Surgical Porcine Model of Chronic Myocardial Ischemia Treated by Exosome-laden Collagen Patch and Off-pump Coronary Artery Bypass Graft
Summary
This study presents a surgical porcine model of chronic myocardial ischemia due to progressive coronary artery stenosis, resulting in impaired cardiac function without infarction. Following ischemia, animals undergo off-pump coronary artery bypass graft with epicardial placement of stem cells-derived exosomes-laden collagen patch. This adjunctive therapy improves myocardial function and recovery.
Abstract
Chronic myocardial ischemia resulting from progressive coronary artery stenosis leads to hibernating myocardium (HIB), defined as myocardium that adapts to reduced oxygen availability by reducing metabolic activity, thereby preventing irreversible cardiomyocyte injury and infarction. This is distinct from myocardial infarction, as HIB has the potential for recovery with revascularization. Patients with significant coronary artery disease (CAD) experience chronic ischemia, which puts them at risk for heart failure and sudden death. The standard surgical intervention for severe CAD is coronary artery bypass graft surgery (CABG), but it has been shown to be an imperfect therapy, yet no adjunctive therapies exist to recover myocytes adapted to chronic ischemia. To address this gap, a surgical model of HIB using porcine that is amenable to CABG and mimics the clinical scenario was used. The model involves two surgeries. The first operation involves implanting a 1.5 mm rigid constrictor on the left anterior descending (LAD) artery. As the animal grows, the constrictor gradually causes significant stenosis resulting in reduced regional systolic function. Once the stenosis reaches 80%, the myocardial flow and function are impaired, creating HIB. An off-pump CABG is then performed with the left internal mammary artery (LIMA) to revascularize the ischemic region. The animal recovers for one month to allow for optimal myocardial improvement prior to sacrifice. This allows for physiologic and tissue studies of different treatment groups. This animal model demonstrates that cardiac function remains impaired despite CABG, suggesting the need for novel adjunctive interventions. In this study, a collagen patch embedded with mesenchymal stem cell (MSC)-derived exosomes was developed, which can be surgically applied to the epicardial surface distal to LIMA anastomosis. The material conforms to the epicardium, is absorbable, and provides the scaffold for the sustained release of signaling factors. This regenerative therapy can stimulate myocardial recovery that does not respond to revascularization alone. This model translates to the clinical arena by providing means of physiological and mechanistic explorations regarding recovery in HIB.
Introduction
Globally, severe CAD affects over a hundred million patients, and although the mortality rate has decreased, it remains one of the leading causes of death1,2. CAD has a wide clinical spectrum from myocardial infarction (MI) to ischemia with preserved viability. Most pre-clinical research focuses on MI, characterized by the presence of infarcted tissue as it is possible to study in small and large animal models. However, that model does not address patients with preserved viability and amenable to revascularization. Most patients undergoing CABG have decreased blood supply and limited function while maintaining variability in contractile reserve and viability3. Without treatment, these patients can progress to advanced heart failure and sudden death, especially during increased workload4. Among these patients, coronary artery bypass graft (CABG) is an effective therapy but may not result in complete functional recovery5. Importantly, diastolic dysfunction, which is a marker for worse clinical outcomes, fails to recover after revascularization suggesting the need for novel adjuvant therapies during CABG6,7. Currently, there are no clinically available adjuvant interventions used with CABG to restore cardiomyocytes to full functional capacity. This is a major therapeutic gap given that many patients progress to advanced heart failure despite appropriate revascularization8.
An innovative porcine model of chronic myocardial ischemia that is amenable to CABG, to mimic clinical CAD experience was created9. Swine provide a good model of heart disease over other large animals as they do not have epicardial bridging collaterals so stenosis of the LAD alone results in regional ischemia10. In this study, 16-week-old female Yorkshire-Landrace pigs were used. In this model, the LAD was revascularized with off-pump CABG using the left internal mammary artery (LIMA) graft (Supplementary Table 1). Percutaneous coronary intervention (PCI) is not possible to open the stenosis as the constrictor is a rigid device. Cardiac magnetic resonance imaging (MRI) is used to assess global and regional function, coronary anatomy, and tissue viability. Cardiac MRI analysis showed diastolic function, characterized by peak filling rate (PFR) remains impaired despite CABG6. The mechanism of diastolic dysfunction likely relates to impaired mitochondrial bioenergetics and collagen formation in HIB that persist following CABG11.
Mesenchymal stem cells (MSC) provide therapeutic signaling through exosomes to improve myocardial recovery when applied during CABG. In this swine model and parallel in vitro studies, it was shown that placement of an epicardial MSC vicryl patch during CABG recovers contractile function with increase in key mitochondrial proteins namely PGC-1α12, an important regulator of mitochondrial energy metabolism13. The in vitro model allowed us to investigate the signaling mechanism of MSCs on impaired mitochondrial function. Exosomes are secreted stable microvesicles (50-150 nm) that contain protein or nucleic acids including microRNA (miRNA)14. Recent in vitro data suggest that MSC-derived exosomes are an important signaling mechanism necessary for recovery of mitochondrial respiration.
Stem cell derived exosomes are promising adjunctive therapeutics as they are readily accessible, can be commercially produced, and lack ethical conflicts. In consideration of clinical translation, a collagen patch embedded with MSC-derived exosomes was created that can be surgically sutured to the hibernating region of myocardium. It was demonstrated that there is sustained delivery of exosomes using this patch and it provides a cell-free regenerative therapy with paracrine signaling mechanism that targets mitochondrial recovery and enhance mitochondrial biogenesis15. This procedure provides the pre-clinical model to study the impact of MSC-derived therapies to improve cardiac function by means of enhancing mitochondrial function and reducing inflammation at the time of revascularization and reverse the myocyte adaptations to chronic ischemia.
In this study, a surgical method of off-pump CABG using LIMA to LAD anastomosis to bypass the area of proximal LAD stenosis mimicking the standard treatment for patients with CAD is shown. As an adjunctive therapy with CABG, the surgical application of MSC-derived exosome embedded collagen patch on the ischemic region of the myocardium was demonstrated. This surgical model can be used to study the physiologic responses to the paracrine effect seen with use of an exosome patch as well as the molecular mechanisms of recovery.
Protocol
The Institutional Animal Care and Use Committees (IACUC) of the Minneapolis VA Medical Center and the University of Minnesota have approved all of the animal studies. The current National Institutes of Health (NIH) guidelines for the use and care of laboratory animals were followed.
1. Isolation of mesenchymal stem cells and preparation and characterization of exosomes
- Isolation of bone marrow derived mesenchymal stem cells (MSCs)
- Obtain 30-50 mL of sterile bone marrow from the sternum or tibia of a 20-week-old female Yorkshire-Landrace swine. To do this, introduce a 25 mm 15G interosseous needle into the sternum or tibia and draw the sample into a 60 mL syringe with 10 mL of heparin.
NOTE: For further details on the collection of bone marrow refer to Pittenger et al. and Hocum-Stone et al.12,16. - In brief, pass the bone marrow specimen through a Vacutainer CPT tube with heparin for 30 min at 1800 x g.
- Remove the buffy coat containing the mononuclear cells and wash with Hank's balanced salt solution. Pellet mononuclear cells by centrifugation and resuspend in growth medium (10% fetal bovine serum [FBS]).
- Transfer the mononuclear cells to cell culture flasks for adherent growth. Isolate the MSCs from the mononuclear fraction by their adherent nature.
- Wash all non-MSCs within 24 h, leaving a monolayer of MSCs in the tissue culture flask. Confirm the MSC phenotype by flow cytometry, ensuring negativity for CD45, a hematopoietic marker, and positivity for CD90 and CD105, markers of MSCs.
- Obtain 30-50 mL of sterile bone marrow from the sternum or tibia of a 20-week-old female Yorkshire-Landrace swine. To do this, introduce a 25 mm 15G interosseous needle into the sternum or tibia and draw the sample into a 60 mL syringe with 10 mL of heparin.
- Preparation and characterization of exosomes from porcine mesenchymal stem cells
- Seed 1 x 104 H9C2 rat cardiomyocytes and culture in 1x DMEM+ 10% FBS and 1x Pen/strep. Seed 2 x 104 porcine MSCs in advanced DMEM + 5% FBS and 1x Pen/strep.
- Once both cell lines are at least 80% confluent, change the media to exosome depleted H9C2 and MSC media.
- Expose H9C2 cardiomyocytes to mild hypoxia (1% O2 for 24 h). Remove flasks from hypoxia after 24 h and pipette out H9C2 media.
- Remove and discard the MSC media from MSC flask. Add purified H9C2 media to MSC flask. Incubate the flask for 6 h in normoxic conditions (5% CO2, 20% O2, and 37 °C).
- Extract the exosomes from the co-cultured conditioned media using the total exosome isolation reagent following manufacturer's instructions.
- Verify the identification of exosomes by western blot detection of common exosomal proteins with antibodies against CD-63 (1:1000)17.
- Perform nanoparticle tracking analysis (NTA) to quantify the exosomes and the assessment of nanoparticle size and its distribution. To do this, dissolve total protein (50 μg) of exosomes in 500 μL of PBS to determine the concentration and size distribution of exosomes by using nanoparticle tracking analyzer.
- Analyze the data using nanoparticle tracking software.
2. Off pump coronary artery bypass graft surgery
- Animal preparation
- Weigh the animal (16-week-old female Yorkshire-Landrace pigs) 3 days before scheduled for surgery. Fast the animal for 12 h before surgery while having access to water during fasting.
- Give buprenorphine 0.18 mg/kg via intramuscular route 2-4 h before surgery.
- Induction of the animal
- Sedate the animal by giving intramuscular injection of 6.6 mg/kg tiletamine-zolazepam/xylazine.
- Wait for 15 min to ensure adequate sedation by assessing the jaw tone followed by 22G catheter placement in the central ear vein.
NOTE: Another peripheral vein may be considered (i.e., cephalic vein) if ear vein is inadequate. - Administer ophthalmic ointment topically to each eye. Administer 1-2 mg/kg of propofol via intravenous route to induce general anesthesia. Jaw tone most reliably reflects the depth of anesthesia and should be assessed throughout the procedure.
- Intubate the animal with an endotracheal tube of appropriate size.
- Surgery
- Shave the sternum and groin of the animal in preparation for surgical procedure.
- Set mechanical ventilation at 10-15 breaths per min, oxygen 1-4 L/min, and isoflurane 1.0-3.0% as needed to maintain deep anesthesia for surgery. Check for absent eye or jaw reflex to confirm deep anesthesia.
- Position monitoring equipment (Electrocardiogram, end tidal CO2, heart rate, Oxygen saturation, blood pressure, and temperature) on the animal.
- Connect the IV catheter to a bag of normal saline or lactate ringers' solution to administer maintenance fluids continuously.
- Prepare the skin using aseptic technique with povidone Iodine scrub and solution 3x for adequate sterility and to minimize the risk of surgical site infection.
- Give Lidocaine via intravascular route (loading dose of 2 mg/kg or continuous infusion at dose of 50 mcg/kg/min) to prevent arrhythmias.
- Position the animal dorsally and drape with sterile towels.
- Perform either left or right femoral artery cut down for arterial line placement by Seldinger technique followed by connecting the catheter to the transducer for continuous blood pressure monitoring at the time of surgery.
- Use monopolar electrocautery to make a 20 cm incision extending from the sternal notch proximally down to the xyphoid process distally, and to incise layers of muscles, subcutaneous fat, and connective tissue down to the sternum.
- Perform median sternotomy by using oscillating saw.
NOTE: Standard saw is avoided for repeat sternotomy as it carries higher risk for myocardial injury from previous pericardial adhesions from left thoracotomy procedure done to place the LAD constrictor. - Divide the posterior sternal plate using a pair of scissors. Use a specialized chest retractor for adequate visualization of the mediastinum.
- Dissect adhesions using either monopolar electrocautery or the Metzenbaum scissors. Carefully dissect the peristernal muscle and fat to expose the left internal mammary artery (LIMA).
- Once LIMA is exposed lateral to the sternal edge, gently separate it from the chest wall using blunt dissection with electrocautery tip. Use the LIMA as a skeletonized graft.
- Start dissection at the level of 3rd intercostal space. Gently elevate the left sternal border for optimal visualization.
- Use gentle traction on the adventitia to expose the arterial and venous branches of LIMA. Clip the LIMA side of the branches using hemoclips and cauterize the chest wall side of the branches.
NOTE: Care must be taken not to cauterize the clip on the LIMA, because this may cause conduit narrowing. - Once an initial segment of LIMA has been mobilized, continue the dissection proximally toward the level of subclavian vein and distally until the LIMA bifurcation.
- Once dissection is finished, administer heparin via intravenous route at a dose of 100-300 U/kg. Wait for 3 min after the heparin is administered.
- After 3 min, clip the distal end of the LIMA, just before the level of the LIMA bifurcation, and divide the conduit. Sew the distal end with a free 2-0 silk suture tie.
- Prepare the proximal end for grafting. Inspect the flow quality visually by letting the graft bleed for a few seconds.
- Gently clamp the distal end of LIMA conduit with an atraumatic bulldog clamp to avoid bleeding. Open the pericardium with an inverted-T making an approximately 5-6 cm incision. Place 3-0 size sutures on the pericardium for traction at both sides of the slit.
- Stabilize the LAD with silicone retraction tapes and tissue stabilizer, which is secured to the sternal retractor. Make an arteriotomy in LAD artery distal to the stenosis (caused by constrictor band) with an 11-blade and extend with an iris scissors.
- Place an appropriately sized coronary shunt in the LAD. Perform the LIMA to LAD anastomosis with 7-0 running non-absorbable suture using an off-pump bypass technique. Release the bulldog occluder on the LIMA and confirm the hemostasis.
- Preparation of Mesenchymal Stem Cell (MSC)-derived exosome patch
- Following successful isolation of exosomes from MSCs, suspend roughly 3 x 108 exosomes in 3 mL of normal saline and add to collagen sponge.
- Bring 3 mL of exosome suspension to room temperature at around 22 °C for 10 min. Place 2 absorbable collage sponges (each 1.27 cm x 2.54 cm) into a medium Petri dish.
- Use a 5 mL syringe with an 18G needle to gently mix exosome suspension. Slowly pipette 1.5 mL suspension onto each collagen sponge and wait for 5 min for full absorption.
- Placement of exosome patch
- Place the Exosome-laden sponge upside down onto the hibernating region of the heart, which is the epicardium of the anterior septal region in the distribution of the LAD.
- Gently place two sponges to cover the hibernating region of the heart. Use one 3.5 cm x 1.0 cm polyglactin mesh to cover each collagen sponge.
- Sew the mesh onto the epicardium with fine 7-0 interrupted sutures.
- Chest tube placement
- Place a chest tube through separate stab incision, near the inferior aspect of the sternotomy incision. Place the chest tube cautiously over the anterior aspect of the heart.
- Once the tube is in place, place a purse string suture with 3-0 suture using a horizontal mattress stitch to allow for closure of the wound upon removal of the tube.
- The chest tube is maintained until complete chest closure.
- Chest closure
- Approximate the sternum with non-absorbable sutures using a figure eight pattern. Administer 1 mg/kg bupivacaine via intramuscular route along the entire length of the incision.
NOTE: Suture is used rather than wires to avoid interference with MRI imaging. - Close layers of muscle and skin in the standard fashion using 2-0 and 3-0 absorbable suture, respectively.
- Perform a breath hold and suction to evacuate all the air out of the thoracic cavity. Monitor the airway pressure on the ventilator cautiously and maintain the pressure between 15-22 mmHg and release when complete.
- Once all the air is evacuated, remove the chest tube while closing the wound using the purse string suture. Apply adhesive glue topically to cover the sternal incision.
- Approximate the sternum with non-absorbable sutures using a figure eight pattern. Administer 1 mg/kg bupivacaine via intramuscular route along the entire length of the incision.
- Post operative care after surgery
- Gradually wean the animal off the ventilator as skin incision is being closed. Ensure that the animal is able to spontaneously breathe and protect reflexes before disconnecting the animal from anesthesia equipment.
- Remove the endotracheal tube after confirming that animal is able to protect its airway. Cover the skin incision with sterile and non-adherent dressing embedded with antibiotic ointment to minimize surgical site infection.
- Continue to monitor vital signs including heart rate, respiratory rate, body temperature every 15 min until animal is able to hold its position without assistance.
- Ensure animal is not left unattended until able to lift and hold its head up and can stand without assistance. Administer meloxicam at a dose of 0.2 mg/kg via subcutaneous route before transporting the animal to the recovery unit.
- Transport the animal to the recovery unit when animal is stable. Keep the surgical site dressing on the incision until postoperative day 3. Replace the dressing if it becomes soiled.
- Continue to monitor the level of the pain, skin incision and overall well-being of the animal for the first 5 days after surgery. Administer half a dose of meloxicam (0.1 mg/kg) as needed once daily for breakthrough pain.
- Single house the animal for first 5 days after surgery while the incision(s) heal to reduce the risk of surgical site infection by another animal. Return the animal to group housing after 5 days.
- Report any complications or changes in the animal's condition (fever, ascites, weight loss, inappetence etc.) to the veterinarian or appropriate staff.
3. Coronary angiography using femoral access
- Secure the animal on the operating table in the dorsal recumbency. Initiate mechanical ventilation at 10-15 breaths per min. Set oxygen at 2-4 L/min, isoflurane at 1% and 4%, as needed to maintain a deep plane of anesthesia.
- Place ECG leads on the animal's limb to monitor for heart rhythm. Evaluate the animal for the depth of anesthesia. Consider the animal deeply anesthetized when the eye or jaw reflex is absent.
- Clean the chest and neck area with povidone iodine scrub and then drape the animal with towels.
- Access the femoral artery via surgical cut-down and expose the femoral artery and vein. Make a 1-2 mm longitudinal incision with a no. 11 blade in femoral artery and cannulate the artery using an 11 Fr introducer sheath in the vessel lumen.
- After obtaining access, advance the catheter to perform coronary angiography to assess the anatomy patency of LIMA-LAD graft.
Representative Results
Following revascularization, coronary angiography is performed to assess for LAD stenosis (greater than 80%) and patency of the LIMA-LAD graft (Figure 1). Four weeks following the revascularization surgery and placement of the exosome-laden collagen patch, cardiac MRI is performed to assess for systolic and diastolic function of the heart at rest and under stress using low-dose dobutamine infusion at 5µg/kg/min. Systolic function is analyzed by measuring wall thickness percentage (wall thickness at end systole – wall thickness at end diastole). Diastolic function is analyzed by measuring peak filling rate over end diastolic volume (PFR/EDV; Figure 2). Delayed contrast imaging was performed to confirm lack of myocardial infarction in the LAD territory. If an infarct in the LAD region is present, it is likely due to the occluded artery secondary to thrombosis caused by the constrictor. Absence of regional wall motion abnormalities demonstrate lack of hibernating phenotype.
At low dose of dobutamine infusion, HIB animals demonstrate significant decrease in diastolic function, measured by PFR/EDV, compared to control group (5.5 ± 0.8 vs. 6.9 ± 1.5, respectively, p < 0.05). CABG group demonstrates a trend towards improvement in PFR/EDV compared to HIB group (6.3 ± 0.9 vs. 5.5 ± 0.8, respectively, p = 0.06). However, CABG + MSC group demonstrate a significant increase in PFR/EDV when compared to HIB group (6.6 ± 1.1 vs. 5.5± 0.8, respectively, p = 0.03; Figure 3). Cardiac MRI was used to confirm lack of necrosis and patency of the Left internal mammary artery (LIMA) to left anterior descending artery (LAD) bypass graft distal to the area of stenosis18.
At rest, CABG + MSC group does not alter regional systolic function (measured by percentage wall thickness) compared with CABG alone (26.3% ± 7.0% vs. 34.9% ± 6.3%; p = 0.19). Under stress, CABG + MSC group shows significant improvement in regional systolic function compared with CABG alone (78.3% ± 19.6% vs. 39.2% ± 5.6%; p = 0.05)12 (Figure 4).
At necropsy, appropriately sized coronary dilators were used to ensure the LAD stenosis and LIMA patency. Myocardium was grossly inspected to ensure the tissue viability is present in all regions especially in the ischemic region. Triphenyltetrazolium chloride (TTC) stain confirmed absence of scar.
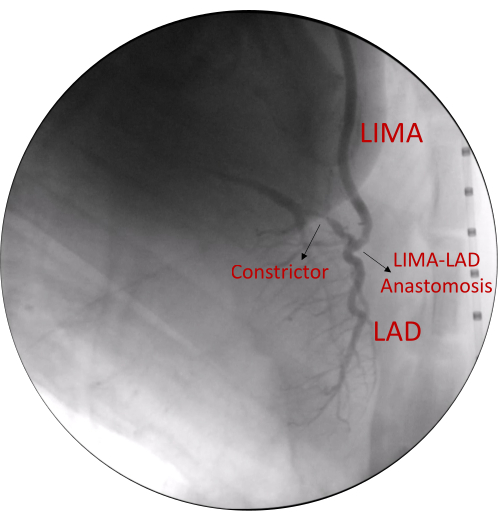
Figure 1. Cardiac angiogram demonstrating the anatomy. Coronary angiography demonstrates >80% stenosis of proximal LAD artery and patent LIMA-LAD graft anastomosis. Abbreviations: LIMA= Left internal mammary artery, LAD= Left anterior descending Please click here to view a larger version of this figure.
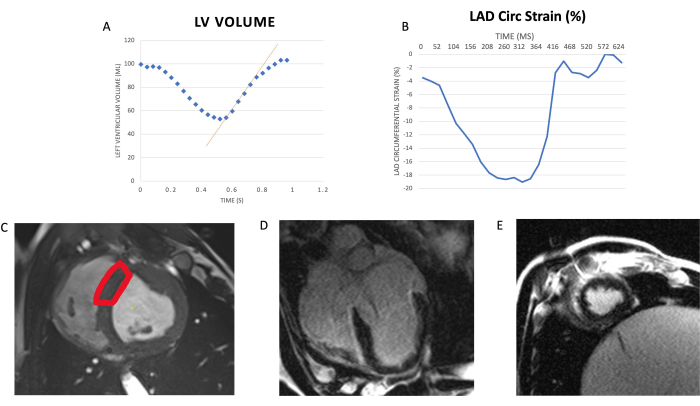
Figure 2. Assessment of diastolic relaxation, global contractile function, and viability using cardiac MRI. (A) Diastolic relaxation: Relationship of left ventricular (LV) volume during a cardiac cycle. The x-axis is time in s; y-axis is volume of left ventricle in mL. The red line indicates peak filling rate (fastest rate at which the LV increases volume). PFR is normalized to the animal's end-diastolic volume (PFR/EDV) to account for size variance across animals. (B) Global contractile function: Segmental circumferential strain (Circ strain) during the cardiac cycle (x-axis: time in ms; y-axis: percentage change in circumferential length of left ventricle segment compared with end-diastolic measurement). Peak circumferential strain is represented by the most negative value of cycle. (C) Representative cardiac MRI image of LAD distribution: LAD distribution is highlighted in red represents the anteroseptal wall. There was no evidence of infarction based on enhanced gadolinium contrast on 4 chamber (D) long axis and (E) short axis views. Abbreviations: LV= left ventricle/ventricular; LAD= left anterior descending; MRI= magnetic resonance imaging. This figure has been modified from 6. Please click here to view a larger version of this figure.
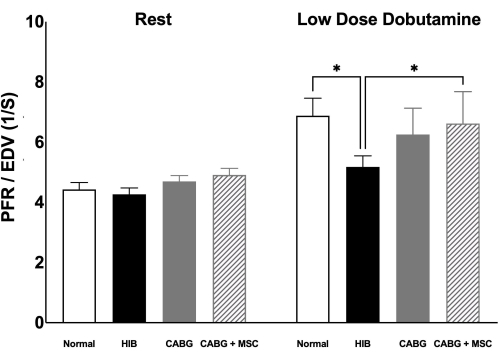
Figure 3. MRI assessment of Peak filling rate/End diastolic volume. Diastolic function, measured by PFR/EDV, was compared among four groups (Control, HIB, CABG, and CABG + MSC). At rest, PFR/EDV is comparable among four animal groups. However, under stress using low dose dobutamine infusion (5µg/kg/min), HIB group showed a significant decrease in PFR/EDV when compared to control (p < 0.05) with trend towards improvement in CABG group (p = 0.06) and significant increase in CABG + MSC group (p < 0.05). Statistical analyses were performed using one-way analysis of variance (ANOVA) test. Data are presented as means ± SD. Abbreviations: CABG= Coronary artery bypass graft, PFR= Peak filling rate, EDV= end diastolic volume; MRI= Magnetic resonance imaging, MSC= Mesenchymal Stem Cells, SD= Standard deviation. Please click here to view a larger version of this figure.
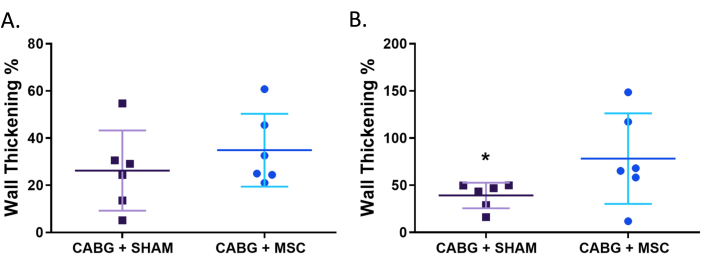
Figure 4. MRI assessment of regional systolic function by wall thickening %. Treatment with MSC patch shows improvement in regional cardiac function compared to sham patch. (A) Regional systolic function, measured by wall thickening percentage, does not significantly improve at rest with MSC patch treatment (n = 6) compared with sham (n = 6). (B) Under stress using low dose dobutamine infusion (5µg/kg/min), there is significant improvement in regional systolic function following treatment with the MSC patch compared with sham animals (P<.05). statistical analyses were performed using Mann-Whitney test. Horizontal bars indicate mean standard deviation. *P<.05. Abbreviations: MSC= Mesenchymal stem cells. This figure has been modified from 12. Please click here to view a larger version of this figure.
Supplementary table 1. Overview of procedures and timeline of each procedure. Please click here to download this File.
Discussion
This study presents the first porcine model of chronically ischemic myocardium, in which it was shown that treatment with an MSC-derived exosome laden collagen patch during surgical revascularization recovers diastolic and systolic function upon inotropic stimulation potentially by targeting mitochondrial recovery. Previously, it was demonstrated that in a large animal model of HIB the diastolic and systolic function, as measured by cardiac MRI, remains impaired and only slightly improves with revascularization without complete recovery6,19. The dysfunction occurred despite preserved left ventricular ejection fraction. These findings accurately mimic the clinical experience seen in patients with chronic ischemic myocardium in a single vessel territory with preserved left ventricular function.
There are several critical and technical challenges during revascularization surgery especially in the setting of previous thoracotomy. Cardiovascular injury on sternal entry is a risk as the pericardium has already been breached and adhesions may be present. Sternotomy may cause cardiac injury due to proximity or adherence to the sternum. This risk can be mitigated by use of an oscillating saw, which has been shown to promote an uneventful sternal re-entry.
A key aspect to obtain successful CABG is graft quality. Meticulous LIMA harvesting is an important technical aspect in successfully performing high-quality CABG and is associated with improved graft patency. LIMA can be harvested using two techniques: pedicled and skeletonized. Pedicled technique includes dissecting the LIMA from the sternum along with its veins, fascia, fat, and lymphatics. Skeletonized technique includes dissecting the LIMA free of all surrounding tissue, and therefore yielding the artery only20. In this model, the skeletonization technique was implemented as it can minimize sternal ischemia and the graft is longer than a pedicled LIMA20. LIMA is a delicate structure, any undue stretching, clamping, or misplaced clips can result in vascular injury and unsatisfactory results. During dissection, the cautery tip should be used with caution and at low voltage. When separating the artery from its perforating branches, the LIMA side of the branches are clipped using hemoclips. Care must be taken not to cauterize the clips as it can result in narrowing of the conduit. Confirm pulsatile flow prior to grafting.
Ensure that reasonable amounts of anesthesia and paralytics are maintained to minimize movement during the surgery especially while sewing the anastomosis. It is crucial to use the appropriate doses of lidocaine and heparin (200-300 units/kg) to eliminate the risk of arrhythmia and thrombosis, respectively. A second dose of lidocaine might be indicated if the animal experiences any arrhythmias during surgery. Use of femoral arterial line allows continuous hemodynamic monitoring. When performing the anastomosis, it is useful to either put 1-2 surgical sponges behind the heart or place sutures on either side of pericardium to lift the left ventricle upward. In this model, we use silastic tapes and the tissue stabilizer that utilizes suction pressure to effectively immobilize the target site. A mild drop in arterial blood pressure in addition to ST depression on the EKG may be noted once the stabilizer is placed and the heart is lifted. These hemodynamic derangements are usually well tolerated without requiring any interventions. In situations where hemodynamic instability is significant, a dose of phenylephrine (5-20 µg/kg) via intravenous route may be administered to raise the arterial blood pressure. If hemodynamic instability is life threatening, a dose of Epinephrine (0.1 µg/kg; diluted 1:10,000) can be administered via intravenous route as an emergency rescue drug. Once the LAD is exposed with a beaver blade, an arteriotomy is performed with an 11-blade and completed with microsurgical scissors. One must be careful not to injure the posterior wall of the LAD during this maneuver. It is critical to keep the arteriotomy site bloodless during off-pump CABG to allow for accurate suturing and several techniques have been described including intermittent irrigation with saline solution, use of CO2 blower, and intraluminal coronary shunts21. In this study, the CO2 blower along with an appropriately sized intraluminal coronary shunt were used as both are routinely used in the off-pump CABG operations. A potentially lethal complication of the CO2 mister blower is an air embolism. However, the risk of air embolism can be negated by using coronary shunt, which can act as a physical barrier within the arteriotomy. Additionally, the use of coronary shunt helps to keep the surgical field bloodless, which permits the use of a lower gas flow and further minimizes the risk of air embolism. Shunts also improve the technical precision for anastomosis and prevent the inadvertent injury to back wall of the artery while suturing22.
In this well-established porcine model, an off-pump rather than on-pump technique during CABG surgery was employed. The advantages of using this technique, instead of the on-pump, is to minimize the operative time and avoid the central cannulation of the aorta and right atrium with full heparinization. In addition, it helps in faster recovery of the animal after surgery by reducing the risk of post-operative bleeding and/or cardiac temponade. These are presumed advantages based on clinical experience in patients undergoing CABG on both on and off pump.
This exosome-laden collagen patch is novel in that it can be quantified and surgically secured to the region of ischemia that has been revascularized. This allows sustained release of exosomes from the patch over the course of several days resulting in continuous and direct treatment of the ischemic region. Histopathology of the hibernating tissue 4 weeks following treatment with CABG and exosome demonstrated lack of inflammatory response of myocardium to the patch itself, although some inflammation was noted at the site of sutures as evident by staining for inflammatory cells. While a variety of methods has been suggested for exosome delivery into the myocardium, common techniques such as direct injection of exosome result in low retention of therapeutic product in the injured area, as up to 90% of exosomes wash away or disperse after injection23. Analysis of exosomes retention following injection has been completed for up to 3 h post injection and has shown significant decreases in the exosome content24. Exosomes are easy to isolate and have more flexibility in storage conditions over long periods of time, presenting an opportunity for off-the-shelf products that can be used in the acute setting making it more translatable to patients.
This study has several limitations including age and sex of animals. Given surgical and logistic limitations, considerations around animal welfare regulations and staff safety, only juvenile female swine were studied. Although, CABG surgery adds to the complexity of the model, it was a required intervention as other less invasive interventions (percutaneous coronary intervention or PCI) would not allow to open the stenotic region of LAD due to the rigid nature of the constrictor18. Furthermore, this model of single vessel stenosis without any comorbidities does not fully simulate the extent and effects of long-standing coronary atherosclerosis as seen in human population. Future studies will focus on using a multivessel disease model of hibernating myocardium by surgically placing the constrictor on the circumflex artery and LAD. However, this two-vessel disease model would result in hibernating myocardium with reduced ejection fraction. Animal mortality would likely increase, and revascularization surgically is more complex and requires on-pump bypass support. In future, if there were problems with the practical applications of patch type, other scaffold material options will be explored, such as decellularized extracellular matrix, or alternate form of hydrogels.
Divulgaciones
The authors have nothing to disclose.
Acknowledgements
This work was supported by the VA Merit Review #I01 BX000760 (RFK) from the United States (U.S.) Department of Veterans Affairs BLR&D and U.S. Department of Veterans Affairs grant #I01 BX004146 (TAB). We also gratefully acknowledge the support of the University of Minnesota Lillehei Heart Institute. The contents of this work do not represent the views of the U.S. Department of Veterans Affairs of the United States Government.
Materials
| 5 Ethibond | Ethicon | MG46G | Suture |
| # 40 clipper blade | Oster | 078919-016-701 | Remove hair from surgery sites |
| 0 Vicryl | Ethicon | J208H | Suture |
| 1 mL Syringe | Medtronic/Covidien | 1188100777 | Administer injectable agents |
| 1" medical tape | Medline | MMM15271Z | Secure wound dressing and IV catheters |
| 1000mL 0.9% Sodium chloride | Baxter | 2B1324X | IV replacement fluid |
| 12 mL Syringe | Medtronic/Covidien | 8881512878 | Administer injectable agents |
| 18 ga needles | BD | 305185 | Administration of injectable agents |
| 20 ga needles | BD | 305175 | Administration of injectable agents |
| 20 mL Syringe | Medtronic/Covidien | 8881520657 | Administer injectable agents |
| 2-0 Vicryl | Ethicon | J317H | Suture |
| 250 mL 0.9% saline | Baxter | UE1322D | Replacement IV Fluid |
| 3 mL Syinge | Medtronic/Covidien | 1180300555 | Administer injectable agents |
| 3-0 Vicryl | Ethicon | VCP824G | Suture |
| 36” Pressure monitoring tubing | Smith’s Medical | MX563 | Connect art. Line to transducer |
| 4.0 mm ID endotracheal tube | Medline | DYND43040 | Establish airway for Hibernation |
| 4-0 Tevdek II Strands | Deknatel | 7-922 | Suture to secure constrictor around LAD |
| 48” Pressure monitoring tubing | Smith’s Medical | MX564 | Connect art. Line to transducer |
| 500mL 0.9% Sodium chloride | Baxter | 2B1323Q | Drug delivery, Provide mist for Blower Mister |
| 6 mL Syringe | Medtronic/Covidien | 1180600777 | Administer injectable agents |
| 6.0 mm ID endotracheal tube | Mallinckrodt | 86049 | Establish airway for Revasc,MRI and Termination |
| 6.5 mm ID endotracheal tube | Medline | DYND43065 | Establish airway for Revasc,MRI and Termination |
| 6” pressure tubing line | Smith’s Medical | MX560 | Collect bone marrow |
| 60 mL Syringe | Medtronic/Covidien | 8881560125 | Administer injectable agents |
| 7.0 mm ID endotracheal tube | Medline | DYND43070 | Establish airway for Revasc,MRI and Termination |
| 7-0 Prolene | Ethicon | M8702 | Suture |
| Advanced DMEM (1X) | ThermoFisher Scientific | 12491023 | |
| Alcohol Prep pads | MedSource | MS-17402 | Skin disinfectant |
| Amicon Ultra-15 Centrifugal Filter Unit | Millipore Sigma | UFC910024 | |
| Anesthesia Machine | Drager | Fabious Trio | maintains general anesthesia |
| Anesthesia Machine + ventilator | DRE Drager- Fabius Tiro | DRE0603FT | Deliver Oxygen and inhalant to patient |
| Anesthesia Monitor | Phillips Intellivue | MP70 | Multiparameter for patient safety |
| Arterial Line Kit | Arrow | ASK-04510-HF | Femoral catheter for blood pressure monitoring |
| Artificial Tears | Rugby | 0536-1086-91 | Lubricate eyes to prevent corneal drying |
| Bair Hugger | 3M | Model 505 | Patient Warming system |
| Basic pack | Medline | DYNJP1000 | Sterile drapes and table cover |
| Blood Collection Tubes- green top | Fisher Scientific | 02-689-7 | Collect microsphere blood samples |
| Blower Mister Kit | Medtronic/Covidien | 22120 | Clears surgical field for vessel anastomosis |
| BODIPY TR Ceramide | ThermoFisher Scientific | D7540 | |
| Bone marrow needle- 25mm 15 ga IO needle | Vidacare | 9001-VC-005 | Collect bone marrow |
| Bone Wax | Medline | ETHW31G | Hemostasis of cut bone |
| Bovie Cautery hand piece | Covidien | E2516 | Hemostasis |
| Bupivicaine | Pfizer | 00409-1161-01 | Local Anesthetic |
| Buprenorphine 0.3 mg/mL | Sigma Aldrich | B9275 | Pre operative Analgesic for survivial procedures |
| Cell Scrapers | Corning | 353085 | |
| Cephazolin 1 gr | Pfizer | 00409-0805-01 | Antibiotic |
| Chest Tube | Covidien | 8888561043 | Evacuates air from chest cavity |
| Cloroprep | Becton Dickenson | 260815 | Surgical skin prep |
| Corning bottle-top vacuum Filter System (500mL) | Millipore Sigma | 430758 | |
| CPT tube | BD | 362753 | MSC isolation from bone marrow |
| Delrin Constrictor | U of MN | Custom made | Creates stenosis of LAD |
| Dermabond | Ethicon | DNX12 | Skin adhesive |
| DMEM (1X) Dulbecco's Modified Eagle Medium, HEPES | ThermoFisher Scientific | 12430062 | |
| Dobutamine 12.5 mg/mL | Pfizer | 00409-2344-01 | Increases blood pressure and heart rate during the second microsphere blood collection |
| ECG Pads | DRE | 1496 | Monitor heart rhythm |
| Exosome-Depleted FBS | ThermoFisher Scientific | A2720801 | |
| Falcon Disposable Polystyrene Serological Pipets, Sterile, 10mL | Fisher Scientific | 13-675-20 | |
| Femoral and carotid introducer | Cordis- J&J | 504606P | femoral and carotis cannulas |
| Fetal Bovine Serum, Heat Inactivated, Gibco FBS | ThermoFisher Scientific | 16140089 | |
| Flo-thru 1.0 | Baxter | FT-12100 | used to anastomos LIMA to L |
| Flo-thru 1.25 | Baxter | FT-12125 | FT-12125 |
| Flo-thru 1.5 | Baxter | FT-12150 | FT-12150 |
| Flo-thru 2.0 | Baxter | FT-12200 | FT-12200 |
| GlutaMAX Supplement | ThermoFisher Scientific | 35050061 | |
| Hair Clipper | Oster | 078566-011-002 | Remove hair from surgery sites |
| Helistat collagen sponge | McKesson | 570973 1690ZZ | Sponge for embedding exosomes |
| Heparin | Pfizer | 0409-2720-03 | anticoaggulant |
| Histology Jars | Fisher Scientific | 316-154 | Formalin for tissue samples |
| HyClone Characterized Fetal Bovine Serum (FBS) | Cytiva | SH30071.03 | |
| Hypafix | BSN Medical | 4210 | Secure wound dressing and IV catheters |
| Isoflurane | Sigma Aldrich | CDS019936 | General Anesthestic- Inhalant |
| IV Tubing for Blower Mister | Carefusion | 42493E | Adapts to IV Fluids for Blower/Mister |
| Jelco 18 ga IV catheter | Smiths medical | 4054 | IV access in Revasc, MRI and Term |
| Lidocaine 2% | Pfizer | 00409-4277-01 | Local Anesthetic/ antiarrthymic |
| Ligaclips | Ethicon | MSC20 | Surgical Staples for LIMA takedown |
| Long blade for laryngoscope | DRE | 12521 | Allows for visualization of trachea for intubation |
| Meloxicam 5 mg/mL | Boehringer Ingelheim | 141-219 | Post operative Analgesic |
| Microsphere pump | Collect blood samples from femoral introducer | ||
| Monopolar Cautery | Covidien | Valleylab™ FT10 | Hemostasis |
| Nanosight NS 300 | Malvern Panalytical | MAN0541-03-EN | |
| NTA 3.1.54 software | Malvern Panalytical | MAN0520-01-EN-00 | |
| OPVAC Synergy II | Terumo Cardiovascular System | 401-230 | Heart positioner and Stabilizer |
| Oxygen Tank E cylinder | various | various | Used for Blower Mister if anesthesia machine doesn't have auxiliary flow meter |
| PBS, pH 7.2 | ThermoFisher Scientific | 20012050 | |
| Penicillin-Streptomycin-Neomycin (PSN) Antibiotic Mixture | ThermoFisher Scientific | 15640055 | |
| Pigtail 145 catheter 6 French | Boston Scientific | 08641-41 | Measure LV pressures |
| Pressure Transducer | various | Must adapt to anesthesia monitor | Monitor direct arterial pressures |
| Propofol | Diprivan | 269-29 | Induction agent |
| Roncuronium | Mylan | 67457-228-05 | Neuromuscular blocking agent |
| SR Buprenorphine 10 mg/mL | Abbott Labs | NADA 141-434 | Post operative Analgesic |
| Sterile Saline 20 mL | Fisher Scientific | 20T700220 | Flush for IV catheters |
| Sternal Saw/ Necropsy Saw | Thermo Fisher | 812822 | Used to open chest cavity |
| Stop Cocks | Smith Medical | MX5311L | 2 to connect to pig tail |
| Succinylcholine 20 mg/mL | Pfizer | 00409-6629-02 | Neuromuscular blocking agent |
| Suction tubing | Medline | DYND50223 | |
| Suction Container | Medline | DYNDCL03000 | |
| Surgery pack with chest retractor | various | See pack list | Femoral cut down and median sternotomy |
| Surgical Instruments | various | See pack list | Femoral and carotid cutdowns and sternotomy |
| Surgical Spring Clip | Applied Medical | A1801 | Clamp end of LIMA after takedown |
| Syringe pump | Harvard | Delivers IV Dobutamine infusion | |
| SYTO RNASelect Green Fluorescent cell Stain – 5 mM Solution in DMSO | Millipore Sigma | S32703 | |
| Telazol 100 mg/mL | Fort Dodge | 01L60030 | Pre operative Sedative |
| Telpha pad | Covidien | 2132 | Sterile wound dressing |
| Timer | Time collection of blood samples | ||
| Total Exosome Isolation Reagent (from cell culture media) | ThermoFisher Scientific | 4478359 | |
| TPP Tissue Culture Flask, T75, Filter Cap w/ 0.22uM PTFE | ThermoFisher Scientific | TP90076 | |
| Triple Antibiotic Ointment | Johnson & Johnson | 23734 | Topical over wound |
| Vicryl mesh | Ethicon | VKML | Patch for epicardial cell application |
| Vortex | Mix microspheres | ||
| Xylazine 100 mg/mL | Vedco | 468RX | Pre operative Sedative/ analgesic |
Referencias
- Dai, H., et al. Global, regional, and burden of ischaemic heart disease and its attributable risk factors, 1990-2017: results from the Global Burden of Disease Study 2017. European heart journal. Quality of care & clinical outcomes. 8 (1), 50-60 (2022).
- Tsao, C. W., et al. Heart Disease and Stroke Statistics-2022 Update: A Report From the American Heart Association. Circulation. 145 (8), e153-e639 (2022).
- Rahimtoola, S. H. The hibernating myocardium. American Heart Journal. 117 (1), 211-221 (1989).
- Canty, J. M., Fallavollita, J. A. Hibernating myocardium. Journal of Nuclear Cardiology. 12 (1), 104-119 (2005).
- Page, B. J., et al. Revascularization of chronic hibernating myocardium stimulates myocyte proliferation and partially reverses chronic adaptations to ischemia. Journal of the American College of Cardiology. 65 (7), 684-697 (2015).
- Aggarwal, R., et al. Persistent diastolic dysfunction in chronically ischemic hearts following coronary artery bypass graft. The Journal of Thoracic and Cardiovascular Surgery. 165 (6), e269-e279 (2023).
- Olsen, F. J., et al. Prognostic Value and Interplay Between Myocardial Tissue Velocities in Patients Undergoing Coronary Artery Bypass Grafting. The American Journal of Cardiology. 144, 37-45 (2021).
- Virani, S. S. Heart Disease and Stroke Statistics-2021 Update: A Report From the American Heart Association. Circulation. 143 (8), e254-e743 (2021).
- Hocum Stone, L., et al. Surgical Swine Model of Chronic Cardiac Ischemia Treated by Off-Pump Coronary Artery Bypass Graft Surgery. Journal of Visualized Experiments:JoVE. (133), e57229 (2018).
- White, F. C., Carroll, S. M., Magnet, A., Bloor, C. M. Coronary collateral development in swine after coronary artery occlusion. Circulation Research. 71 (6), 1490-1500 (1992).
- Righetti, A., et al. Interventricular septal motion and left ventricular function after coronary bypass surgery: evaluation with echocardiography and radionuclide angiography. The American Journal of Cardiology. 39 (3), 372-377 (1977).
- Hocum Stone, L. L., et al. Recovery of hibernating myocardium using stem cell patch with coronary bypass surgery. The Journal of Thoracic and Cardiovascular Surgery. 62 (1), e3-e16 (2021).
- Puigserver, P., Spiegelman, B. M. Peroxisome proliferator-activated receptor-gamma coactivator 1 alpha (PGC-1 alpha): transcriptional coactivator and metabolic regulator. Endocrine Reviews. 24 (1), 78-90 (2003).
- Henning, R. J. Cardiovascular Exosomes and MicroRNAs in Cardiovascular Physiology and Pathophysiology. Journal of Cardiovascular Translational Research. 14 (2), 195-212 (2021).
- Chen, Y., Liu, Y., Dorn, G. W. 2nd. Mitochondrial fusion is essential for organelle function and cardiac homeostasis. Circulation Research. 109 (12), 1327-1331 (2011).
- Pittenger, M. F., Martin, B. J. Mesenchymal stem cells and their potential as cardiac therapeutics. Circulation Research. 95 (1), 9-20 (2004).
- Campos-Silva, C., et al. High sensitivity detection of extracellular vesicles immune-captured from urine by conventional flow cytometry. Scientific Reports. 9 (1), 2042 (2019).
- Hocum Stone, L. L., et al. Magnetic resonance imaging assessment of cardiac function in a swine model of hibernating myocardium 3 months following bypass surgery. The Journal of Thoracic and Cardiovascular Surgery. 153 (3), 582-590 (2017).
- Stone, L. L. H., et al. Mitochondrial Respiratory Capacity is Restored in Hibernating Cardiomyocytes Following Co-Culture with Mesenchymal Stem Cells. Cell Medicine. 11, 2155179019834938 (2019).
- Lamy, A., et al. Skeletonized vs Pedicled Internal Mammary Artery Graft Harvesting in Coronary Artery Bypass Surgery: A Post Hoc Analysis From the COMPASS Trial. JAMA Cardiology. 6 (9), 1042-1049 (2021).
- Shim, J. K., Choi, Y. S., Yoo, K. J., Kwak, Y. L. Carbon dioxide embolism induced right coronary artery ischaemia during off-pump obtuse marginalis artery grafting. European Journal of Cardio-Thoracic Surgery. 36 (3), 598-599 (2009).
- Aklog, L. Future technology for off-pump coronary artery bypass (OPCAB). Seminars in Thoracic and Cardiovascular Surgery. 15 (1), 92-102 (2003).
- Hou, D., et al. Radiolabeled cell distribution after intramyocardial, intracoronary, and interstitial retrograde coronary venous delivery: implications for current clinical trials. Circulation. 112 (9 Suppl), I150-I156 (2005).
- Gallet, R., et al. Exosomes secreted by cardiosphere-derived cells reduce scarring, attenuate adverse remodelling, and improve function in acute and chronic porcine myocardial infarction. European Heart Journal. 38 (3), 201-211 (2017).

