The Development and Application of Biophysical Assays for Evaluating Ternary Complex Formation Induced by Proteolysis Targeting Chimeras (PROTACS)
Summary
Here we describe protocols for the biophysical characterization of ternary complex formation induced by proteolysis targeting chimeras (PROTACS) that involve the ubiquitin ligases Von Hippel-Lindau E3 ligase (VHL) and Cereblon (CRBN). Biophysical methods illustrated herein include surface plasmon resonance (SPR), biolayer interferometry (BLI), and isothermal titration calorimetry (ITC).
Abstract
E3 ligases and proteins targeted for degradation can be induced to form complexes by heterobifunctional molecules in a multi-step process. The kinetics and thermodynamics of the interactions involved contribute to efficiency of ubiquitination and resulting degradation of the protein. Biophysical techniques such as surface plasmon resonance (SPR), biolayer interferometry (BLI), and isothermal titration calorimetry (ITC) provide valuable information that can be used in the optimization of those interactions. Using two model systems, a biophysical assay tool kit for understanding the cooperativity of ternary complex formation and the impact of the 'hook effect' on binding kinetics was established. In one case, a proteolysis targeting chimera (PROTAC) molecule that induced ternary complex formation between Brd4BD2 and VHL was evaluated. The heterobifunctional molecule, MZ1, has nM affinities for both the Brd4BD2 protein (SPR KD = 1 nM, ITC KD = 4 nM) and the VHL complex (SPR KD = 29 nM, ITC KD = 66 nM). For this system, robust SPR, BLI, and ITC assays were developed that reproduced published results demonstrating the cooperativity of ternary complex formation. In the other case, a molecule that induced ternary complexes between a 46.0 kDa protein, PPM1D, and cereblon [CRBN (319-442)] was studied. The heterobifunctional molecule, BRD-5110, has an SPR KD = 1 nM for PPM1D but much weaker binding against the truncated CRBN (319-442) complex (SPR KD= ~ 3 µM). In that case, the binding for CRBN in SPR was not saturable, resulting in a "hook-effect". Throughput and reagent requirements for SPR, BLI, and ITC were evaluated, and general recommendations for their application to PROTAC projects were provided.
Introduction
The polyubiquitination of proteins in the cell is a tightly regulated process that involves enzymes in the Ubiquitin Ligase family1,2. The terminal enzymes in the pathway are the E3 ubiquitin ligases that covalently attach ubiquitin molecules to their protein-binding partners3. The polyubiquitination of those protein binding partners targets them for proteolytic degradation by the proteasome4. This system is part of the protein homeostasis process that has been therapeutically leveraged to induce the degradation of proteins involved in disease5. Small molecules that induce the interaction between E3 ubiquitin ligases, such as Von Hippel-Lindau E3 ligase (VHL) or cereblon (CRBN), are typically composed of an E3 ligase binding warhead connected by a flexible linker to a warhead that binds to the protein being targeted for degradation. These heterobifunctional molecules are commonly referred to as proteolysis targeting chimeras or PROTACS6.
The development of PROTACS involves evaluating the ability of molecules to induce the degradation of proteins in cells. Many cellular assay systems have been developed that monitor the induced interaction between the target protein and E3 ligase components, such as VHL or CRBN, upon treatment of the cells with a PROTAC molecule. One such cellular assay, the nanoluc-Halotag system7, involves an E3 ligase fused to the Halotag acceptor and a target protein tagged with a nanoluc donor. Ternary complex formation brings the nanoluc donor and Halotag acceptor into proximity allowing the transfer of energy from the donor to the acceptor resulting in the emission of light. Variations of this system can be used to assess the cellular permeability of PROTACS molecules8 or changes in the relative level of target protein ubiquitination9. While these cellular systems are essential for driving the optimization of PROTACS, the formation of complexes between E3 ligases and proteins targeted for degradation is a multi-step process10,11. The kinetics and thermodynamics of the binary and ternary interactions involved contribute to efficiency ubiquitination and resulting degradation of the protein12,13,14.
Herein are described protocols that can be adapted for the biophysical characterization of ternary complex formation induced by PROTACS using surface plasmon resonance (SPR), biolayer interferometry (BLI), and isothermal titration calorimetry (ITC). SPR and ITC protocols for the MZ1 PROTAC molecule that induces ternary complex formation between Brd4BD2 and VHL derived from literature reports13,15 and described here were able to recapitulate the reported results with some modification of the reported procedures, which will be discussed. A description of a BLI assay used to evaluate ternary complex formation between MZI, Brd4BD2, and VHL is included in this report. Affinity measurements from BLI were consistent with those observed in SPR and ITC. A previously published protocol in which an SPR assay was developed for assessing the PROTAC-induced ternary complex formation between PPM1D, a Ser/Thr protein phosphatase whose expression is induced in a p53-dependent manner16, and CRBN is also described. In this instance, the PROTAC molecule has a nanomolar affinity for PPM1D but only a micromolar affinity for CRBN. In this case, the binding of the PROTAC molecule to CRBN is not saturable, resulting in the commonly observed "hook effect". The hook effect is a property of three body systems in which there are two species that can form a heterotrimeric complex when both are bound to a bridging molecule (Figure 1)17. The hook effect is observed when the bridging species is in excess concentration relative to the two other species. The resulting state is one in which the binary interactions outcompete the ternary interactions. The systems where the hook effect is observed require specific experimental design considerations discussed in this report. General concepts and reagent requirements for evaluating the utilization of biophysical assays for the evaluation of PROTAC-induced ternary complex formation are provided.
Protocol
All the proteins were overexpressed in E.coli with good yield and purity (>80%) following the literature protocols18. Biotinylation was carried out using a BirA-catalyzed reaction18. All small molecules were prepared at 1 mM stock solutions in 100% DMSO. The procedures described herein do not require specialized laboratory safety equipment or precautions. Standard laboratory personal protective equipment (PPE) should be used (i.e., lab coat, safety goggles, and gloves).
Proteins applied in this study are listed below:
VHL: biotinylated VHL(53-213)/ElonginB (1-104)/ElonginC(17-112) complex with Avi-tag at the C-terminus of ElonginB.
Brd4BD2: Non-tagged Brd4BD2(333-460)
CRBN: biotinylated CRBN(319-442) with Avi-tag at the N terminus
PPM1D: non-tagged or double His8-tagged PPM1D(1-420) at the N terminus
Small molecules applied in this study are listed below:
MZ1 (MW = 1002.6 Da): PROTAC that binds to VHL and Brd4BD2
BRD-2512 (MW = 841.4 Da): CRBN KD ~3 µM, doesn't bind to PPM1D
BRD-5110 (MW = 872.0 Da): CRBN KD ~3 µM, PPM1D KD = 1-2 nM
BRD-4761 (MW = 476.6 Da): doesn't bind to CRBN, PPM1D KD = 1-2 nM
1. Method 1: ITC (isothermal titration calorimetry)
NOTE: Titrations are performed using a micro-calorimeter with auto-injection.
- Buffer preparation: Prepare 3 L of buffer containing 20 mM HEPES, 150 mM NaCl, 1 mM TCEP, pH 7.5.
- Dialysis: Dialyze VHL and Brd4BD2 (~ 500 µL at 150 µM each) against 1 L of the buffer prepared in step 1.1, 3 times at 4 ˚C, for 4 h, 2 h, and approximately 16 h, respectively. Save the buffer after the last dialysis for use in subsequent steps.
- Prepare samples on a 96-well plate with a plastic cover.
- For each titration, prepare 400 µL of solution for the cell, 125 µL for the syringe, and 400 µL of buffer for cleaning. Add samples to three consecutive wells on the plate. Since compound stock is prepared at 1 mM in 100% DMSO, add the same percentage of DMSO to the protein solutions to ensure the matching buffer in the cell and syringe. Add 2% DMSO to the final solutions.
- Sample for the titration of VHL into MZ1: Prepare cell solution containing 392 µL of buffer, 4 µL of MZ1 at 1 mM (10 µM final concentration), and 4 µL of 100% DMSO. Prepare a syringe solution containing 122.5 µL of VHL at 85.7 µM, 2.5 µL of 100% DMSO (84 µM final concentration).
- Samples for the titration of VHL into the buffer (data will be used for background subtraction of data generated from sample 1.3.1.1): Prepare cell solution containing 392 µL buffer, 8 µL 100% DMSO. Prepare a syringe solution containing 122.5 µL of VHL at 85.7 µM, (84 µM final concentration) and add 2.5 µL of 100% DMSO.
- Samples for the titration of VHL into MZ1 and Brd4BD2: Prepare cell solution containing 392 µL of 17.1 µM Brd4BD2 (16.8 µM final concentration), 3.36 µL of MZ1 at 1 mM (8.4 µM final concentration), and 4.64 µL of 100% DMSO. Prepare a syringe solution containing 122.5 µL of VHL at 85.7 µM (84 µM final concentration) and 2.5 µL of 100% DMSO.
- Samples for the titration of VHL into Brd4BD2 (background of 1.3.1.3): Prepare cell solution containing 392 µL of 17.1 µM Brd4BD2 (16.8 µM final concentration) and 8 µL of 100% DMSO. Prepare a syringe solution containing 122.5 µL of VHL at 85.7 µM (84 µM final concentration) and 2.5 µL of 100% DMSO.
- For each titration, prepare 400 µL of solution for the cell, 125 µL for the syringe, and 400 µL of buffer for cleaning. Add samples to three consecutive wells on the plate. Since compound stock is prepared at 1 mM in 100% DMSO, add the same percentage of DMSO to the protein solutions to ensure the matching buffer in the cell and syringe. Add 2% DMSO to the final solutions.
- Run all four titrations on the micro-calorimeter. Each consists of 19 injections of 2 µL syringe solution at a rate of 2 µL/s at 120 s time intervals. Make an initial injection of protein (0.4 µL) and discard it during data analysis. Perform all experiments at 25 ˚C, while stirring at 600 rpm.
- Data analysis: Fit the data to a single-binding-site model to obtain the stoichiometry (n), the dissociation constant (KD), and the enthalpy of binding (ΔH) using the analysis software provided by the manufacturer (Figure 2).
2. Method 2: BLI (biolayer interferometry)
- Perform BLI experiments using streptavidin (SA) coated sensors at room temperature (RT) with an acquisition rate of 5 Hz. During the automated BLI experiment, ensure the sensors are stationary within a single column and move between different columns of a 96-well, flat bottom, black plate with a maximum well volume of 392 µL. Fill each well of the plate used in the experiment with 200 µL of solution.
NOTE: BLI is only useful to detect protein-protein interactions (i.e., ternary complex formation). It is not sensitive enough to detect interactions between proteins and small molecules.- Buffer preparation: Prepare 100 mL of buffer containing 20 mM HEPES, 150 mM NaCl, 1 mM TCEP, 0.05% P20, pH 7.5.
- Optimization for the immobilization step: Load the test sensor to 1-3 nm, as recommended by the manufacturer. A loading of ~1.0 nm is used for the procedures described here. To achieve this, dip the sensor into a solution containing VHL at 1.5 µg/mL for 80 s.
- Carry out BLI kinetic measurements by applying seven SA sensors using the following sequence:
- For the first baseline phase, dip into buffer for 60 s.
- For the immobilization phase, dip into a solution of VHL at 1.5 µg/mL for 80 s.
- For the second baseline phase, dip into buffer for 60 s.
- For the association phase, dip into solution of a fixed concentration of Brd4BD2 at 2 µM, fixed concentration of DMSO at 2%, and varying concentrations of MZ1 at 100 nM, 50 nM, 25 nM, 12.5 nM, 6.3 nM, 3.1 nM, and 0 nM (reference sensor) for 300 s.
- For the dissociation phase, dip into buffer for 600 s.
- Perform data analysis using the manufacturer's software. kon, koff, and KD are reported for data fitting (Figure 3).
3. Method 3: SPR (surface plasmon resonance)
NOTE: All SPR experiments are carried out using streptavidin (SA) coated sensor chips at RT. Although the NTA chip is used for the detection between protein and small molecules, it is to be used with caution when applied to the ternary complex, as a much higher background than the SA chip is observed, possibly due to electrostatic interactions between the charged chip surface and protein in the analyte.
- SPR for VHL-MZ1 interaction
- Buffer preparation.
- Prepare a 1 L buffer containing 20 mM HEPES, 150 mM NaCl, 1 mM TCEP, 0.005% P20, pH7.5, and pass it through a 0.2 µm filter unit.
- Remove 20 mL of buffer for future use (DMSO-free buffer) and refill with 20 mL of DMSO (2% DMSO in the final running buffer). Keep DMSO at 2% in all the samples described below (steps 3.1 and 3.2)
- Activate the SA chip following the manufacturer's protocol and immobilize VHL to ~2000 RU (inject protein solution of 5 µg/mL at 5 µL/min).
- Prepare MZ1 in a 3-fold, 8-point serial dilution with the top concentration at 10 µM on a 384-well, conical bottom, translucent, polypropylene plate with a maximum volume of 130 µL. Cover the plate with self-adhesive, transparent plastic foils compatible with polypropylene microplates.
- Prepare the top concentration with the DMSO-free buffer to ensure the 2% DMSO at the final concentration. To 147 µL of DMSO-free buffer, add 1.5 µL of MZ1 stock at 1 mM and 1.5 µL of 100% DMSO.
- Prepare the 3-fold serial dilution with the running buffer containing 2% DMSO. To 100 µL of the running buffer, add 50 µL of solution at the top concentration prepared in step 3.1.3.1, and mix well to prepare the 2nd highest concentration.
- Then transfer 50 µL of the solution prepared in step 3.1.3.2 to the next 100 µL of running buffer, mix well to prepare the 3rd highest concentration, and so on.
- Run SPR using the multi-cycle setup: Mode: High-performance; contact time: 120 s; dissociation time: 300 s; flow rate: 50 µL/min.
- Perform data analysis using the evaluation software provided by the instrument manufacturer. Steady-state analysis indicated KD = 26 (± 3) nM and Rmax of ~91% (± 5%) binding achieved (Figure 4A)
- Buffer preparation.
- SPR for VHL: MZ1: Brd4BD2 ternary complex
- Prepare the buffer as described in step 3.1.1.
- Activate the SA chip following the manufacturer's protocol and immobilize VHL to ~100 RU. Inject a protein solution of 0.5 µg/mL VHL at a flow rate of 5 µL/min and contact time between 1-5 min until a surface density of ~100 RU is reached.
- Prepare samples for two 5-fold, 5-point single-cycle injections in a 96-well,clear, polystyrene, round bottom plate with a maximum volume of 323 µL and cover it with self-adhesive, transparent, plastic foils that are compatible with polystyrene microplates as described in steps 3.2.3.1-3.2.3.2.
- Negative control: analyte contains Brd4BD2 only.
- Prepare top concentration at 25 µM in running buffer with a volume of 200 µL (#A5)
- Add 160 µL each of Brd4BD2 at 2 µM in the running buffer to the next 4 wells on the left (#A1-A4)
- Transfer 40 µL of A5 to A4. Mix well.
- Transfer 40 µL of A4 to A3. Mix well. Keep doing so until A1.
- Ternary complex formation: analyte contains MZ1 and Brd4BD2.
- Prepare the top concentration by adding 4 µL of MZ1 at 20 µM in 100%-DMSO solution to 196 µL containing 25.5 µM Brd4BD2 in DMSO-free buffer (#B5). The final concentrations are 25 µM Brd4BD2, 100 nM MZ1, and 2% DMSO.
- Add 160 µL each of Brd4BD2 at 2 µM in the running buffer to the next 4 wells on the left (#B1-B4)
- Transfer 40 µL of B5 to B4. Mix well.
- Transfer 40 µL of B4 to B3. Mix well. Keep doing so until B1.
- Negative control: analyte contains Brd4BD2 only.
- Run SPR using the single-cycle setup: Contact time: 100 s; dissociation time: 720 s; flow rate: 50 µL/min. Apply three injections of buffer before each sample to ensure a stable background.
- Data analysis: Apply the third injection of the buffer as the background for subtraction. As the negative control, when MZ1 is not present, the SPR response between VHL and Brd4BD2 is negligible. When MZ1 is present, kinetic fitting indicates the interaction between VHL and MZ1-Brd4BD2 complex has kon = 7.9 (± 1.5) *107 /M/s, koff = 0.014 s-1, and KD = 1 nM. (Figure 4B)
- SPR for CRBN and small molecule interactions
- Buffer preparation.
- Prepare a 1 L buffer containing 50 mM Tris, 100 mM NaCl, 1 mM MgCl2, 0.5 mM TCEP, 0.005% P20, pH 7.5, and pass it through a 0.2 µm filter unit.
- Remove 20 mL of buffer and refill 20 mL of 100% DMSO (2% DMSO in the final running buffer). It is important to keep DMSO at 2% in all the samples described below (steps 3.3, 3.4, and 3.5).
- Activate the SA chip following the manufacturer's protocol and immobilize CRBN to ~800 RU.
- Prepare compounds in 3-fold, 6-pt serial dilution on a 384-well plate with a top concentration of 30 µM as described in 3.1.3.
- Run SPR using the multi-cycle setup: Mode: High-performance, contact time: 60 s, dissociation time: 90 s; flow rate: 50 µL/min. For this system, three buffer injections are sufficient to obtain a stable background. For other assay systems, it may be necessary to perform additional buffer injections.
- Perform data analysis using the manufacturer's software.
NOTE: Steady-state analysis indicates KD of BRD-2512 and BRD-5110 are both around 3 µM. However, binding only reached ~ 70% Rmax binding at the top concentration. Both weak affinity and the shape of the sensorgram indicate that compound insolubility at high concentrations is likely to happen. Thus, the actual KD might be higher than 3 µM. BRD-4761, which does not bind to CRBN, was included as a negative control.
- Buffer preparation.
- SPR for PPM1D and small molecule interactions
- Prepare buffer as described in step 3.3.1. Use a nitriloacetic acid (NTA) sensor chip.
- Apply single-cycle because the PPM1D warhead has a slow kon and koff. Regenerate the NTA chip using the manufacturer's default setup and immobilize PPM1D to ~ 1000 RU after each compound injection (inject protein solution of 5 µg/mL at 5 µL/min).
- Prepare compounds in 3-fold, 5-point serial dilution with the top concentration at 400 nM as described in 3.1.3.
- Run SPR using the single-cycle setup: Mode: high-performance; contact time: 90 s; dissociation time: 600 s; flow rate: 50 µL/min. Apply three injections of buffer before each compound to ensure a stable background.
- Data analysis. Apply the third injection of the buffer as the background for subtraction. As the negative control, BRD-2512 does not show binding to PPM1D. For BRD-4761 and BRD-5110, kinetic fitting indicated the KD for both to be 1-2 nM.
- SPR for CRBN:PROTAC: PPM1D ternary complex
- Prepare buffer as described in step 3.3.1.
- Activate the SA chip following the manufacturer's protocol and immobilize CRBN to ~35 RU (inject a protein solution of 0.5 µg/mL at 5 µL/min).
- Prepare three compounds in 3-fold, 6-point serial dilution with a top concentration of 30 µM while keeping [PPM1D] at 1 µM in all samples, including blanks, using the method described in step 3.2.3.
- Run SPR using the multi-cycle setup: Mode: High-performance; contact time: 60 s; dissociation time: 90 s; flow rate: 50 µL/min.
- Perform data analysis using the manufacturer's software.
NOTE: While BRD-2512 and BRD-4761 show no/negligible response, BRD-5110 clearly shows the "hook effect" at the steady state with fast on/off kinetics (Figure 5A-C). The experimental binding response from BRD-5110 (Figure 5C) is in between the predicted response from the simulation when assuming KD (CRBN, cpd) is 3 or 10 µM (Figure 5E), suggesting the ternary KD and binary KD are very similar. There is no apparent cooperativity of PPM1D: BRD-5110: CRBN complex formation.
Representative Results
Characterization of VHL: MZ1 binary complex and VHL: MZ1: Brd4BD2 ternary complex can be found in Figure 2 (ITC), Figure 3 (BLI), and Figure 4 (SPR) using a very similar buffer. The KD extracted from orthogonal assays is consistent. The cooperativity can be calculated by KD (binary) / KD (ternary), which is highly positive (15 from ITC or 26 from SPR).
Characterization of the CRBN:PROTAC: PPM1D system was performed by SPR (Figure 5A–D). CRBN was immobilized to ~35 RU's to facilitate the observation of ternary complex formation. The binding of PROTAC alone resulted in a signal of <2 RU's which is below the noise. PPM1D in the analyte gives a high background signal on the SA chip surface, and the highest concentration that can be applied is around 1 µM. This value is lower than the KD between CRBN and its warhead ≥3 µM) thus "hook effect" is expected. SPR is sensitive enough to detect it, which has good agreement with the simulation (Figure 5E). The simulation was done using the non-cooperative equilibria in literature19 combined with the classic SPR calculation [Responsemax = (ResponseLigand × MassImmobilization)/MassLigand]. Since the KD between CRBN and compound is not accurately determined due to the insolubility of the compound at high concentration, simulation was done using four assumptive KD's: 1 µM, 3 µM, 10 µM, or 30 µM. The experimental results fell in between the simulated 3 µM and 10 µM curves, which is almost identical to the KD in the binary system, suggesting there is no cooperativity.
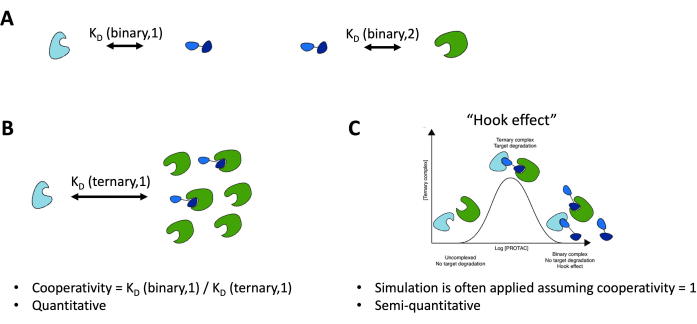
Figure 1: Illustration of three binding scenarios and definition of different KD's. (A) Classic two-component systems. (B) Three-component system in which one end of PROTAC can be saturated thus, it can be evaluated as a two-component system. (C) Three-component system in which the "hook effect" is observed. Please click here to view a larger version of this figure.
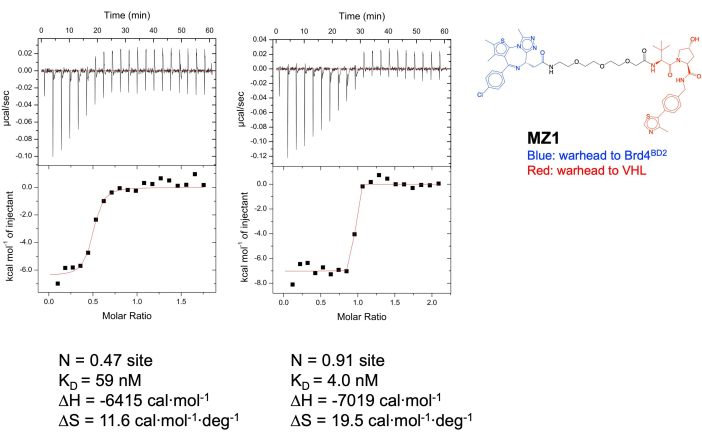
Figure 2: ITC results. Titrating VHL into MZ1 (left) or MZ1:Brd4BD2 complex (right). Please click here to view a larger version of this figure.
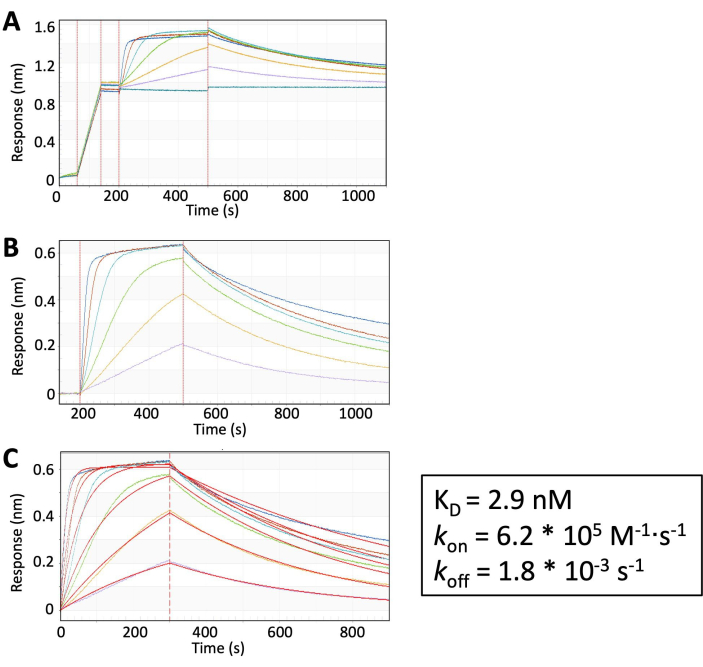
Figure 3: BLI results. MZ1 mediates the formation of VHL: MZ1: Brd4BD2 ternary complex. (A) Raw data. (B) Subtraction of background signals where [MZ1] = 0. (C) Kinetic fitting of B to extract kon, koff, and KD. Please click here to view a larger version of this figure.
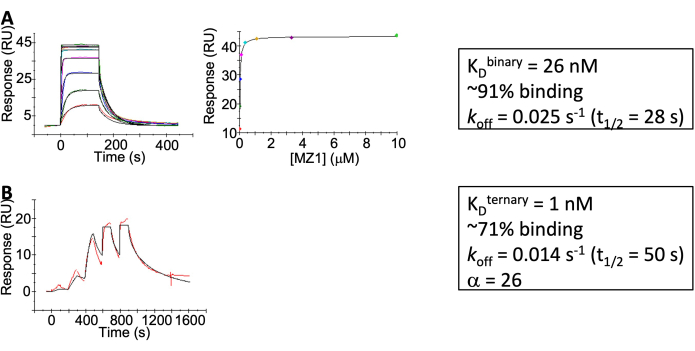
Figure 4: SPR results. (A) MZ1 binding to VHL. (B) MZ1:Brd4BD2 binary complex binding to VHL. Please click here to view a larger version of this figure.
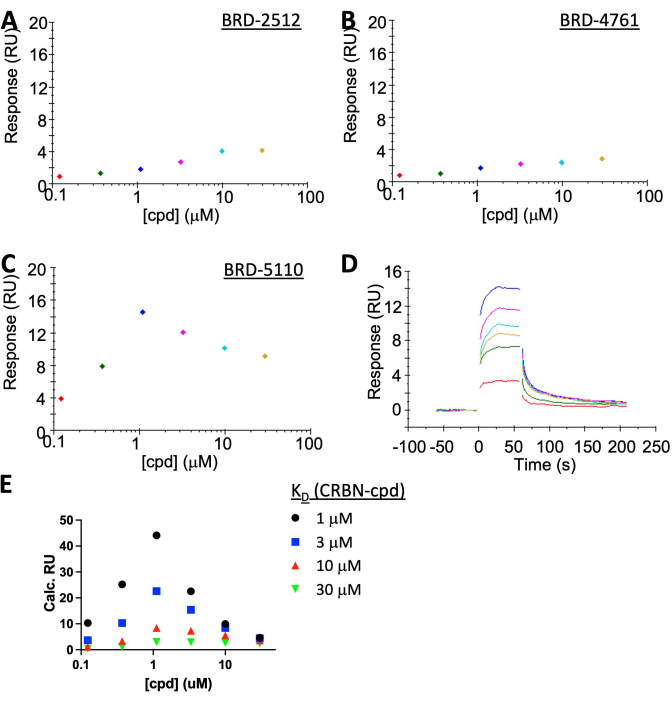
Figure 5: SPR results showing the "hook effect" of a representative PPM1D-PROTAC. CRBN was immobilized on the SA chip surface while [PPM1D] was kept at 1 µM in the analyte for all cases. (A) BRD-2512, a compound that only binds to CRBN, gives almost no response. (B) BRD-4761, a compound that only binds to PPM1D, also gives no response. (C,D) BRD-5110, a PROTAC with the warhead of CRBN in BRD-2512 and the warhead of PPM1D in BRD-4761, induced the formation of the ternary complex. (E) A simulation of SPR results assuming the KD between CRBN and compound is 1 µM (black), 3 µM (blue), 10 µM (red), or 30 µM (green). BRD-2512 curve is between 3 µM and 10 µM, which is very close to the measured binary KD, suggesting no cooperativity (cooperativity = 1). Please click here to view a larger version of this figure.
Discussion
Biophysical characterization of the binary and ternary interactions between PROTAC molecules and their protein binding partners can provide unique and complementary insights relative to widely used cellular systems. Understanding the affinity between each warhead of a PROTAC molecule and its protein binding partners can help guide medicinal chemistry efforts toward the optimization of those interactions. Previously published crystal structures of ternary PROTAC complexes have revealed that atoms in the linker region can form interactions with one or both of the protein binding partners16,20. Experimentally determining the cooperativity of ternary complex formation can support linker optimization.
Described in this report is the utilization of three different biophysical techniques that can provide information about the binding affinities between PROTAC molecules and their protein binding partners. Method 1 details the isothermal titration calorimetry (ITC) experimental set-up for the PROTAC molecule, MZ1, the VHL E3 ligase complex, and the Brd4BD2 bromodomain. ITC results showed KD's of 59 nM for the binary interaction between MZ1 and VHL and 4 nM for the ternary interaction between VHL and pre-mixed MZ1 and Brd4BD2. The affinities were consistent with those observed in SPR (immobilized VHL binding to MZ1 KD = 26 nM, immobilized VHL binding to pre-mixed MZ1 and Brd4BD2 KD=1 nM) and BLI (KD= 2.8 nM). While the ITC KD results for VHL binding to MZ1 are consistent with reported values16, the stoichiometry obtained is different. One potential explanation for this result is the poor solubility of MZ1 in the HEPES-based buffer used in the protocol described here, while the results from the literature were generated using a Bis-tris-based buffer. The authors preferred to use the same buffer components across SPR, ITC, and BLI.
Method 2 describes the experimental setup for the BLI analysis of the interaction of immobilized VHL, a fixed concentration of Brd4BD2, and varying concentrations of MZ1. Because of the sensitivity limitations of the technique, KD, kon, and koff values for ternary complex formation could be generated, but not for the binary interaction between MZ1 and the proteins.
Method 3 describes multiple SPR assays. SPR is more sensitive than BLI and can be applied to observe both the protein-small molecule (binary) and protein-protein (ternary) interactions. In the latter case, background signals should be carefully monitored as protein in the analyte could give high and unstable signals. SPR is very sensitive to reagents with a high refractive index, including DMSO, glycerol, and detergents. If the protein is stored in the buffer containing glycerol or detergent, the running buffer must contain matching concentrations of those components. Alternatively, applying size-exclusion chromatography completely removes them before any SPR experiment. Care should be taken to match the DMSO concentrations between buffer and analyte samples closely. The DMSO solvent corrections are performed according to the manufacturer's instructions.
The method in step 3.1 describes the SPR assay for the binary VHL-MZ1 interaction. Method 3.2 describes the SPR assay for the VHL: MZ1: Brd4BD2 ternary complex where VHL is immobilized, and the analyte is either Brd4BD2 alone or the MZ1:Brd4BD2 complex. In this system, the interaction between Brd4BD2 and VHL is negligible. The ternary complex formation is highly cooperative (ɑ = 26). The off-rate for ternary complex formation is 0.014 s-1, which requires the use of single-cycle kinetics. Results from ITC also show a highly cooperative ternary complex formation (ɑ=15). SPR methods in steps 3.3, 3.4, and 3.5 describe assays for evaluating the formation of a complex between CRBN and PPM1D induced by the presence of a PROTAC molecule, BRD-5110. The PROTAC molecule has a weak affinity for CRBN (KD ~3 µM) and a strong affinity for PPM1D (KD = 1-2 nM). As a result, the weak binding to CRBN is not saturated and results in an observed "hook-effect". While it is possible to increase ligand solubility by increasing the DMSO concentration used in the experiment, it is important in those instances to carefully monitor protein stability which can be negatively impacted by high concentrations of DMSO. Additionally, DMSO has a high heat of dissolution which can obscure the heat of binding of ligands to protein. Care should be taken to match the DMSO concentrations of the solution in the syringe and the solution in the cell. The authors recommend dialysis of the two solutions against the same buffer preparation.
General recommendations and guidelines are provided based on the experiments performed and reported here. When the affinities of binary interactions between PROTAC molecules and their protein binding partners are strong (KD <1 µM), SPR provides reliable and reproducible affinities along with valuable information on the cooperativity of ternary complex formation. When the affinities of the binary interaction between one of the protein binding partners and the PROTAC molecule are weak (KD >1 µM), the assay setup will need to be modified. In those instances, the use of molecular simulations where the binding constants are fixed, and the concentrations of ligand and analyte are varied can be valuable in guiding assay design and interpreting experimental results. ITC assays provide important information on the stoichiometry of binding but require significantly more protein and compound reagents relative to SPR and BLI. Additionally, the solubility of the PROTAC molecule can be limiting for ITC experiments. BLI has higher throughput than ITC and requires less protein and compound reagents. However, due to sensitivity limitations, BLI can only be used to assess the ternary complex formation and not binary interactions between PROTAC molecules and their protein binding partners. It is recommended that SPR be used for routine testing of both binary and ternary PROTAC binding assays and BLI and ITC assays used for orthogonal validation of results from SPR.
Divulgaciones
The authors have nothing to disclose.
Acknowledgements
This work was supported by an Innovation and Technology Development award from the Center for the Development of Therapeutics at the Broad Institute of MIT and Harvard. The authors wish to thank the members of the senior leadership team and the review committee for their support of this work.
Materials
| 96-plate | Greiner | 655076 | flat-bottom, black plates used In BLI experiments |
| 96-well plate | Nunc | 73520-120 | Plate use for ITC sample preparation |
| 96-well plate | Greiner | 650101 | Plate used to prepare samples for SPR experiments |
| Auto iTC200 micro-calorimeter | Malvern Panalytical | Instrument used to perform ITC experiments. Product discontinued. | |
| Biacore S200 | Cytiva | 29136649 | Instrument used to perform SPR experiments |
| MZ1 | ProbeChem | PC-60099 | PROTAC that binds to VHL and Brd4BD2 |
| NTA sensor chip | Cytiva | BR100532 | SPR chip used to perform SPR experiments involving PPM1D |
| Octet Red-384 | Sartorius | Instrument used to perform BLI experiments. Product discontinued. | |
| Plate cover | Malvern | PQA0001 | Cover for Nunc 96-well plate (73520-120) |
| Plate cover | Cytiva | 28975816 | Plate cover for Greiner plate (650101) |
| Series S SA sensor chip | Cytiva | BR100531 | SPR chip used to perform SPR experiments involving MZ1:VHL:BRD4 |
| Streptavidin (SA) Dip and Read Biosensors | Sartorius | 18-509 | Coated sensors used in BLI experiments |
Referencias
- Balaji, V., Hoppe, T. Regulation of E3 ubiquitin ligases by homotypic and heterotypic assembly. F1000Research. 9, (2020).
- Song, L., Luo, Z. -. Q. Post-translational regulation of ubiquitin signaling. Journal of Cell Biology. 218 (6), 1776-1786 (2019).
- Yang, Q., Zhao, J., Chen, D., Wang, Y. E3 ubiquitin ligases: styles, structures and functions. Molecular Biomedicine. 2 (1), 23 (2021).
- Grice, G. L., Nathan, J. A. The recognition of ubiquitinated proteins by the proteasome. Cellular and Molecular Life Sciences: CMLS. 73 (18), 3497-3506 (2016).
- Chirnomas, D., Hornberger, K. R., Crews, C. M. Protein degraders enter the clinic – a new approach to cancer therapy. Nature Reviews Clinical Oncology. 20 (4), 265-278 (2023).
- Toure, M., Crews, C. M. Small-molecule PROTACS: New approaches to protein degradation. Angewandte Chemie (International ed. In English). 55 (6), 1966-1973 (2016).
- Ottis, P., Toure, M., Cromm, P. M., Ko, E., Gustafson, J. L., Crews, C. M. Assessing different E3 ligases for small molecule induced protein ubiquitination and degradation. ACS Chemical Biology. 12 (10), 2570-2578 (2017).
- Riching, K. M., et al. Quantitative live-cell kinetic degradation and mechanistic profiling of PROTAC mode of action. ACS Chemical Biology. 13 (9), 2758-2770 (2018).
- Nabet, B., et al. The dTAG system for immediate and target-specific protein degradation. Nature Chemical Biology. 14 (5), 431-441 (2018).
- Paiva, S. -. L., Crews, C. M. Targeted protein degradation: elements of PROTAC design. Current Opinion in Chemical Biology. 50, 111-119 (2019).
- Hershko, A., Ciechanover, A. The ubiquitin system. Annual Review of Biochemistry. 67, 425-479 (1998).
- Chan, K. -. H., Zengerle, M., Testa, A., Ciulli, A. Impact of target warhead and linkage vector on inducing protein degradation: Comparison of bromodomain and extra-terminal (BET) degraders derived from triazolodiazepine (JQ1) and tetrahydroquinoline (I-BET726) BET inhibitor scaffolds. Journal of Medicinal Chemistry. 61 (2), 504-513 (2018).
- Roy, M. J., et al. SPR-measured dissociation kinetics of PROTAC ternary complexes influence target degradation rate. ACS Chemical Biology. 14 (3), 361-368 (2019).
- Pierce, N. W., Kleiger, G., Shan, S., Deshaies, R. J. Detection of sequential polyubiquitylation on a millisecond timescale. Nature. 462 (7273), 615-619 (2009).
- Gadd, M. S., et al. Structural basis of PROTAC cooperative recognition for selective protein degradation. Nature Chemical Biology. 13 (5), 514-521 (2017).
- Nahta, R., Castellino, R. C. Phosphatase magnesium-dependent 1 δ (PPM1D), serine/threonine protein phosphatase and novel pharmacological target in cancer. Biochemical Pharmacology. 184, 114362 (2021).
- Douglass, E. F., Miller, C. J., Sparer, G., Shapiro, H., Spiegel, D. A. A comprehensive mathematical model for three-body binding equilibria. Journal of the American Chemical Society. 135 (16), 6092-6099 (2013).
- Zorba, A., et al. Delineating the role of cooperativity in the design of potent PROTACs for BTK. Proceedings of the National Academy of Sciences. 115 (31), E7285-E7292 (2018).
- Fairhead, M., Howarth, M. Site-specific biotinylation of purified proteins using BirA. Methods in Molecular Biology. 1266, 171-184 (2015).
- Nowak, R. P., et al. Plasticity in binding confers selectivity in ligand-induced protein degradation. Nature Chemical Biology. 14 (7), 706-714 (2018).

