Journal of Medicine. 378 (4), 370-379 (2018).
- Dehmer, J. J., Adamson, W. T. Massive transfusion and blood product use in the pediatric trauma patient. Seminars in Pediatric Surgery. 19 (4), 286-291 (2010).
- Fülöp, A., Turóczi, Z., Garbaisz, D., Harsányi, L., Szijártó, A. Experimental models of hemorrhagic shock: a review. European Surgical Research. 50 (2), 57-70 (2013).
- Moochhala, S., Wu, J., Lu, J. Hemorrhagic shock: an overview of animal models. Frontiers in Bioscience. 14 (12), 4631-4639 (2009).
- Lomas-Niera, J. L., Perl, M., Chung, C. S., Ayala, A. Shock and hemorrhage: an overview of animal models. Shock. 24, 33-39 (2005).
- Hildebrand, F., Andruszkow, H., Huber-Lang, M., Pape, H. C., van Griensven, M. Combined hemorrhage/trauma models in pigs-current state and future perspectives. Shock. 40 (4), 247-273 (2013).
- Chum, H., Pacharinsak, C. Endotracheal intubation in swine. Lab Animal. 41 (11), 309-311 (2012).
- Leibowitz, A., Oren-Grinberg, A., Matyal, R. Ultrasound guidance for central venous access: Current evidence and clinical recommendations. Journal of Intensive Care Medicine. 35 (3), 303-321 (2020).
- Lockwood, J., Desai, N. Central venous access. British Journal of Hospital Medicine. 80 (8), C114-C119 (2019).
- Operator’s Manual PiCCO2. Version 3.1. Pulsion Medical Systems Available from: https://www.manualslib.com/manual/2821743/Pulsion-Picco2.html#manual (2013)
- Acker, S. N., Ross, J. T., Partrick, D. A., Tong, S., Bensard, D. D. Pediatric specific shock index accurately identifies severely injured children. Journal of Pediatric Surgery. 50 (2), 331-334 (2015).
- Urbano, J., et al. Comparison of normal saline, hypertonic saline and hypertonic saline colloid resuscitation fluids in an infant animal model of hypovolemic shock. Resuscitation. 83 (9), 1159-1165 (2012).
- Urbano, J., et al. Comparison of normal saline, hypertonic saline albumin and terlipressin plus hypertonic saline albumin in an infant animal model of hypovolemic shock. PLoS One. 10 (3), e0121678 (2015).
- González, R., et al. Microcirculatory alterations during haemorrhagic shock and after resuscitation in a paediatric animal model. Injury. 47 (2), 335-341 (2016).
- López-Herce, J., Rupérez, M., Sánchez, C., García, C., García, E. Haemodynamic response to acute hypovolaemia, rapid blood volume expansion and adrenaline administration in an infant animal model. Resuscitation. 68 (2), 259-265 (2006).
- Gil-Anton, J., et al. Addition of terlipressin to initial volume resuscitation in a pediatric model of hemorrhagic shock improves hemodynamics and cerebral perfusion. PLoS One. 15 (7), e0235084 (2020).
- Williams, A. M., et al. Complete and partial aortic occlusion for the treatment of hemorrhagic shock in swine. Journal of Visualized Experiments. (138), e58284 (2018).
- Schüttler, D., et al. A practical guide to setting up pig models for cardiovascular catheterization, electrophysiological assessment, and heart disease research. Lab Animal. 51 (2), 46-67 (2022).
- Chiesa, O. A., et al. Minimally invasive ultrasound-guided technique for central venous catheterization via the external jugular vein in pigs. American Journal of Veterinary Research. 82 (9), 760-769 (2021).
- Anderson, J. H., et al. Ultrasound guided percutaneous common carotid artery access in piglets for intracoronary stem cell infusion. Laboratory Animals. 52 (1), 88-92 (2018).
- Hughes, H. C. Swine in cardiovascular research. Laboratory Animal Science. 36 (4), 348-350 (1986).
- Mayer, A. R., et al. A systematic review of large animal models of combined traumatic brain injury and hemorrhagic shock. Neuroscience and Biobehavioral Reviews. 104, 160-177 (2019).
- Velik-Salchner, C., et al. Normal values for thrombelastography (ROTEM) and selected coagulation parameters in porcine blood. Thrombosis Research. 117 (5), 597-602 (2006).
- Siller-Matula, J. M., Plasenzotti, R., Spiel, A., Quehenberger, P., Jilma, B. Interspecies differences in coagulation profile. Thrombosis and Haemostasis. 100 (3), 397-404 (2008).
- Reisz, J. A., et al. All animals are equal but some animals are more equal than others: Plasma lactate and succinate in hemorrhagic shock-A comparison in rodents, swine, nonhuman primates, and injured patients. The Journal of Trauma and Acute Care Surgery. 84 (3), 537-541 (2018).

