Calcium Imaging In Electrically Stimulated Flat-Mounted Retinas
Summary
Retinal prostheses have the ability to generate visual perceptions. To advance the development of new prostheses, ex vivo methods are needed to test devices before implantation. This article provides a comprehensive protocol for studying calcium activity in the retinal ganglion cell layer when subjected to electrical stimulation.
Abstract
Retinal dystrophies are a leading cause of blindness worldwide. Extensive efforts are underway to develop advanced retinal prostheses that can bypass the impaired light-sensing photoreceptor cells in the degenerated retina, aiming to partially restore vision by inducing visual percepts. One common avenue of investigation involves the design and production of implantable devices with a flexible physical structure, housing a high number of electrodes. This enables the efficient and precise generation of visual percepts. However, with each technological advancement, there arises a need for a reliable and manageable ex vivo method to verify the functionality of the device before progressing to in vivo experiments, where factors beyond the device’s performance come into play. This article presents a comprehensive protocol for studying calcium activity in the retinal ganglion cell layer (GCL) following electrical stimulation. Specifically, the following steps are outlined: (1) fluorescently labeling the rat retina using genetically encoded calcium indicators, (2) capturing the fluorescence signal using an inverted fluorescence microscope while applying distinct patterns of electrical stimulation, and (3) extracting and analyzing the calcium traces from individual cells within the GCL. By following this procedure, researchers can efficiently test new stimulation protocols prior to conducting in vivo experiments.
Introduction
The retina contains photoreceptors, which are cells responsible for sensing light. They capture photons and convert them into nerve impulses. These impulses are then processed within the retina and transmitted to the visual cortex, resulting in the formation of a visual image1. Retinitis Pigmentosa (RP) and Age-related Macular Degeneration (AMD) are degenerative diseases characterized by the progressive loss of photoreceptors. These retinopathies are among the leading causes of blindness worldwide1, impacting millions of individuals and having significant medical, personal, and socioeconomic consequences for patients, healthcare systems, and society as a whole. Furthermore, with the aging population, it is projected that AMD cases will increase by 15% by 20502.
Currently, numerous research efforts are underway to restore vision in patients affected by these conditions3. One promising approach is the use of retinal prostheses, which have demonstrated effectiveness in partially restoring vision4,5. These devices capture light from the visual scene and convert it into electrical pulses. These pulses are delivered through electrodes within a microelectrode array (MEA) implanted in the eye, stimulating the surviving neurons and bypassing the function of the lost photoreceptors. The activated retinal ganglion cells (RGCs) transmit the output to the brain, where it is interpreted as visual perception. However, the main limitations of current implants lie in the resolution of the electrode-tissue interface6 and the non-selective stimulation of different cell types. Therefore, to optimize the design of new implantable devices for more efficient vision restoration, it is crucial to understand how stimulation paradigms can be developed to selectively activate cells in close proximity to the electrodes.
Calcium imaging is a widely employed technique for studying neural activity, offering several advantages over non-optical methods7,8. Firstly, it provides cellular and subcellular resolution. Secondly, calcium markers can target specific cell types. Thirdly, it allows for long-term tracking, and fourthly, it enables the observation of entire cell populations while distinguishing between active and inactive cells. This method provides indirect evidence of cellular activity with a temporal resolution in the range of hundreds of milliseconds. Genetically-encoded fluorescent calcium indicators, such as GCaMP sensors, undergo a conformational change upon binding to calcium, resulting in increased fluorescence9. Recombinant adeno-associated viral vectors (AAVs) are an effective means of transducing retinal cells with GCaMP10.
This protocol presents an efficient method that utilizes calcium imaging for testing stimulation protocols of retinal implants. Specifically, we focus on ex vivo rat retinal tissue and provide detailed step-by-step instructions, from sample acquisition to data analysis. By offering this comprehensive guide, researchers from various backgrounds can embark on electrical stimulation experimentation with confidence.
Protocol
All animal procedures were conducted in accordance with standard animal ethical guidelines (European Communities Directive 86/609/EU) and approved by the local animal ethics committees. 8 weeks-old Long Evans rats were used for the present study. The animals were obtained from a commercial source (see Table of Materials).
1. Preparation of media and flat-mounting assembly
- Ames' Medium (1 L)
- In a 1 L glass bottle, combine the Ames' Medium powder, 1.9 g/L NaHCO3, 10 ml of Penicillin/Streptomycin 100x, and 1 L of deionized water (see Table of Materials). Adjust the pH to 7.4 and the osmolarity to 280 mOsm with deionized water or NaHCO3. Sterilize the solution by filtering it through a 0.2 µm pore size filter under a hood.
NOTE: Store the sterilized medium at 4 °C. This solution remains stable and can be used for up to 1 month.
- In a 1 L glass bottle, combine the Ames' Medium powder, 1.9 g/L NaHCO3, 10 ml of Penicillin/Streptomycin 100x, and 1 L of deionized water (see Table of Materials). Adjust the pH to 7.4 and the osmolarity to 280 mOsm with deionized water or NaHCO3. Sterilize the solution by filtering it through a 0.2 µm pore size filter under a hood.
- Mounting membranes
- Attach one PTFE porous membrane (see Table of Materials) to a washer using small drops of glue. Allow it to dry for at least 15 min.
- To achieve translucency, immerse the membranes in 70% ethanol for 1 min.
- Rinse the membranes twice with deionized water to completely remove the ethanol. Store them in deionized water to prevent opacity.
2. GCL labeling and rat retina flat-mounting
NOTE: This labeling method does not differentiate RGCs from displaced amacrine cells. If selective labeling of RGCs is desired, consider using AAVs with RGC-specific promoters11 and/or retrograde labeling through the optic nerve12. To discriminate between classes of ON- and OFF-center RGCs, classify RGCs based on their light response13,14, and utilize newer versions of genetically encoded calcium indicators that offer increased sensitivity and the ability to measure single action potentials15.
- Intravitreal injection
- Anesthetize the 8-week-old Long Evans rat with 2% isoflurane/1% O2 until there is no pedal reflex, and maintain anesthesia with a rat nasal mask (see Table of Materials).
NOTE: During anesthesia, position the animal on a heating pad to maintain body temperature. - Administer one drop of commercially available eye drops (see Table of Materials) to dilate the pupil.
- Before proceeding with surgery, examine the eye for abnormalities using fundoscopy and Optical Coherence Tomography (OCT) with an in vivo retinal imaging system. Apply one drop of Methocel 2% to facilitate cornea-objective contact (see Table of Materials).
NOTE: If any abnormalities are detected, do not proceed with the subsequent steps for that eye. - Apply one drop of Prescaine as a local anesthetic. Fix the eyelid and limbal conjunctiva with a commercially available suture filament (see Table of Materials). Create a 1 mm sclerotomy 4 mm from the limbus using a 30 G needle.
- Attach a 36 G blunt needle to a precision syringe and inject the AAV particles carrying the genetically encoded calcium indicator into the vitreous for 30 s, at a 45° angle. In this study, we used AAV2-CAG-GCaMP5G (7.5 x 1011 GC/mL in HBSS) (see Table of Materials).
NOTE: AAV constructs that do not encode potentially tumorigenic gene products or toxin molecules and are produced without a helper virus can be handled in Biosafety Level 1 (BSL-1) facilities. Otherwise, if considered biohazardous material under BSL-2 containment, proper precautions must be taken16. AAVs encoding for GCaMP are considered BSL-1 and do not require manipulation under biosafety cabinets.
- Attach a 36 G blunt needle to a precision syringe and inject the AAV particles carrying the genetically encoded calcium indicator into the vitreous for 30 s, at a 45° angle. In this study, we used AAV2-CAG-GCaMP5G (7.5 x 1011 GC/mL in HBSS) (see Table of Materials).
- Apply one drop of Tobradex (see Table of Materials) to prevent inflammation and as an antibiotic prophylaxis.
- If desired, repeat steps 2, 3, and 4 with the other eye.
NOTE: Check on the animals 12-24 h after surgery to ensure there are no adverse reactions. - Three days after injection, examine the retinal structure using fundoscopy and OCT with an in vivo retinal imaging system (see Table of Materials).
- Two weeks after injection, the GCL should emit fluorescence. Assess retinal structure and AAV expression by fluorescence fundoscopy using an in vivo retinal imaging system.
NOTE: According to Weitz et al.12, fluorescence from AAV2-CAG-GCaMP5G becomes noticeable at 1-week post-injection and intensifies by 2 weeks. Starting from the fourth week, overexpression of GCaMP induces cytomorbidity. Dying cells exhibit a high baseline fluorescence signal in the nucleus and cytoplasm that does not fluctuate in response to stimulation. In healthy cells, GCaMP expression is confined to the cytoplasm and excluded from the nucleus7,8,12,17,18. These features can be observed ex vivo during microscopy imaging. The window of gene expression may vary depending on the viral vector and chosen promoter.
- Anesthetize the 8-week-old Long Evans rat with 2% isoflurane/1% O2 until there is no pedal reflex, and maintain anesthesia with a rat nasal mask (see Table of Materials).
- Retina excision and flat-mounting
NOTE: Two to three weeks after injection, intravitreally-injected rats are euthanized immediately before the electrophysiology protocol begins, in accordance with standard ethical guidelines (European Communities Directive 86/609/EU) and approved by local ethical committees. Carbon dioxide (CO2) inhalation is used as the method of euthanasia in this protocol.- Eye enucleation
- Gently press the exterior of the orbit using a pair of curved forceps to slightly protrude the eye from the eye socket.
- Use a pair of spring scissors to cut the muscles holding the eye and enucleate it, taking care not to puncture the eyeball.
NOTE: Starting from this step, dissect the retina under a stereo microscope in oxygenated (95% O2 / 5% CO2) Ames' Medium.
- Retina excision
- Use a pair of small curved forceps and fine spring scissors to remove all surrounding tissue from the eyeball.
- Take a piece of approximately 3 cm x 3 cm filter paper and place it on the lid of a 3.5 cm dish. Soak the paper with Ames' Medium.
- Place the eyeball on top of the paper, with the anterior segment facing the operator. Use a pair of straight forceps to hold the eyeball, positioning them on top of the ora serrata at approximately a 45° angle from the dish surface. Make a small cut with a blade, using the space between the straight forceps as a reference.
- Reimburse the eyeball into Ames' Medium. Use a pair of straight forceps and fine spring scissors to separate the anterior and posterior segments of the eye.
- Carefully remove the lens using two pairs of straight forceps. Then, separate the retina from the sclera.
- Cut the sclera toward the optic nerve using fine spring scissors until the retina is isolated from the eyecup.
- Use a fluorescence stereo microscope to identify the region of the retina with the best calcium indicator expression.
NOTE: The extent of viral spread depends on the success of the intravitreal injection. Achieving fluorescence across large portions of the retina may require practice. The experience of the investigator plays a crucial role in obtaining optimal results. - Using a cut-tip plastic pipette, transfer the selected piece of the retina onto the mounting membrane (mounting membranes steps). Use a pair of straight forceps to flat-mount the retina with the GCL facing up.
- With a plastic pipette attached to a 100 µL pipette tip, remove the media to allow the retina piece to adhere to the porous membrane. Flip the assembly onto the MEA so that the GCL rests on top of the electrodes.
- Fill the sample bath with oxygenated Ames' Medium.
- Eye enucleation
3. Ex vivo calcium imaging upon electrical stimulation
NOTE: In this work, a proof-of-concept MEA was used for ex vivo experimentation. The custom MEAs were fabricated with 25 µm diameter porous graphene-based electrodes on 500 µm thick borosilicate glass with Ti/Au traces and later insulated with silicon nitride and SU-8 photoresist12. However, the calcium imaging methods are valid irrespective of the electrode material used for stimulation.
- Set the perfusion system so the oxygenated Ames' Medium constantly perfuses the sample bath at 33 °C at a constant flow rate of 5 mL/min.
- Using an inverted fluorescence microscope equipped with a fluorescent lamp, a FITC filter cube, and a CMOS camera, inspect the retina for an area where the stimulating electrodes and the fluorescence from GCaMP-expressing cells are visible. A 20x NA 0.75 air objective was used for this study.
NOTE: To effectively stimulate (and record) the cells with the electrodes, the retina and the electrode need to be in close contact. Thus, cells are visibly in the same focal plane as the electrodes. If it is not the case, repeat the steps of retina excision from step 8 onwards. When using retinas from healthy animal models (with functioning photoreceptors), note that every time the fluorescent lamp is turned on, there will be some evoked responses generated by the light since the retina is light-sensitive to the wavelength used for exciting the GCaMP sensor. These light-induced calcium changes can be used to assess the health status of the tissue. To avoid mixing light with electrical-evoked responses, turn on the fluorescent lamp at least 1 min before starting the image acquisition. - To elicit electrical-evoked responses in the GCL, select an electrode to send current-controlled pulses. Set the electrical stimulation parameters in the software of the pulse generator device, such as: shape, amplitude, duration, phase delay, and frequency of pulses to be applied.
NOTE: Effective stimulus parameters can vary widely from pulse widths of 50 µs to 100 ms, with amplitudes ranging from 0.1 µA to 10 µA. These parameters, along with stimulus frequency, stimulus polarity, number of pulses, and interphase delays, can influence the spatiotemporal response observed by calcium imaging19,20,21,22. A train of 40 biphasic pulses delivering 1 ms, 2 µA stimulation often generates a visible response in labeled neurons. - To synchronize the image acquisition with the stimulation delivery, use the pulse generator as an external trigger to control the start of image acquisition. Connect the camera (see Table of Materials) with the pulse generator using the output trigger signal and set the "Capture Mode" of the camera software to "External Start Trigger". Press Start in the camera software so that it awaits an external trigger to start. Start the image acquisition with the pulse generator software.
NOTE: The external trigger control might be set up differently for different cameras. This study typically acquired images (512 x 512 pixels, 16-bit grayscale) at 10 frames per second for 1 min while providing bursts of biphasic pulse trains every 10 s. Pulse delivery starts after 10 s, so the first frames in all experiments correspond to spontaneous activity. Depending on the GCaMP sensor and the analysis one will perform, one might need to adjust the recording rate according to the rise and decay times of your calcium indicator8. Consider the sensitivity to detect single action potentials of the calcium indicator15. - Save the images with a file name that includes the electrical stimulation parameters applied, such as [Electrode number]_[Pulse Amplitude]_[Pulse duration]_[Pulse Frequency]_Image001.
4. Data analysis
- ImageJ/FIJI to extract the fluorescence intensity profile over time and the spatial coordinates from the cell somas
- Segment the region of interest (ROI) with the "Area Selection Tools" and add it to the ROI Manager (Analyze > Tools > ROI Manager > Add). From the ROI Manager menu, save it as a .zip folder (More > Save).
NOTE: Typically, the same ROIs can be applied to all stimulation experiments since they correspond to the same FOV. - Select the "Mean gray value" as the parameter to be extracted (Analyze > Set measurements).
- Extract the "Mean gray value" from the cell somas by clicking More > Multi Measure. A dialog box will appear. Enable the Measure all 600 slices and One row per slice options to obtain a single table in which columns correspond to ROIs and rows correspond to time frames. Save the generated table as a .xls spreadsheet.
- Select the "Centroid" as the parameter to be extracted (Analyze > Set measurements).
- Extract the "Centroid" from the ROIs by clicking Measure. The generated table corresponds to the coordinates (X,Y) of the ROIs. Save it as a .xls spreadsheet.
- Segment the region of interest (ROI) with the "Area Selection Tools" and add it to the ROI Manager (Analyze > Tools > ROI Manager > Add). From the ROI Manager menu, save it as a .zip folder (More > Save).
- Custom-built script to identify cells that respond to the stimuli
NOTE: MATLAB (see Table of Materials) was used here, but the steps described can be achieved in any programming language. Users can obtain our custom-built script by requesting the corresponding author.- Photobleaching effect correction: To mitigate the background and photobleaching effect, take 15-20 frames from the non-stimulating periods before each burst and fit them to a linear curve [fit (poly1)].
NOTE: In this case, for a total 600-frame movie in which periodic bursts of pulse trains were sent every 10 s, frames 1:90, 170:190, 270:290, 370:390, 470:490, 570:590 were considered as non-stimulating periods. - Normalize using the formula: (X-min) / (max-min)
- Identification of responding cells
- Calculate the root mean square (RMS) of the non-stimulating periods from the normalized data. This will be considered as the baseline signal.
- Calculate the maximum of the stimulating periods (frames between the non-stimulating periods). In this case, for a total 600-frame movie in which periodic bursts of pulse trains were sent every 10 s, frames 91:169, 191:269, 291:369, 391:469, 491:569 were considered as the stimulating periods.
- If the maximum value surpasses the baseline signal by 2.5 times for a specific ROI, tag the cell as responding to that stimulating period. If the cell responds to three out of the five stimulating periods, classify it as a responding cell.
- Photobleaching effect correction: To mitigate the background and photobleaching effect, take 15-20 frames from the non-stimulating periods before each burst and fit them to a linear curve [fit (poly1)].
Representative Results
The protocol described in this study is based on the fluorescence imaging and electrical stimulation studies conducted by Weitz et al.12. The protocol consists of three main parts: (1) fluorescent labeling of the GCL and flat-mounting of the retina on the MEA (Figure 1-left), (2) visualization of calcium activity in the GCL during electrical stimulation (Figure 1-middle), and (3) extraction, processing, and interpretation of the imaging data (Figure 1-right).
First, as depicted in Figure 1-left, Long Evans rats are intravitreally injected with AAV2-CAG-GCaMP5G prior to the imaging session. The optimal viral expression for this vector occurs 2 to 3 weeks after injection12,18. After fully anesthetizing the animal, a pilot hole is made using a 30 G needle, and then 5 µL of AAV2-CAG-GCaMP5G is slowly injected into the vitreous using a 36 G blunt needle attached to a precision syringe to prevent reflux. During viral expression, an in vivo retinal imaging system is used to assess the condition of the retina post-surgery, with OCT images providing detailed visualization of the retinal layers. Once gene expression is achieved, the retina is carefully extracted from the eyecup using a stereo microscope and high-precision dissection tools. From this point onwards, the tissue is manipulated in oxygenated media to preserve the sample. The excised retina, with the GCL facing up, is then mounted on a platform designed for flat-mounting to ensure stability and prevent sample floating. The sample is mounted on the MEA surface with the GCL facing the electrodes.
Next, the MEA is mounted on its interface board on an inverted fluorescent microscope (Figure 1-middle). The retinal sample is perfused with oxygenated media at 33 °C using a perfusion system. The sample can be maintained in this configuration for several hours. The desired stimulation scheme is programmed, and images are acquired at a rate of 10 frames per second. It is recommended to name the movies according to the applied electrical stimulation parameters. Image acquisition should begin before the initiation of stimulation to obtain some baseline frames without stimulation, which will serve as a negative control.
Finally, as illustrated in Figure 1-right, the data is extracted from the time-lapse images by segmenting the cell somas. Photobleaching effects are corrected by fitting the data, and responsive cells are identified. Responsive cells are defined as those with fluorescence peaks during stimulation that exceeds their baseline by 2.5 times. If a cell responds to three out of the five bursts of stimulation, it is considered responsive to that specific train of stimulation.
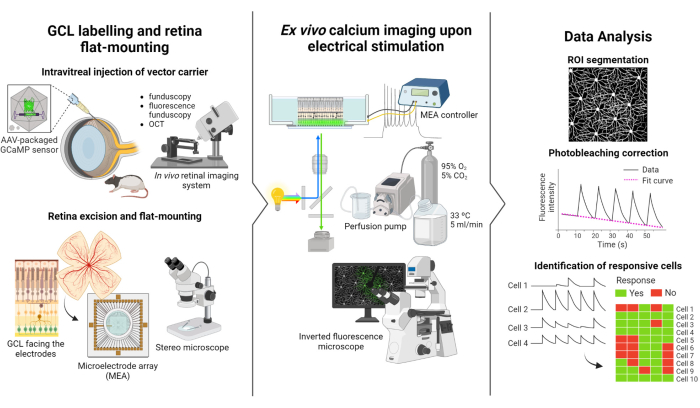
Figure 1: Overview of the study. Schematic illustration of the protocol for (left) fluorescently labeling the GCL of the retina and sample mounting, (middle) set up preparation for ex vivo recordings with electrical stimulation provided by a MEA, and (right) analyzing the calcium imaging data to classify responsive cells. Please click here to view a larger version of this figure.
Intravitreal-injected retina
The incidence of complications associated with intravitreal injections is very low. However, there are some complications that can arise from the surgery itself, regardless of the injected component. These complications include cataract formation, vitreous hemorrhage, elevation of intraocular pressure, and endophthalmitis23. To determine whether these complications are caused by the surgery, the animal needs to undergo evaluation before the procedure using funduscopy and OCT. Three days after the injection, the animals should be followed up. In Figure 2A–D, the retina of a healthy injected animal is shown. After two weeks of injection, the RGCs begin to express fluorescence, which can be visualized using fluorescence fundoscopy (Figure 2B,C). OCT images provide detailed visualization of the disposition and thickness of retinal layers (Figure 2D), offering higher resolution compared to funduscopy, particularly when assessing retinal detachment. Once the retina is flat-mounted and imaged using an inverted fluorescence microscope, it becomes possible to distinguish the cells and axon bundles. Unlike other calcium indicators, the GCaMP indicator is restricted to the cytoplasm7, and fluorescence is excluded from the nucleus (Figure 2E).
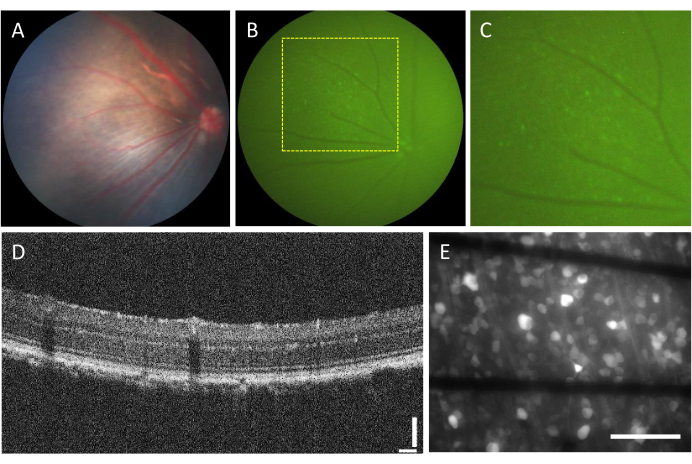
Figure 2: Representative images of the intravitreal-injected retina. (A) Fundoscopy, (B) fluorescence fundoscopy, (C) zoom-in of the fluorescence fundoscopy, (D) OCT image, and (E) epi-fluorescence image of the excised retina mounted on a custom MEA with graphene-based electrodes on 500 µm thick borosilicate glass. In (E), black lines correspond to Ti/Au traces. Scale bars: 115 µm (D) and 100 µm (E). Please click here to view a larger version of this figure.
Electrodes and GCL contact
In order to evoke neural responses effectively, it is crucial to ensure that the flat-mounted retina is in close contact with the surface of the MEA. A simple way to verify this is by visually confirming whether the cells and electrodes are located in the same focal plane (Figure 3A). If the cells are not in the same focal plane as the electrodes (Figure 3B), it indicates that the contact is suboptimal, which will result in less effective stimulation.
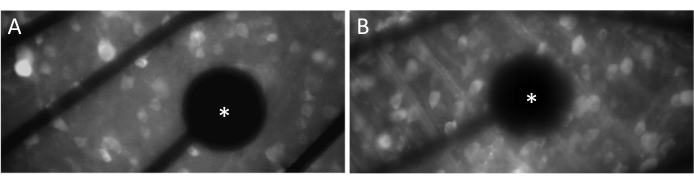
Figure 3: Electrodes and GCL contact. (A) Cells and the electrode (asterisk) in the same focal plane. (B) Cells and electrodes not in the same focal plane, indicating suboptimal contact for electrical stimulation in that area. Please click here to view a larger version of this figure.
Ex vivo calcium imaging upon electrical stimulation provided by a MEA
The resulting data from calcium imaging consist of time-lapse images that monitor the neural activity of hundreds of cells in response to electrical stimulation. Suprathreshold stimuli cause a calcium influx into the cell somas, resulting in a sudden change in fluorescence intensity (Video 1). This protocol enables determining whether an electrode, MEA, and/or stimulation algorithm elicits the desired response in neural tissue. The size and pitch of the electrodes on the MEA, as well as the proportion of tissue being studied, will determine the appropriate objective magnification to choose. Typically, for single-electrode stimulation studies with diameters ranging from 5 µm to 100 µm, a 20-25x objective magnification is suitable (Figure 4A), providing a FOV of approximately 600 µm x 600 µm. For experiments involving stimulation with multiple electrodes, a 4-10x objective magnification may be necessary to assess a wider area of around 2 mm x 2 mm. Responsive cells can be easily identified by generating a standard deviation image projection of the time-lapse movie (Figure 4B and Video 1).
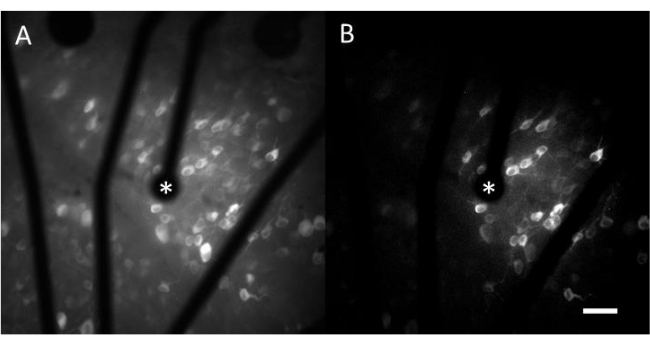
Figure 4: Calcium imaging of the GCL with electrical stimulation provided by a 25 µm-diameter electrode. (A) Maximum projection of a 60 s time-lapse movie and (B) standard deviation projection clearly depicting cells that respond to electrical stimuli from a 25 µm diameter porous graphene-based electrode. The stimulating electrode is indicated with an asterisk. Scale bar: 50 µm. Please click here to view a larger version of this figure.
Analysis of the calcium dynamics over time upon controlled stimulation
For each identified cell soma, the mean intensity values were extracted over time. Figure 5A shows the photobleaching-corrected calcium traces from the responsive cells. In this example, five bursts of biphasic pulse trains (cathodic-first, 40 cycles, 1 ms duration, 2 µA amplitude) were delivered every 10 s (indicated by black lines) during a 60 s image acquisition. Within a given experiment, the same five pulse trains are applied to test the consistency of the response. The frames captured during the non-stimulating periods (highlighted in red) are used to perform a linear fit, correcting for the photobleaching effect.
Once the responding cells are identified, and their coordinates (x,y) are known relative to the stimulating electrode, one can examine the relationship between the current required to activate the cells and the distance from the stimulating electrode (Figure 5B). As expected, cells located closer to the stimulating electrode require lower current values to evoke a response.
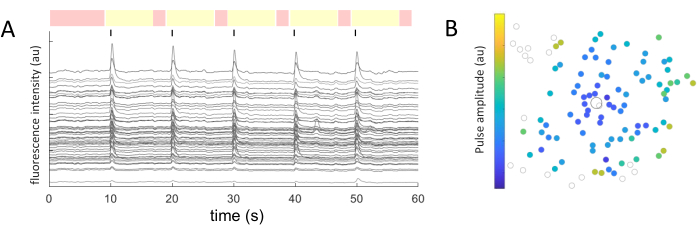
Figure 5: Representation of the electrical-evoked responses. (A) Calcium traces of cell somas upon 5 bursts of pulse trains (biphasic, cathodic-first, 40 cycles, 1 ms duration, 2 µA amplitude) every 10 s (black lines) during a 60 s image acquisition. Non-stimulating (red-highlighted frames) and stimulating periods (yellow-highlighted frames) are shown. Traces surpassing the baseline signal (root mean square of the non-stimulating periods) by 2.5 times are considered evoked responses. Cells responding in three out of the five stimulating periods are classified as responding cells. (B) Calcium activity distribution map showing the stimulating electrode (black outlined circle) and cells (gray outlined circle). The color code represents the minimum pulse amplitude necessary to evoke a cellular response. Please click here to view a larger version of this figure.
Video 1: Calcium imaging of the GCL with electrical stimulation provided by a 25 µm-diameter electrode. The video displays differences in fluorescence intensity due to electrical stimulation from a 25 µm diameter porous graphene-based electrode. The left side shows the original movie, and the right side shows the standard deviation projection where responding cells can be easily identified. Scale bar: 50 µm. Please click here to download this Video.
Discussion
The protocol described here serves to study the calcium dynamics occurring in the rat retinal GCL upon electrical stimulation provided with a MEA. It is a reliable and manageable method but requires some training, particularly to uniformly label the GCL efficiently and to mount the retina properly to ensure optimal tissue-electrode contact. This protocol is specific to rodents and needs to be adapted if applied to a different laboratory species. The critical points, modifications, and limitations of the methodology are presented in detail.
Intravitreal injections
Injections are widely used for ocular gene delivery, with intravitreal injections being the preferred procedure. They have been proven to be safer and less invasive compared to subretinal injections, which introduce the molecules of interest directly between the photoreceptors and the retinal pigment epithelium (RPE), risking retinal detachment10. However, there are limitations, especially when performing these injections in rodent models. The vitreous humor is gelatinous, hindering viral diffusion. Moreover, the lens in rodent eyes is large, making it non-trivial to insert the needle without scratching it. The precision syringe needles are delicate and need to be replaced often. To prevent obstruction, wash them with deionized water before and after every use and replace them regularly. Additionally, inject the content slowly to prevent solution reflux and changes in intraocular pressure. Achieving large and uniform fluorescence across the retina may require practice.
Retinal cell transduction
Viral vectors are an excellent method for in vivo gene delivery, and AAVs have been widely used for transducing retinal cells10. They have been approved as a treatment for some retinopathies causing human blindness24. However, their carrier capacity is limited to 5 kb, including the required regulatory elements (e.g., the promoter)10,25. There are multiple serotypes available, each with different tropism. Choose the most suitable AAV based on the genes to be delivered and the cells to be transduced26. For labeling the RGCs, it is recommended to use AAV227.
Gene expression window
The optimal viral expression for AAV2-CAG-GCaMP5G is 2 to 3 weeks after injection12,18. Beyond that timeframe, the nuclei from transfected cells become fluorescent, cells stop responding to stimuli, and ultimately die7,28,29. This is due to the overexpression of the GCaMP indicator, which is translocated into the nucleus. The time window for optimal gene expression will vary depending on the viral vector and the chosen promoter30 and needs to be determined experimentally before proceeding with this protocol.
Tissue-electrode contact
For optimal and reproducible results, achieving good tissue-electrode contact is crucial. Poor contact is typically due to the natural curvature of the retina. One approach is to cut the retina into quarters, mount and image one section at a time. Small portions of the retina can be better flattened, resulting in more effective contact with the surface of the MEA. Another potential reason for poor contact is the presence of vitreous humor. When conducting stimulation experiments simulating an epi-retinal implant, it is important to carefully remove the vitreous humor during retina excision as it can act as an insulator to current. Here, a simple method is described to check if the contact is sufficient by visualizing the electrode and cells in the same focal plane.
An alternative to ex vivo retinal measurements is to grow neurons directly on the surface of the electrodes. Primary culture of neurons, such as hippocampal neurons31, can be useful for initial tests to evaluate the functionality of the novel stimulating device. However, this approach still requires the use of laboratory animals and does not represent the complexity of the retinal network, which is important for evaluating synaptic responses to stimulation.
To visualize the cells underneath the electrode and electrode traces, MEAs fabricated with transparent materials such as indium tin-oxide (ITO) can be used19,20,32. In addition to optical measurements, GCL activity upon electrical stimulation can be assessed through electrical recordings. The MEA can be used to record the local field potential (LFP) of the tissue. However, this compromises spatial resolution, as each electrode captures activity from multiple cells simultaneously (depending on the electrode dimensions). Optical recording overcomes this limitation and offers higher spatial resolution mapping. Its main advantage is the ability to distinguish between active and inactive cells while measuring a large FOV with single-cell resolution. Among all cellular activity reporters, calcium indicators are well-described and most commonly used33.
Divulgaciones
The authors have nothing to disclose.
Acknowledgements
We are thankful to Merche Rivas, Angel Sandoval, Jesús Planagumà, Jordi Cortés, Sandra Ortonobés Lara and Alina Hirschmann (ICFO-Institut de Ciències Fotòniques) for their technical support, to Anna Duarri (VHIR, Vall d'Hebron Institute of Research) from the Ophthalmology Research group for their support with the intravitreal injections and the in vivo retinal imaging.
The funding entities that supported this work are: Fundació CELLEX; Fundació Mir-Puig; Ministerio de Economía y Competitividad – Severo Ochoa program for Centres of Excellence in R&D (CEX2019-000910-S, [MCIN/AEI/10.13039/501100011033]); Generalitat de Catalunya through CERCA program; Laserlab-Europe (EU-H2020 GA no. 871124); La Caixa Foundation (LCF/HR19/52160003); and Fondo Social Europeo (PRE2020-095721, M.C.).
Materials
| 20x NA 0.75 S Fluor air objective | Nikon | CFI Super Fluor 20X | – |
| 3.5 cm Cell culture dish | Nunc | 12-565-90 | – |
| 30 G needle | VWR | 613-5373 | – |
| 36 G blunt needle | World Precision Instruments | NF36BL-2 | – |
| 6 cm Cell culture dish | Nunc | 12-565-94 | – |
| AAV2-CAG-GCaMP5G | Vector Biolabs | – | – |
| Ames' Medium | Sigma Aldrich | A1420 | – |
| Blade | Swann-morton | 0308 | – |
| Camera | Hamamatsu | ORCA Flash v4.0 | – |
| Carbogen | Air liquide | – | – |
| Curved-forceps | – | – | – |
| Fine spring-scissors | FST | 91501-09 | – |
| FITC filter cube | Nikon | Standard series | – |
| Fluorescent lamp | Nikon | C-HGFI | – |
| Fluorescent stereomicroscope | Nikon | SMZ25 | – |
| HBSS | Capricorn | HBSS-1A | – |
| ImageJ/FIJI | NIH | v1.50i | – |
| In vivo retinal imaging system | Phoenix Research Laboratories | Micron III | – |
| Inverted fluorescence microscope | Nikon | Eclipse Ti | – |
| Isofluorane | Arrane Baxter Laboratories | – | – |
| Long-Evans rat | Janvier | – | – |
| MATLAB (Version R2021b) | Mathworks | – | – |
| Media filters | Merckmillipore | SCGPS02RE | – |
| Methocel 2% | Omni Vision | – | – |
| Microelectrode array (MEA) | – | Custom-made | |
| NaHCO3 | Thermofisher | 42427 | – |
| Penicillin/Streptomycin 100x | Thermofisher | 15140122 | – |
| Phenylephrine | Alcon Cusí Laboratories | 653437.3 | 100 mg/mL |
| Plastic pipette | VWR | 612-1793 | – |
| Porous membrane | Merckmillipore | #JVWP01300 | – |
| Precision syringe | World Precision Instruments | 10 µl Nanofil | – |
| Prescaina | Llorens | – | Oxybuprocaine chlorhydrate (2 mg/mL), local anesthetic |
| Rat nasal mask | Xenotec | XRK-RA | – |
| Small curved-forceps | Bbraun | AESCBD311R | – |
| Spring-scissors | FST | 15040-11 | – |
| Stereo microscope | Zeiss | Stemi 2000 | – |
| Straight forceps | FST | 11252-20 | – |
| Suture filament | Vitrex Medical | 4328 | Nilon monofilament, 7/0, DS12 |
| Tobradex | Alcon Cusí Laboratories | – | Tobramycin (3 mg/mL) and dexamethasone (1 mg/mL) |
| Tropicamide | Alcon Cusí Laboratories | 653486 | 10 mg/mL |
| Washer | Thorlabs | W8S038 | – |
Referencias
- Bourne, R. R. A., et al. Prevalence and causes of vision loss in high-income countries and in Eastern and Central Europe in 2015: Magnitude, temporal trends and projections. British Journal of Ophthalmology. 102 (5), 575-585 (2018).
- Li, J. Q., et al. Prevalence and incidence of age-related macular degeneration in Europe: a systematic review and meta-analysis. British Journal of Ophthalmology. 104 (8), 1077-1084 (2020).
- Roska, B., Sahel, J. A. Restoring vision. Nature. 557 (7705), 359-367 (2018).
- Hornig, R., Velikay-Parel, M. Retina implants. Implantable Sensor Systems for Medical Applications. , 469-496 (2013).
- Lewis, P. M., et al. Advances in implantable bionic devices for blindness: a review. ANZ Journal of Surgery. 86 (9), 654-659 (2016).
- Weiland, J. D., Walston, S. T., Humayun, M. S. Electrical stimulation of the retina to produce artificial vision. Annual Review of Vision Science. 2, 273-294 (2016).
- Tian, L., et al. Imaging neural activity in worms, flies and mice with improved GCaMP calcium indicators. Nature Methods. 6, 875-881 (2009).
- Chen, T. -. W., et al. Ultrasensitive fluorescent proteins for imaging neuronal activity. Nature. 499, 295-300 (2013).
- Nakai, J., Ohkura, M., Imoto, K. A high signal-to-noise Ca2+ probe composed of a single green fluorescent protein. Nature Biotechnology. 19, 137-141 (2001).
- Sahu, B., Chug, I., Khanna, H. The ocular gene delivery landscape. Biomolecules. 11 (8), 1135 (2021).
- Hanlon, K. S., et al. A novel retinal ganglion cell promoter for utility in AAV vectors. Frontiers in Neuroscience. 11, (2017).
- Weitz, A. C., et al. Imaging the response of the retina to electrical stimulation with genetically encoded calcium indicators. Journal of Neurophysiology. 9 (7), 1979-1988 (2013).
- Briggman, K. L., Euler, T. Bulk electroporation and population calcium imaging in the adult mammalian retina. Journal of Neurophysiology. 105 (5), 2601-2609 (2011).
- Baden, T., Berens, P., Franke, K., Román Rosón, M., Euler, T. The functional diversity of retinal ganglion cells in the mouse. Nature. 529, 345-350 (2016).
- Zhang, Y., et al. Fast and sensitive GCaMP calcium indicators for imaging neural populations. Nature. 615, 884-891 (2023).
- Meyer, E. L., Jenkins, C., Rengarajan, K. NIH Guidelines April 2019. Applied Biosafety. 24, (2019).
- Akerboom, J., et al. Optimization of a GCaMP calcium indicator for neural activity imaging. Journal of Neuroscience. 32 (40), 13819-13840 (2012).
- Chang, Y. -. C., Walston, S. T., Chow, R. H., Weiland, J. D. GCaMP expression in retinal ganglion cells characterized using a low-cost fundus imaging system. Journal of Neural Engineering. 14 (5), 056018 (2017).
- Weitz, A. C., et al. Improving the spatial resolution of epiretinal implants by increasing stimulus pulse duration. Science Translational Medicine. 7 (318), 1-12 (2015).
- Chang, Y. C., Ghaffari, D. H., Chow, R. H., Weiland, J. D. Stimulation strategies for selective activation of retinal ganglion cell soma and threshold reduction. Journal of Neural Engineering. 16 (2), 026017 (2019).
- Im, M., Fried, S. I. Temporal properties of network-mediated responses to repetitive stimuli are dependent upon retinal ganglion cell type. Journal of Neural Engineering. 13 (2), 025002 (2016).
- Jensen, R. J., Ziv, O. R., Rizzo, J. F. Thresholds for activation of rabbit retinal ganglion cells with relatively large, extracellular microelectrodes. Investigative Ophthalmology & Visual Science. 46 (4), 1486-1496 (2005).
- Ramos, M. S., et al. Patient-reported complications after intravitreal injection and their predictive factors. Ophthalmology Retina. 5 (7), 625-632 (2021).
- Vandenberghe, L. H., Auricchio, A. Novel adeno-associated viral vectors for retinal gene therapy. Gene Therapy. 19 (2), 162-168 (2012).
- Zin, E. A., Ozturk, B. E., Dalkara, D., Byrne, L. C. Developing new vectors for retinal gene therapy. Cold Spring Harbor Perspectives in Medicine. , a041291 (2023).
- Haggerty, D. L., Grecco, G. G., Reeves, K. C., Atwood, B. Adeno-associated viral vectors in neuroscience research. Molecular Therapy-Methods & Clinical Development. 17, 69-82 (2020).
- Hellström, M., et al. Cellular tropism and transduction properties of seven adeno-associated viral vector serotypes in adult retina after intravitreal injection. Gene Therapy. 16 (4), 521-532 (2009).
- Resendez, S. L., et al. Visualization of cortical, subcortical and deep brain neural circuit dynamics during naturalistic mammalian behavior with head-mounted microscopes and chronically implanted lenses. Nature Protocols. 11, 566-597 (2016).
- Yang, Y., et al. Improved calcium sensor GCaMP-X overcomes the calcium channel perturbations induced by the calmodulin in GCaMP. Nature Communications. 9, 1504 (2018).
- Nieuwenhuis, B., et al. Improving adeno-associated viral (AAV) vector-mediated transgene expression in retinal ganglion cells: comparison of five promoters. Gene Therapy. 30, 503-519 (2023).
- Cunquero, M., Aguilar, E., Loza-Alvarez, P., Planagumà, J. Hippocampal neuronal cultures to detect and study new pathogenic antibodies involved in autoimmune encephalitis. Journal of Visualized Experiments. 184, e63829 (2022).
- Behrend, M. R., Ahuja, A. K., Humayun, M. S., Chow, R. H., Weiland, J. D. Resolution of the epiretinal prosthesis is not limited by electrode size. IEEE Transactions on Neural Systems and Rehabilitation Engineering. 19 (4), 436-442 (2011).
- Grienberger, C., Konnerth, A. Imaging calcium in neurons. Neuron. 73 (5), 862-885 (2012).

