Induction of Acute Ischemic Stroke in Mice Using the Distal Middle Artery Occlusion Technique
Summary
Here, we present a protocol to establish a distal middle cerebral artery occlusion (dMCAO) model through transcranial electrocoagulation in C57BL/6J mice and evaluate the subsequent neurological behavior and histopathological features.
Abstract
Ischemic stroke remains the predominant cause of mortality and functional impairment among the adult populations globally. Only a minority of ischemic stroke patients are eligible to receive intravascular thrombolysis or mechanical thrombectomy therapy within the optimal time window. Among those stroke survivors, around two-thirds suffer neurological dysfunctions over an extended period. Establishing a stable and repeatable experimental ischemic stroke model is extremely significant for further investigating the pathophysiological mechanisms and developing effective therapeutic strategies for ischemic stroke. The middle cerebral artery (MCA) represents the predominant location of ischemic stroke in humans, with the MCA occlusion serving as the frequently employed model of focal cerebral ischemia. In this protocol, we describe the methodology of establishing the distal MCA occlusion (dMCAO) model through transcranial electrocoagulation in C57BL/6 mice. Since the occlusion site is located at the cortical branch of MCA, this model generates a moderate infarcted lesion restricted to the cortex. Neurological behavioral and histopathological characterization have demonstrated visible motor dysfunction, neuron degeneration, and pronounced activation of microglia and astrocytes in this model. Thus, this dMCAO mouse model provides a valuable tool for investigating the ischemiastroke and worth of popularization.
Introduction
Stroke is a common acute cerebrovascular disease characterized by high incidences of disability and fatality1. Of all stroke cases, nearly 80% belong to ischemic stroke2. Up to now, intravenous thrombolysis remains one of a limited number of productive approaches for the treatment of acute ischemic stroke. However, the effectiveness of thrombolytic treatment is restricted by the narrow effective time window and the occurrence of hemorrhagic transformation3. In the long-term rehabilitation phase following an ischemic stroke, a considerable number of patients are likely to experience durable neurological dysfunctions4. Further investigation is urgently required to unravel the underlying pathophysiological mechanisms of ischemic stroke, as well as to facilitate the development of novel therapeutic strategies targeting ischemic stroke. The establishment of a dependable and replicable model of ischemic stroke is crucial for basic research as well as subsequent translational research in the field of ischemic stroke.
In 1981, Tamura et al. developed a focal cerebral ischemia model by employing transcranial electrocoagulation at the proximal site of the middle cerebral artery (MCA)5. Since then, numerous researchers have utilized various methodologies such as ligation, compression, or clipping to induce distal MCA occlusion (dMCAO) for establishing transient or permanent ischemic stroke models6,7,8. Compared to the filament model, the dMCAO model exhibits notable advantages such as smaller infarct size and higher survival rate, rendering it more suitable for investigating long-term functional recovery subsequent to ischemic stroke9. In addition, the dMCAO model demonstrates a higher survival rate in aged rodents compared to the filament model, making it an advantageous tool for investigating ischemic stroke in elderly and comorbid animal models10. The photothrombotic (PT) stroke model has been demonstrated to possess the characteristics of less surgical invasiveness and a significantly low mortality rate. However, the PT model exhibits a greater degree of cellular necrosis and tissue edema compared to the dMCAO model, leading to the absence of collateral circulation11. Furthermore, it is noteworthy that the ischemic lesions observed in the PT model predominantly arise from microvascular occlusion, which differs substantially from the cerebral ischemia induced by large vessel embolism in the dMCAO model12.
In this paper, we present the methodology for inducing the murine dMCAO model by coagulating the distal MCA via small bone window craniotomy. Additionally, we conducted histological examinations and behavioral evaluations to comprehensively characterize the ischemic insults and stroke outcomes in this experimental model. We aim to acquaint researchers with this model and facilitate further investigations into the pathologic mechanisms of ischemic stroke.
Protocol
The experimental protocol was approved by the Institutional Animal Care and Use Committee of Jianghan University and was conducted in accordance with Experimental Animals Ethical Guidelines issued by the Center for Disease Control of China. Adult male C57BL/6J mice, 10 weeks old, weighted 24-26 g, were used in this protocol. All mice were housed under a 12-h light/dark cycle controlled environment with food and water ad libitum.
1. Preoperative preparation
NOTE: The key instruments and equipment required for this protocol are shown in Figure 1.
- Prior to the procedure, sterilize the surgical instruments by autoclaving.
- Administer meloxicam subcutaneously at a dose of 5 mg/kg for analgesia 60 min before surgery.
- Anesthetize the mouse with 5% isoflurane in an induction chamber.
- Wipe the heating surgery board with 75% ethanol and activate the heating switch with the temperature set to 37 °C. Then, position the mouse in a left lateral position on the board, with the head positioned away from the surgeon and the tail facing towards the surgeon.
- Attach a mask to the mouse face that provides a constant supply of 2% isoflurane and 0.3 L/min oxygen for maintenance anesthesia. Apply the eye ointment on the cornea to protect them from drying during the procedure.
- Shave the fur from the left orbit to the left ear canal with a shaver. Use depilatory creams to remove the remaining fur for sterilization.
- Prepare the surgical area by applying povidone-iodine disinfectant and 75% ethanol three times.
2. Distal MCAO model
- Check if the mouse is deeply anesthetized by assessing the reflexes of the toe pinch.
- Make a vertical incision between the left orbit and the left ear canal using a sterile scalpel blade.
- Retract the skin's soft tissue with retractors and expose the temporal muscle.
- Use straight microforceps to separate the apical and dorsal segments of the temporal muscle from the skull.
- Identify the bifurcation of the MCA, which assumes a "Y" shape beneath the temporal bone with a bias towards the base of the skull (Figure 2A).
NOTE: If the bifurcation of MCA is not visually discernible (due to an anatomically normal variation), ascertain the most rostral vessel. - Use an electric cranial drill to thin out the skull to make a bone window (4 mm in diameter) until the dura mater, with its translucent texture, becomes visible.
NOTE: Intermittently add drops of normal saline to the skull to prevent overheating during this process. - Remove the dura mater above the artery with curved micro forceps to expose the MCA branch.
- Set the electrocautery to 7 W. Coagulate the artery using the electric coagulation forceps at the sites of proximal and distal to the bifurcation of MCA (Figure 2A). When the bifurcation is not visible due to an anatomical variation, perform the coagulation on the accurately identified MCA branch (as mentioned above) at two separate locations approximately 1 mm apart13.
- Monitor the blood flow of the left MCA cortical branch utilizing a laser speckle blood flow meter.
NOTE: Mice exhibiting a blood flow reduction of less than 40% of the baseline value following electrocoagulation were excluded from the study. - Suture the muscle and the skin separately using 5-0 polyglycolic acid sutures. Apply diclofenac sodium gel and mupirocin ointment to the skin incision using a sterile cotton swab.
- Place the mouse in a recovery chamber. Monitor all mice until they are fully awake. Provide meloxicam (5 mg/kg, SC) for analgesia every 24 h up to 72 h.
3. Sham Operation
- Reproduce the same procedure as those mentioned above, excluding the process of arterial coagulation.
4. Behavioral tests
NOTE: Prior to the dMCAO, the mice underwent behavioral training twice daily for 3 days. On 3rd day post-dMCAO, move the mice to the behavioral testing room for a 2 h environmental adaptation before testing.
- Grip strength test14
NOTE: The grip strength meter comprises a push-pull strain gauge and a metal horizontal bar.- Grasp the base of the mouse tail. Gradually lower the mouse toward the metal grip bar until it grips the horizontal bar with both forepaws (Figure 2B).
- Pull the mouse backward at a constant speed of about 2 cm/s and keep its body horizontal.
- Record the peak force in grams (g) when the mouse releases its forepaws from the bar.
- Pole test15
- Place the mouse on the top of a vertical wooden pole (50 cm high, 1 cm in diameter) with their head facing upwards, and allow them to climb down the pole (Figure 2B).
- Record the time taken by the mouse to turn around (T-turn) and the total time to climb down the pole (T-total) with a 60-s cut-off time.
- Adhesive tape removal test16
- Hold the mouse and attach a patch of adhesive tape (2 × 2 mm) to the right forepaw of the mouse (Figure 2B).
- Put the mouse back into the rearing cage and allow it to move freely.
- Record the time taken by the mouse to remove adhesive with a limit of 60 s.
- Cylinder test17
- Place the mouse in an open-top, clear glass cylinder (diameter: 14 cm; height: 20 cm) and record its spontaneous standing exploratory behavior for 3 min using a camera (Figure 2B).
NOTE: During the experimental procedure, two mirrors were positioned alongside the cylinder to ensure optimal recording of forelimb movements. - Wipe the cylinder with 75% ethanol to eliminate residual olfactory cues before testing the subsequent mouse.
- Reduce the playback speed of the video to 1/5 of the actual speed, and count the number of wall touches with the left (L) or right forepaw (R) or simultaneous use of both forepaws (B). Calculate the usage rate of the right forepaw as (L-R)/(L + R + B) × 100%.
- Place the mouse in an open-top, clear glass cylinder (diameter: 14 cm; height: 20 cm) and record its spontaneous standing exploratory behavior for 3 min using a camera (Figure 2B).
5. Perfusion and sample preparation
- Euthanize the mouse by peritoneal injection overdose of pentobarbital (300 mg/kg). Position the mouse supine and immobilize the limbs.
- Make a midline incision on the skin, starting from the mid-abdominal cavity and extending towards the upper chest region.
- Open the chest using surgical scissors and fold the xiphoid upwards using a hemostat. Carefully remove the lung and the diaphragm to expose the heart.
- Insert the perfusion needle tip into the left ventricle and puncture the right atrium with scissors. Perfuse the heart with 20 mL of normal saline via the left ventricle.
- Decapitate the mouse and cut open the scalp along the midline between the eyes. Cut the bone along both sides, starting from the skull base and opening up to the eye socket.
- Lift the calvarium and gently detach the brain from the base of the skull by using a forceps.
NOTE: Brains from half of the mice were subjected to 2,3,5-triphenyl tetrazolium chloride (TTC) staining, while brains from the other half underwent histological examination. - Soak the brains in 4% paraformaldehyde solution overnight for follow-up histological analysis.
6. Identification of infarction volume
- Place the brain in a -20 °C freezer compartment of the refrigerator for 20 min.
- Position the brain into the 1 mm brain matrix and slice the brain into coronary sections with a thickness of 2 mm by using microtome blades.
- Transfer the brain sections to a 24-well culture plate and add 2% TTC to cover the brain tissue. Put the 24-well culture plate in a constant temperature oven for 30 min with the temperature set at 37 °C.
- Carefully lift out the brain sections and fix them in 4% paraformaldehyde solution for 15 min.
- Perform optical scanning of these brain sections with a resolution of 1200 x 1200 dpi by a scanister.
- Measure the cerebral infarct volume by summing the infarct area of each section (area of the right hemisphere minus uninjured area of the left hemisphere) using Image J software (https://imagej.net/software/imagej/index)18. Calculate the infarct volume percentage as infarct volume/volume of the right hemisphere × 100%.
7. Histopathological and immunofluorescent staining analysis
- Take the brain samples from the paraformaldehyde solution and flush them with running water for 1 h.
- Transfer the brain samples into the automation-tissue-dehydrating machine. Launch the preset program for dehydration, vitrification, and wax immersion. Embed the dehydrated brain samples in paraffin and slice them into 5-µm thick sections by a microtome.
- Hematoxylin and eosin (H&E) staining
- Dewax the sections with xylene 3 times for 8 min each.
- Rehydratethe sections by successively immersing in gradient ethanol (100%, 95%, 90%, 80%, 70%) and distilled water for 5 min each time.
- Stain the sections with the hematoxylin solution for 5 min.
- Rinse the sections with distilled water to wash the residue hematoxylin, then dip them in 5% hydrochloric acid alcohol for a 5-s differentiation. Wash the sections with distilled water for 2 min.
- Stain the sections with eosin solution for 30 s and rinse off excess staining fluid using distilled water.
- Immerse the sections in 90% ethanol, 95% ethanol, 100% ethanol, and xylene (2 times) successively for 5 min each time. Mount the sections with a neutral balsam mounting medium.
- Immunofluorescence staining
- Dewax the sections with xylene 3 times for 8 min each time.
- Rehydrate the sections by successively immersing in gradient ethanol (100%, 95%, 90%, 80%, 70%) and distilled water for 5 min each time.
- Preheat the citrate antigen retrieval solution to boil in the microwave. Immerse the sections in the antigen retrieval solution. Reheat the antigen retrieval buffer with low power (20 W) for 20 min.
- Turn off the microwave and let the antigen retrieval buffer cool to room temperature (RT).
- Wash the sections thrice with 0.1 mM phosphate buffered saline (PBS) for 5 min each time.
- Incubate the sections with a blocking solution (5% bovine serum albumin) at RT for 1 h to block the non-specific binding of immunoglobulin.
- Incubate the sections with rabbit anti-NeuN antibody (1: 300), rabbit anti-Iba-1 antibody (1: 500) and mouse anti-GFAP antibody (1: 500) at 4˚C overnight.
- Wash the sections thrice with 0.1 mM PBS for 5 min each time.
- Incubate the sections with goat anti-rabbit Alexa 594-conjugated IgG (1:500) or goat anti-mouse Alexa 488-conjugated IgG (1:500) for 1 h at RT.
- Wash the sections with 0.1 mM PBS and mount them with an antifade mounting media containing 4',6-diamidino-2-phenylindole (DAPI).
- Take images of three microscopic fields (300 µm × 300 µm) within the ischemic penumbra using a fluorescent microscope at 594 nm and 488 nm, respectively.
- Using the ImageJ software (https://imagej.net/software/imagej/index), estimate the positively stained cell density by averaging counts in ischemic penumbra19.
Representative Results
The key instruments used to perform the dMCAO are the microsurgical instruments set, the isoflurane vaporizer, and the monopolar microsurgical electrocoagulation generator shown in Figure 1. The experimental procedure of this study is illustrated in Figure 2. In brief, a small bone window craniotomy was employed to expose the distal MCA, which was subsequently coagulated to induce permanent focal cerebral ischemia in C57BL/6 mice. Furthermore, the ischemic insults and stroke outcomes were assessed through TTC staining, histological examinations, and behavioral evaluations at 3 days post dMCAO. During the surgical procedure, only 1 mouse died from surgical bleeding. Furthermore, all the remaining mice survived during the 3-day observation period after surgery.
Macroscopic observation revealed that the dMCAO generated visible hyperemia and edema in the cortex (Figure 3A, bottom). No discernible macroscopic alterations were observed in the sham-operated group (Figure 3A, top). Additionally, dMCAO-induced cortical infarction was also verified using TTC staining (Figure 3B). The infarct volume was 16.6% ± 0.8% 3 days after the surgery in the dMCAO group, demonstrating the stability and repeatability of this cerebral ischemia model (Figure 3C).
Several behavioral tests were conducted to evaluate the neurological deficits on 3rd day post dMCAO. As shown in Figure 4A, the fore limb grip strength exhibited a significant reduction in the dMCAO group compared to the sham-operated group (70.8 g ± 4.2 g vs. 114.0 g ± 6.2 g, P < 0.001). The dMCAO group mice exhibited prolonged latencies in both turning around and descending to the ground during the pole test, as compared to the sham-operated group (15.8 s ± 1.7 s vs. 8.7 s ± 1.3 s, P < 0.01, 45.1 s ± 3.3 s vs. 29.2 s ± 2.1 s, P < 0.001) (Figure 4B,C). For the adhesive removal test, a significant increase in the removal time was observed in the dMCAO group compared to the sham operation group (20.5 s ± 2.5 s vs. 7.8 s ± 1.1 s, P < 0.001) (Figure 4D). The statistical analysis of the cylinder test data demonstrated a significant reduction in contralateral forepaw usage rates within the dMCAO group compared to the sham operation group (35.2% ± 2.6% vs. 48.7% ± 2.2%, P < 0.001) (Figure 4E).
Black squares in Figure 5A illustrate the analysis region for immunofluorescent staining. The H&E staining results revealed the disordered arrangement of neuron cells in the peri-infarct area of the dMCAO group, characterized by prominent pyknosis, vacuolization, and nuclear hyperstaining (Figure 5B, bottom). However, the sham-operation group did not exhibit any discernible alterations in neuronal morphology (Figure 5B, top). Figure 5C illustrates that dMCAO resulted in a significant reduction in the density of NeuN-positive cells within the peri-infarct area (319.6 ± 19.0 vs. 765.0 ± 26.0, P < 0.001). Figure 5D,E illustrates that the densities of microglia (665.8 ± 30.6 vs. 207.4 ± 16.2, P < 0.001) and astrocytes (305.2 ± 17.2 vs. 17.2 ± 2.1, P<0.001) increased greatly in dMCAO group compared with the sham-operation group. These findings offer compelling evidence supporting the presence of neuronal loss and the excessive activation of microglia and astrocytes on the 3rd day post-dMCAO.

Figure 1: Key instruments used to establish the dMCAO model. (A) Essential surgical instruments. The Roman numerals I-VII refer to electric coagulation forceps, curved micro forceps, micro scissors, straightmicro forceps, retractors, needle holders, and surgical scissors. (B) Isoflurane vaporizer. (C) Electro-surgical generator. Please click here to view a larger version of this figure.
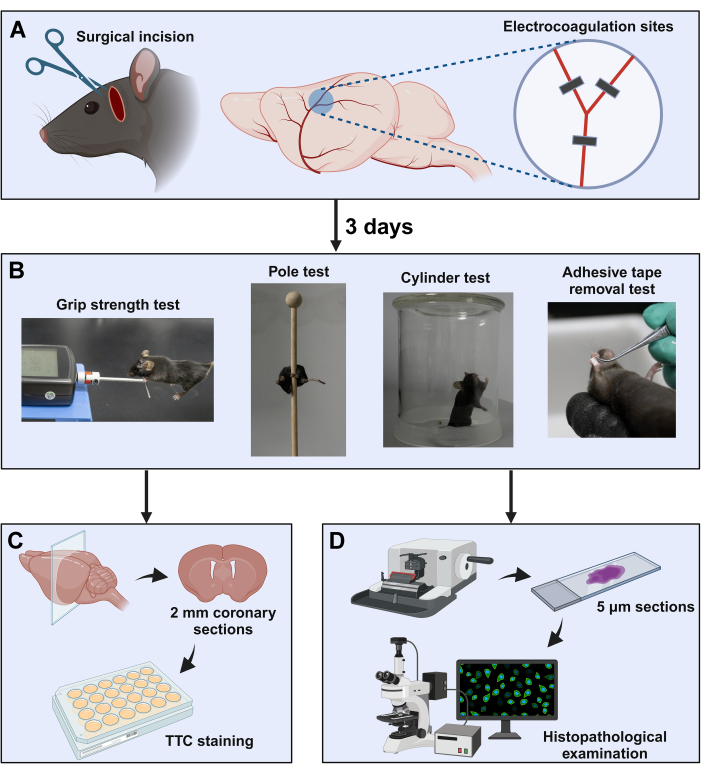
Figure 2: Schematic diagram of the experimental procedure. (A) The surgical window is located between the orbit and the ear canal. The distal MCA was exposed by subtemporal craniotomy, followed by coagulation at three sites near the bifurcation (indicated with black squares). (B) Neurological behavior evaluation paradigms were used in this study. After completion of the neurological behavior tests, the brain samples were collected for (C) TTC staining and (D) histopathology examinations. Please click here to view a larger version of this figure.
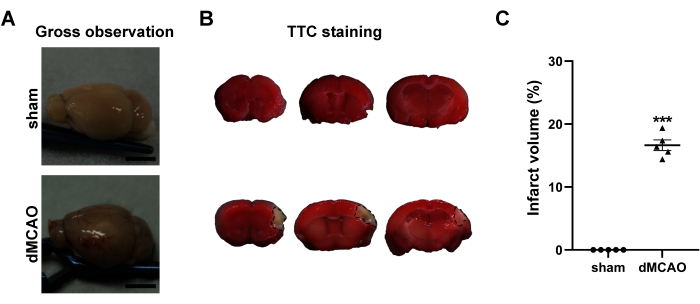
Figure 3: Macroscopic evaluation of the brains at 3rd day post dMCAO. (A) Gross observation of the brains from the sham operation and dMCAO groups. (B) Representative TTC-stained brain coronary sections of sham operation group (top) and dMCAO group (bottom). The black line demarcates the non-infarcted (unstained) cortex of the ipsilateral brain. (C) Quantification of the infarct volume. Scale bar = 5 mm. Data are presented as mean ± SEM. N = 5, ***P < 0.001 compared with the sham group, paired t-test. Please click here to view a larger version of this figure.
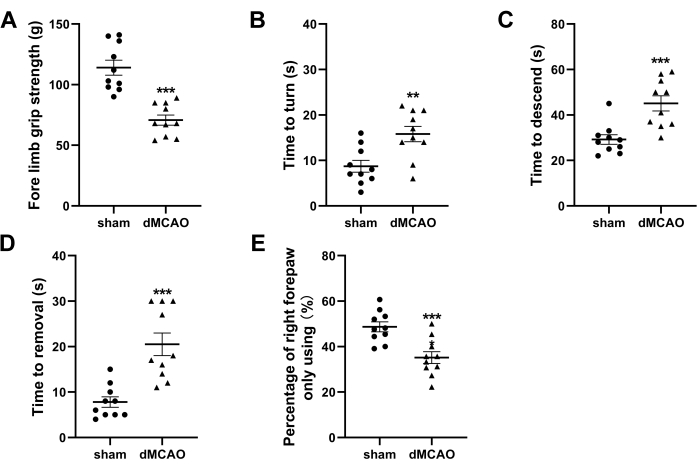
Figure 4: Neurological behavior tests at 3rd day post-dMCAO. (A) Quantification ofthefore limb grip strength. (B,C) Quantification of the time latencies to turn around and descend to the ground in the pole test. (D) Quantification of time required to remove the adhesive tape in the adhesive removal test. (E) Quantification of cylinder test presented as the usage ratio of the right forepaw. Data are presented as mean ± SEM, N = 10, **P < 0.01, ***P < 0.001 compared with the sham group, paired t-test. Please click here to view a larger version of this figure.
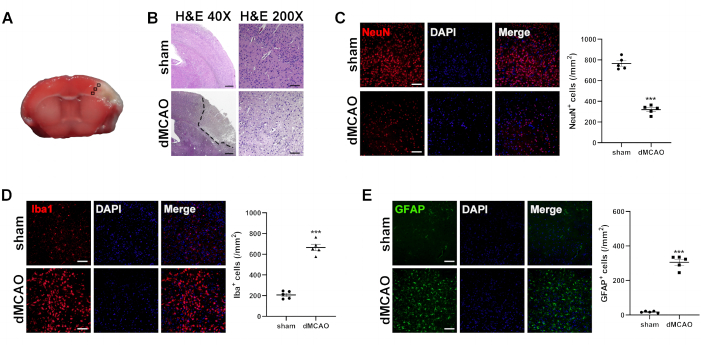
Figure 5: Histological analysis of brains at 3rd day post dMCAO. (A) Immunofluorescence measurement from the cortex of the selected three fields in the ischemic penumbra. (B) Representative images of H&E-stained brain sections from the sham operation and dMCAO groups. Dotted lines surround unstained parts, indicating an infarct lesion. (H&E 40x, Scale bar = 200 µm; H&E 200x, Scale bar = 50 µm). Representative images of immunofluorescence staining and quantitative analysis for (C) NeuN (a marker for neuron), (D) Iba-1 (a marker for microglia), and (E) GFAP(a marker for astrocyte). Scale bar = 50 µm. Data are presented as mean ± SEM, N = 5, ***P < 0.001 compared with the sham group, paired t-test. Please click here to view a larger version of this figure.
Discussion
In the present protocol of the craniotomy electrocoagulation dMCAO model, the surgical procedures are conducted with minimal invasiveness, wherein only a portion of the temporalis muscle is separated to mitigate the adverse effects on masticatory function. The mice all recovered well after the procedure, with no observed instances of feeding difficulties. The MCA can be easily discerned in the temporal bone of the mouse, thereby facilitating precise identification of suitable craniotomy locations. This dMCAO model-induced ischemic lesions was localized in the lateral part of the cortex, consistent with previous reports described by Llovera G et al.13. Furthermore, the infarct volume associated with this model is approximately 16%, closely with the observed infarct volumes in the majority of human ischemic stroke cases20.
Several laboratories have utilized the craniotomy electrocoagulation dMCAO model to investigate the pathophysiology of ischemic stroke and its pharmacological interventions. It is noteworthy that the majority of the above experimental studies solely employed diverse rating scales to evaluate basic reflexes and limb motor function in mice after stroke6,21. From our perspective, these estimates appear to lack precision, rendering them inadequate for the assessment of neurological impairments following a stroke. To address this issue, we devised a series of behavior tests to appraise the neurologic deficits of the mice in this model methodically. As expected, the behavior tests revealed evident impairments in motor coordination and motor symmetry at 3rd day post dMCAO. It should be noted that some other behavioral tests (e.g., grid walk test, rotarod test, corner test, and gait analysis) are available for the evaluation of motor and sensory functions of mice in this model22.
Cerebral ischemia can induce progressive neuronal damage and alterations in synaptic substructure, resulting in the manifestation of neurological deficits. As anticipated, evident neuronal degeneration and reduced neuronal density were observed within the peri-infarct penumbra region. This finding is consistent with previous studies, which have reported the peak occurrence of neuronal apoptosis at 48-72 h after the ischemic insult in murine stroke models23,24. Furthermore, the immunofluorescence findings substantiated the presence of microgliosis and astrogliosis within the peri-infarct penumbra region, consistent with previous studies25,26. These findings demonstrate distinct cellular and histological alterations following dMCAO, thereby providing additional validation for the reliability of the model established in this study.
The challenge in establishing the dMCAO model through craniotomy electrocoagulation lies in the presence of anatomical variations in the MCA, which poses difficulty in selecting an appropriate site for electrocoagulation. During the procedure of this protocol, it was observed that 23 mice exhibited normal MCA routes, while only one mouse displayed anatomical normal variation in MCA characterized by inconspicuous MCA bifurcation. We propose that beginners may consider referring to Llovera’s method, which entails employing multiple occlusion sites (three in the case of bifurcation and two for MCA without bifurcation) to minimize the risk of partial recanalization of MCA13.
Overall, the current approach effectively generated a reliable experimental ischemic stroke model that exhibits high survivability and excellent repeatability. Consequently, this methodology serves as an invaluable tool for both foundation and translational research in the field of stroke and is worth popularization.
Divulgaciones
The authors have nothing to disclose.
Acknowledgements
This study was supported by the Grants from the Nature Science Foundation of Hubei Province (2022CFC057).
Materials
| 2,3,5-Triphenyltetrazolium Chloride (TTC) |
Sigma-Aldrich | 108380 | Dye for TTC staining |
| 24-well culture plate | Corning (USA) | CLS3527 | Vessel for TTC staining |
| 4% paraformaldehyde | Wuhan Servicebio Technology Co., Ltd. |
G1101 | Tissue fixation |
| 5% bovine serum albumin | Wuhan BOSTER Bio Co., Ltd. | AR004 | Non-specific antigen blocking |
| 5-0 Polyglycolic acid suture | Jinhuan Medical Co., Ltd | KCR531 | Material for surgery |
| Anesthesia machine | Midmark Corporation | VMR | Anesthetized animal |
| Antifade mounting medium | Beyotime Biotech | P0131 | Seal for IF staining |
| Automation-tissue-dehydrating machine |
Leica Biosystems (Germany) | TP1020 | Dehydrate tissue |
| Depilatory cream | Veet (France) | 20220328 | Material for surgery |
| Diclofenac sodium gel | Wuhan Ma Yinglong Pharmaceutical Co., Ltd. |
H10950214 | Analgesia for animal |
| Drill tip (0.8 mm) | Rwd Life Science Co., Ltd. | Equipment for surgery | |
| Eosin staining solution | Wuhan Servicebio Technology Co., Ltd. |
G1001 | Dye for H&E staining |
| Eye ointment | Guangzhou Pharmaceutical Co., Ltd | H44023098 | Material for surgery |
| Fluorescence microscope | Olympus (Japan) | BX51 | Image acquisition |
| GFAP Mouse monoclonal antibody | Cell Signaling Technology Inc. (Danvers, MA, USA) |
3670 | Primary antibody for IF staining |
| Goat anti-mouse Alexa 488-conjugated IgG |
Cell Signaling Technology Inc. (Danvers, MA, USA) |
4408 | Second antibody for IF staining |
| Goat anti-rabbit Alexa 594-conjugated IgG |
Cell Signaling Technology Inc. (Danvers, MA, USA) |
8889 | Second antibody for IF staining |
| Grip strength meter | Shanghai Xinruan Information Technology Co., Ltd. | XR501 | Equipment for behavioral test |
| Hematoxylin staining solution | Wuhan Servicebio Technology Co., Ltd. |
G1004 | Dye for H&E staining |
| Iba1 Rabbit monoclonal antibody | Abcam | ab178846 | Primary antibody for IF staining |
| Isoflurane | Rwd Life Science Co., Ltd. | R510-22-10 | Anesthetized animal |
| Laser doppler blood flow meter | Moor Instruments (UK) | moorVMS | Blood flow monitoring |
| Meloxicam | Boehringer-Ingelheim | J20160020 | Analgesia for animal |
| Microdrill | Rwd Life Science Co., Ltd. | 78001 | Equipment for surgery |
| Microsurgical instruments set | Rwd Life Science Co., Ltd. | SP0009-R | Equipment for surgery |
| Microtome | Thermo Fisher Scientific (USA) | HM325 | Tissue section production |
| Microtome blade | Leica Biosystems (Germany) | 819 | Tissue section production |
| Monopolar electrocoagulation generator | Spring Scenery Medical Instrument Co., Ltd. |
CZ0001 | Equipment for surgery |
| Mupirocin ointment | Tianjin Smith Kline & French Laboratories Ltd. |
H10930064 | Anti-infection for animal |
| NeuN Rabbit monoclonal antibody | Cell Signaling Technology Inc. (Danvers, MA, USA) |
24307 | Primary antibody for IF staining |
| Neutral balsam | Absin Bioscience | abs9177 | Seal for H&E staining |
| Paraffin embedding center | Thermo Fisher Scientific (USA) | EC 350 | Produce paraffin blocks |
| Pentobarbital sodium | Sigma-Aldrich | P3761 | Euthanized animal |
| Phosphate buffered saline | Shanghai Beyotime Biotech Co., Ltd | C0221A | Rinsing for tissue section |
| Shaver | Shenzhen Codos Electrical Appliances Co.,Ltd. |
CP-9200 | Equipment for surgery |
| Sodium citrate solution | Shanghai Beyotime Biotech Co., Ltd. | P0083 | Antigen retrieval for IF staining |
Referencias
- Patel, P., Yavagal, D., Khandelwal, P. Hyperacute management of ischemic strokes: JACC Focus Seminar. J Am Coll Cardiol. 75 (15), 1844-1856 (2020).
- GBD 2016 Stroke Collaborators. Global, regional, and national burden of stroke, 1990-2016: a systematic analysis for the Global Burden of Disease study 2016. Lancet Neurol. 18 (5), 439-458 (2019).
- Joo, H., Wang, G., George, M. G. A literature review of cost-effectiveness of intravenous recombinant tissue plasminogen activator for treating acute ischemic stroke. Stroke Vasc Neurol. 2 (2), 73-83 (2017).
- Jones, A. T., O’Connell, N. K., David, A. S. Epidemiology of functional stroke mimic patients: a systematic review and meta-analysis. Eur J Neurol. 27 (1), 18-26 (2020).
- Tamura, A., et al. Focal cerebral ischaemia in the rat: Description of technique and early neuropathological consequences following middle cerebral artery occlusion. J Cereb Blood Flow Metab. 1 (1), 53-60 (1981).
- Kuraoka, M., et al. Direct experimental occlusion of the distal middle cerebral artery induces high reproducibility of brain ischemia in mice. Exp Anim. 58 (1), 19-29 (2009).
- Orset, C., et al. Mouse model of in situ thromboembolic stroke and reperfusion. Stroke. 38 (10), 2771-2778 (2007).
- Fluri, F., Schuhmann, M. K., Kleinschnitz, C. Animal models of ischemic stroke and their application in clinical research. Drug Des Devel Ther. 9, 3445-3454 (2015).
- Candelario-Jalil, E., Paul, S. Impact of aging and comorbidities on ischemic stroke outcomes in preclinical animal models: A translational perspective. J Exp Neurol. 335, 113494 (2021).
- Zuo, X., et al. Attenuation of secondary damage and Aβ deposits in the ipsilateral thalamus of dMCAO rats through reduction of cathepsin B by bis(propyl)-cognitin, a multifunctional dimer. Neuropharmacology. 162, 107786 (2020).
- Shabani, Z., Farhoudi, M., Rahbarghazi, R., Karimipour, M., Mehrad, H. Cellular, histological, and behavioral pathological alterations associated with the mouse model of photothrombotic ischemic stroke. J Chem Neuroanat. 130, 102261 (2023).
- Caleo, M. Rehabilitation and plasticity following stroke: Insights from rodent models. Neurociencias. 311, 180-194 (2015).
- Llovera, G., Roth, S., Plesnila, N., Veltkamp, R., Liesz, A. Modeling stroke in mice: permanent coagulation of the distal middle cerebral artery. J Vis Exp. (89), e51729 (2014).
- Lavayen, B. P., et al. Neuroprotection by the cannabidiol aminoquinone VCE-004.8 in experimental ischemic stroke in mice. Neurochem Int. 165, 105508 (2023).
- Hu, K., et al. Cathepsin B knockout confers significant brain protection in the mouse model of stroke. J Exp Neurol. 368, 114499 (2023).
- Yu, S. P., et al. Optochemogenetic stimulation of transplanted iPS-NPCs enhances neuronal repair and functional recovery after ischemic stroke. J Neurosci. 39 (33), 6571-6594 (2019).
- Lin, Y. H., et al. Opening a new time window for treatment of stroke by targeting HDAC2. J Neurosci. 37 (28), 6712-6728 (2017).
- Shi, X. F., Ai, H., Lu, W., Cai, F. SAT: Free software for the semi-automated analysis of rodent brain sections with 2,3,5-triphenyltetrazolium chloride staining. Front Neurosci. 13, 102 (2019).
- Jensen, E. C., et al. Quantitative analysis of histological staining and fluorescence using ImageJ. Anat Rec. 296 (3), 378-381 (2013).
- Donahue, J., Wintermark, M. Perfusion CT and acute stroke imaging: foundations, applications, and literature review. J Neuroradiol. 42 (1), 21-29 (2015).
- Sun, M., et al. Long-term L-3-n-butylphthalide pretreatment attenuates ischemic brain injury in mice with permanent distal middle cerebral artery occlusion through the Nrf2 pathway. Heliyon. 8 (7), e09909 (2022).
- Balkaya, M. G., Trueman, R. C., Boltze, J., Corbett, D., Jolkkonen, J. Behavioral outcome measures to improve experimental stroke research. Behav Brain Res. 352, 161-171 (2018).
- Hao, T., et al. Inflammatory mechanism of cerebral ischemia-reperfusion injury with treatment of stepharine in rats. Phytomedicine. 79, 153353 (2020).
- Pietrogrande, G., et al. Low oxygen post conditioning prevents thalamic secondary neuronal loss caused by excitotoxicity after cortical stroke. Sci Rep. 9 (1), 4841 (2019).
- Shi, X., et al. Stroke subtype-dependent synapse elimination by reactive gliosis in mice. Nat Commun. 12 (1), 6943 (2021).
- Chen, W. C., et al. Aryl hydrocarbon receptor modulates stroke-induced astrogliosis and neurogenesis in the adult mouse brain. JNeuroinflammation. 16 (1), 187 (2019).

