Measuring Dissolved Methane in Aquatic Ecosystems Using An Optical Spectroscopy Gas Analyzer
Summary
This study demonstrates an approach to measure methane gas concentrations in aqueous samples using portable optical analyzers coupled to an injection chamber in a closed loop. The results are similar to conventional gas chromatography, presenting a practical and low-cost alternative particularly suitable for remote field studies.
Abstract
Measuring greenhouse gas (GHG) fluxes and pools in ecosystems are becoming increasingly common in ecological studies due to their relevance to climate change. With it, the need for analytical platforms adaptable to measuring different pools and fluxes within research groups also grows. This study aims to develop a procedure to use portable optical spectroscopy-based gas analyzers, originally designed and marketed for gas flux measurements, to measure GHG concentrations in aqueous samples. The protocol involves the traditional headspace equilibration technique followed by the injection of a headspace gas subsample into a chamber connected through a closed loop to the inlet and outlet ports of the gas analyzer. The chamber is fabricated from a generic mason jar and simple laboratory supplies, and it is an ideal solution for samples that may require pre-injection dilution. Methane concentrations measured with the chamber are tightly correlated (r2 > 0.98) with concentrations determined separately through gas chromatography-flame ionization detection (GC-FID) on subsamples from the same vials. The procedure is particularly relevant for field studies in remote areas where chromatography equipment and supplies are not readily available, offering a practical, cheaper, and more efficient solution for measuring methane and other dissolved greenhouse gas concentrations in aquatic systems.
Introduction
Ecosystems at the terrestrial-aquatic interphase, like wetlands, lakes, reservoirs, rivers, and creeks, are important sinks and sources of greenhouse gases (GHG) like carbon dioxide (CO2), methane (CH4), and nitrous oxide (N2O)1,2. CH4, specifically, is produced during anaerobic respiration in the saturated pore spaces of sediment pores. Once it is produced, a fraction is oxidized and transformed to CO2, while the rest will eventually diffuse through the water column and vegetation or burst out into bubbles3. The concentration of CH4 in the water saturating the sediment pores (i.e., porewater) at a given time offers a glimpse into the balance between CH4 produced, consumed, and transported4. When measured over vertical profiles or time, porewater concentration also allows for identifying zones more active in CH4 production and consumption and their seasonal variation.
Traditionally, the methods to determine the concentration of GHG from porewater in ecosystems involve processing water samples collected in the field to equilibrate gases in a created headspace. Then, the headspace is analyzed through gas chromatography to determine the concentrations5. While this method is widely applied in ecological studies, it requires bench-top gas chromatography-flame ionization detection (GC-FID) systems that entail allocating conventional lab space and a high degree of expert knowledge to calibrate and operate (for example6). It also requires the use of specialized consumables, such as large tanks of carrier gasses (i.e., Nitrogen (N2) and Helium (He)), which are not readily available in remote locations. These requirements and the associated logistics of sample transport to the lab may constrain sampling design and, in some cases, limit the study's scope when chromatography equipment is unavailable.
This study aimed to develop an alternative method to measure dissolved greenhouse gas concentrations from headspace samples of aqueous solutions using portable optical spectroscopy-based gas analyzers. This type of optical gas analyzer is a cost-effective alternative to standard GC-FID systems, and its portability makes it an ideal choice for fieldwork applications. Portable optical spectroscopy-based gas analyzers produce high-frequency gas concentration measurements (i.e., ~ 1 s-1) with 2 – 5 s response times, depending on the brands and models. These instruments are designed and marketed primarily for determining gas fluxes from GHG-emitting surfaces like soils, water, and vegetation7,8,9. Optical analyzers allow flux calculation from continuous concentration measurements in non-steady state headspace chambers deployed over the emitting surfaces of interest. In their regular intended use with surface chambers, the high-frequency measurements of the rate of change in concentrations observed in the chamber and known chamber dimensions, pressure, and temperature allow for interpretation of those data as for the rate of emission (or uptake) per surface area (i.e., surface fluxes)10. However, portable gas analyzers are neither equipped nor optimized for dissolved concentrations in aqueous media, necessitating additional adaptations and interpretations for that type of analysis.
Leveraging previous demonstrations of the use of optical analyzers to determine concentrations in discrete samples from headspaces8, we designed a small, closed chamber (i.e., no emitting surfaces) that connects to the analyzer in a closed loop. The change in concentrations after the injection of the headspace gas subsample, followed by dilution calculations, allows for determining the original headspace's concentrations. The precision of this approach was evaluated by comparing its results to those obtained through GC-FID in the same samples. The method is further demonstrated through a use case that analyzed the vertical profiles of CH4 in porewater samples collected from experimental sites in a freshwater marsh in Louisiana.
Protocol
1. Porewater sampling and analysis
- Collect the samples using porewater dialysis samplers (peepers)11. Deploy the peepers in relevant locations of the study site. In the demonstration study, 6 peepers were deployed on the two dominant vegetation patches of a freshwater marsh: three on a patch dominated by Sagitaria lancifolia and the other three on a patch co-dominated by S. lancifolia and Typha latifolia vegetation.
- Collect samples 4x in June, September, October, and December. Collect samples at 10 different depths from the soil surface down to ~50 cm below it. The sampling followed the method described in12,13.
- Fill sampling cells with 61 mL of deionized water ~20 days before sampling. Bubble deionized water with N2 for 5 min in the lab to remove the oxygen before using it in the field.
- During sampling, withdraw 25 mL of water from the cell with a syringe connected to the cell's extraction line. Then, refill cells with fresh deionized water pre-bubbled with N2 through a secondary cell recharge line. If concentration measurements are required right after collection, continue to step 2.1.
- Add the extracted water to 10 mL glass vials preserved with 0.2 mL of 0.1 M HCl, cap with septum, and refrigerate until analysis in the laboratory.
NOTE: Users of this method are welcome to modify the collection depths, sampling schedule, and frequency based on specific study needs.
2. Greenhouse gas concentration measurement
- Perform headspace equilibration of porewater samples in a syringe
- Create headspace gas samples using the equilibration technique14. Use a 30 mL syringe to draw 5 mL of water (Vpw) from the samples collected in the field and then add 15 mL of N2 (Ultra High Purity 99.999%) to create the headspace (Vhs).
- Agitate the syringe vigorously and consistently for 5 min, manually or using a rocking shaker (if available). Inject 12 mL of headspace gas subsample into a 10 mL pre-evacuated vial used for gas concentration measurements with the optical analyzer in the following steps (Figure 1). Evacuate the vials with a peristaltic pump15 or manually pull the plunger of a 60 mL syringe on the closed vial and pump the air out 3x.
NOTE: He can be used instead of N2 to create the headspace. The procedure to add N2 or He to the syringe depends on the specific adaptation or regulator of the actual tank available to users. This addition typically involves coupling the syringe to the tank using matching tubing or extraction with the syringe from a sampling port adapted to the tank. Depending on site conditions and associated logistics, users may use ambient air to create the headspace.
- Create the injection chamber
- Manufacture an injection chamber that can accept a small volume of air (~1 – 5 mL) into a sealed volume of air that connects to the gas analyzer inflow and outflow to form a closed loop (Table of Materials). The injection chamber and the analyzer are the two main elements of the system (Figure 2).
- Modify the metallic lid of a 365 mL mason jar by drilling one 11 mm diameter hole to fit one septum as the injection port and two 7 mm diameter holes to insert stopcock valves that connect with the analyzer. Use epoxy glue to tighten the injection port and fittings and ensure chamber sealing.
- Connect the injection chamber to the optical analyzer
- Connect the jar with the inlet and outlet ports of the optical analyzer using 5/32 inch inner diameter (I.D.) and ¼ inch outer diameter (O.D.) PFA plastic tubing and account for their added volume. Ensure the tubing follows the instrument manufacturer's recommendation and is clean and dry without condensation.
- Set the valves that connect the injection chamber to the instrument to open to create a closed-loop air circuit. Wait for gas concentrations to stabilize.
- Inject a sample in the chamber
- When the concentration in the chamber and the signal in the analyzer have stabilized (i.e., standard deviation <0.03 ppm), inject 2 mL of a subsample from the vials containing the headspace sample created in step 2.1.2. Wait for concentrations in the analyzer to stabilize again before injecting the next subsample (Figure 3).
- Inject up to 20 subsamples consecutively or less if the concentration gets closer to 100 ppm (see8for effects on accuracy above this threshold). Analyze CH4 check standards for every 5 samples and evaluate the difference with actual measurements using the relative standard deviation (RSD).
- When done with the set of stacked injections, unplug one of the lines connected to the instrument to reset the chamber to ambient pressure and avoid significant pressure and methane concentration build-up.
NOTE: Based on multiple test runs, it was determined that the signal stabilized ~15 s after the injection, but different times may be required in response to other instrument recording specifications (Figure 3). The flow rate of the instrument used was ~1 L/min with a response time of 5 s and cavity pressure during the different analyses on different days ranging from 720 to 745 Torr.
- Calculate CH4 concentrations in the headspace
- For each subsample, calculate the concentration of the target GHG (e.g., CH4, CO2, N2O) in the headspace (Chs – µmol/mol) with equation 1:
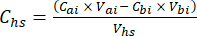 equation [1]
equation [1]
Where Vhs is the volume of the headspace subsample. - Calculate the concentrations before (Cbi – µmol mol-1) and after (Cai – µmol mol-1) injections as the mean of 15 measurements (~15 s) before the injection and 15 after the stabilization of the signal post-injection. Calculate the volumes in mL, before (Vbi) and after (Vai) injections with equations 2 and 3:
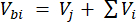 [2]
[2]
and
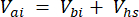 [3]
[3]
Where Vj (mL) is the added volume of the jar (365 mL) and hosing (125.4 mL), and Vi (mL) is the accumulated volume of the subsamples injected.
- For each subsample, calculate the concentration of the target GHG (e.g., CH4, CO2, N2O) in the headspace (Chs – µmol/mol) with equation 1:
- Calculate porewater concentration
- Calculate the partial pressure (Pi – MPa) of CO2, CH4, or N2O in the headspace using the mole fraction of gas in the headspace and the atmospheric pressure (P) during the processing of the sample using equation 4:
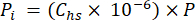 [4]
[4] - Calculate the aqueous equivalent concentration of GHGs in porewater using a dilution equation (equation 5) with headspace and aqueous equivalent concentrations and volumes:
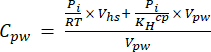 [5]
[5]
Where R is the universal gas constant (0.008314 L MPa mol−1 K−1), is the air temperature (K), Vhs is the volume of the headspace (mL), KHcp is Henry's volatility constant of CO2, CH4, or N2O (2.96, 67.13, and 3.82 L MPa/mol, respectively)16, and Vpw the volume of the liquid subsample used to create the headspace (mL). - Use recorded T and P during the measurements. The average T and P during the analysis of the demonstration project samples were 295.15 K and 0.101325 MPa, respectively.
- Calculate the partial pressure (Pi – MPa) of CO2, CH4, or N2O in the headspace using the mole fraction of gas in the headspace and the atmospheric pressure (P) during the processing of the sample using equation 4:
3. Validation against standard chromatography measurements
- Analyze different known CH4 concentrations in 10 mL vials in a conventional gas chromatograph. Use three identical groups of 20 standards each. The lowest concentrations ranged from 5 to 100 ppm and increased at 5 ppm increments.
- Determine CH4 concentrations by flame ionization detection on a gas chromatograph. The chromatograph used a porous polymer absorbent packed column with He (25 mL/min) as the carrier gas. To run the measurements of the standards' concentrations, calibrate the chromatograph to a curve fit (r2) > 0.99.
- After chromatography, proceed to determine CH4 concentration in the same standard vials using the method described above, using an optical analyzer calibrated by the manufacturer (i.e., precision (300 s, 1σ): 0.3 ppb CH4).
- Assess the accuracy of the method by fitting a linear regression to the chromatography and optical analyzer results of the three standard groups. Assess the replicability of the method by comparing the slopes and intercepts of regressions performed within each group of standards. Evaluate the comparison using a Standard Least Square Model with an emphasis on Effect Leverage. Evaluate all regressions and comparisons at a significance level of 0.05.
Representative Results
Optical analyzer versus gas chromatography
The results obtained through gas chromatography and the optical analyzer for the three groups of standards showed good linear fits (i.e., r2 > 0.98) with slopes close to one (Figure 4). The slopes of the regressions in the three experiments were statistically similar (F(2) = 0.478, p = 0.623), suggesting the reproducibility of the results. It is important to note that the slopes in all three cases were larger than one, indicating that, on average, one can anticipate a slight underestimation of the gas concentrations measured with the optical analyzer compared to concentrations obtained through chromatography. However, the intercepts at zero are different (F(1) = 1648.49, p <0.0001), which warns caution when interpreting low CH4 concentrations (i.e., <10 µmol/mol). In fact, at these lowest concentrations, the optical analyzer and chromatography showed the largest relative errors (Table 1), suggesting the challenges of measuring at low concentrations.
Field test: using the protocol to determine methane concentrations in porewater from two vegetation patches in coastal wetlands of Louisiana
The porewater CH4 concentrations determined from field samples show distinct vertical and temporal dynamics (Figure 5). These patterns are expected in wetland ecohydrological settings like the ones sampled, where diverse wetland vegetation can modulate CH4 production and oxidation and the resulting gas exchange with the atmosphere17,18. The RSD was ± 10.03%, less than the 15% typically used as a threshold in quality control and assurance (QC/QA) protocols employing chromatography on greenhouse gas analyses19.

Figure 1: Protocol overview. Schematic showing the main steps of the protocol. Please click here to view a larger version of this figure.
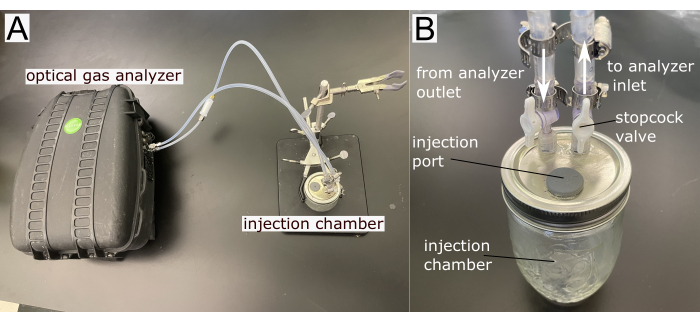
Figure 2: System components. (A) Main components and (B) a detailed view of the injection chamber. Please click here to view a larger version of this figure.
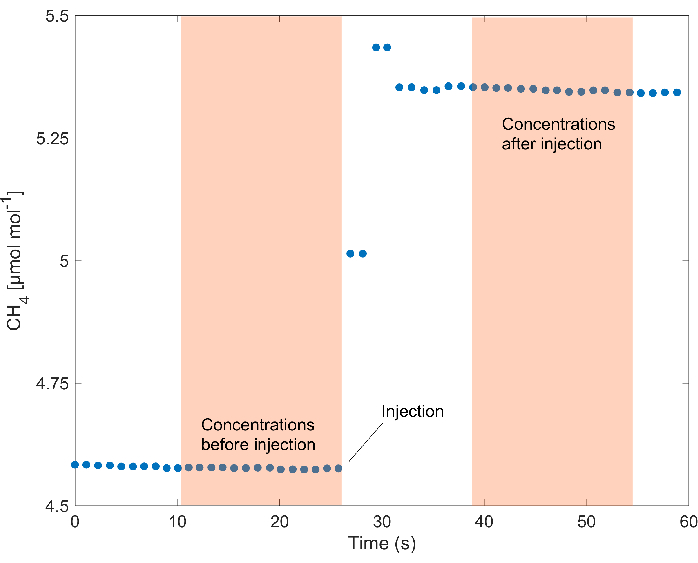
Figure 3: Concentrations during injections. CH4 concentrations in the injection chamber during a selected measurement show the typical response after the injection of the sample. The areas highlighted indicate the concentrations before and after the injection considered for the calculations. Please click here to view a larger version of this figure.
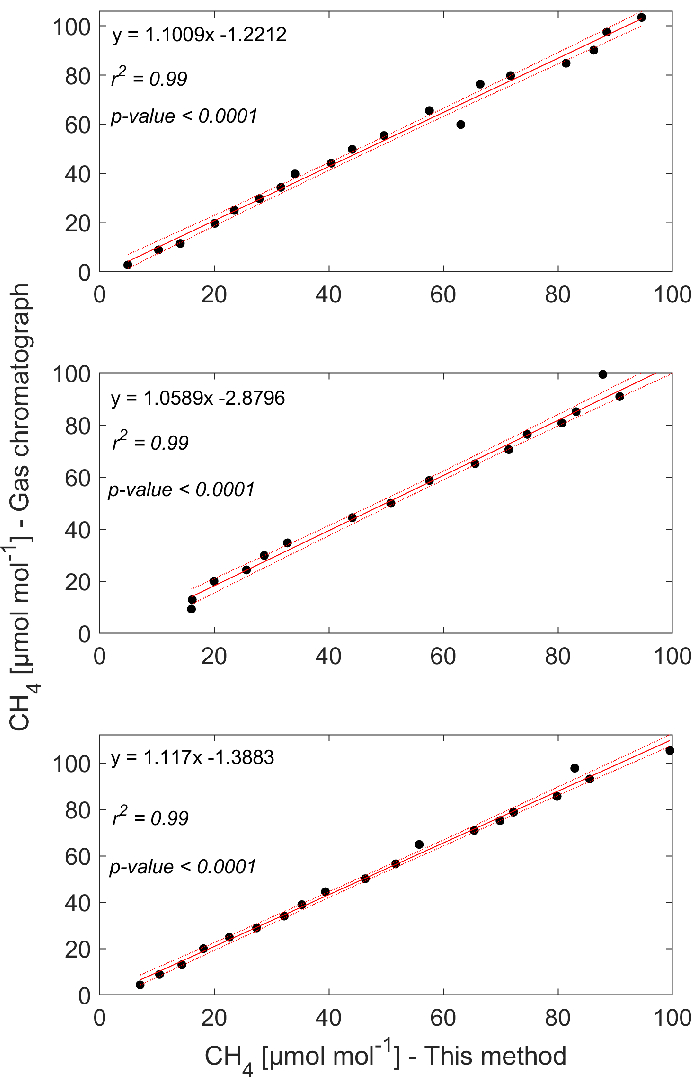
Figure 4: Comparison between measurements with this method and chromatography. The linear relationships between CH4 headspace concentrations determined with gas chromatography (GC-FID) and concentrations determined with this method for the three experiments had good linear fits (i.e., r2 > 0.98). Please click here to view a larger version of this figure.
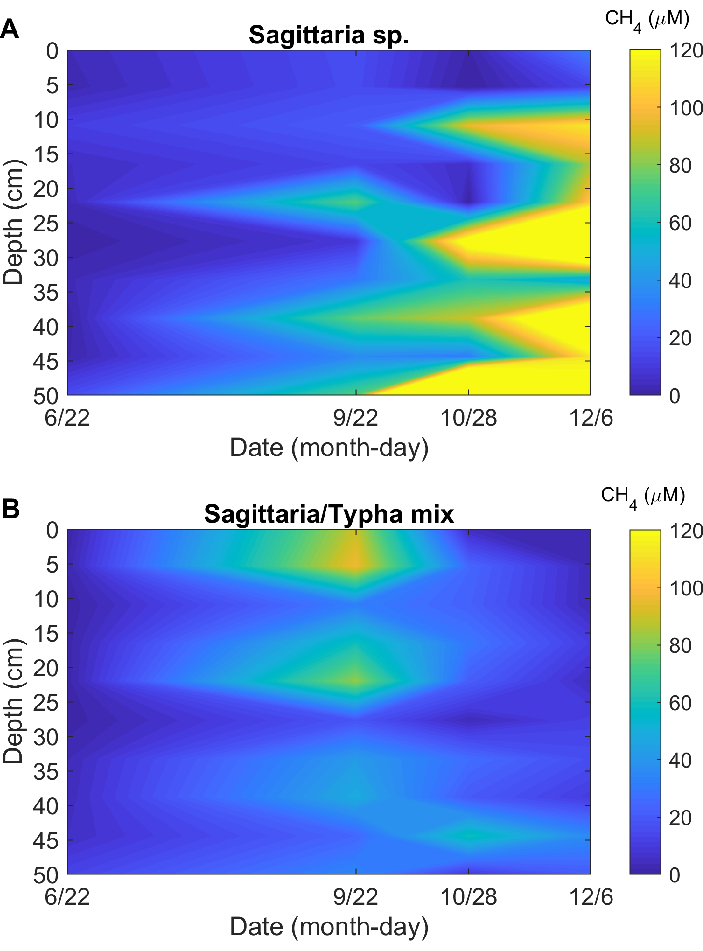
Figure 5: Use case demonstration. Porewater concentrations in patches dominated by (A) Sagittaria lancifolia, and (B) a mix of Sagittaria lancifolia and Typha latifolia. Please click here to view a larger version of this figure.
| CH4 standard [ppm] | GC [%] | Optical analyzer [%] |
| 5 | 24.3 | 22.8 |
| 10 | 9.6 | 3.4 |
| 15 | 16.7 | 6.1 |
| 20 | 0.6 | 3.4 |
| 25 | 1 | 6 |
| 30 | 1.5 | 6.7 |
| 35 | 1.7 | 8 |
| 40 | 1.3 | 13.3 |
| 45 | 1.4 | 8.2 |
| 50 | 0.2 | 6.9 |
| 55 | 1.4 | 7.9 |
| 60 | 1.6 | 4.6 |
| 65 | 0.4 | 8.8 |
| 70 | 2.4 | 4.3 |
| 75 | 1.4 | 6.3 |
| 80 | 0.9 | 7 |
| 85 | 0.4 | 4.1 |
| 90 | 1.6 | 3.3 |
| 95 | 3.4 | 9 |
| 100 | 4.2 | 3.3 |
Table 1: Relative error (%). Gas chromatography (GC) and optical analyzer measurements of the same check standard vial.
Discussion
This study demonstrated the applicability of portable optical spectroscopy-based gas analyzers coupled to a custom-made injection chamber to analyze headspaces created from water samples. The demonstration focused on CH4, but the protocol could be applied to analyzing other relevant GHGs like CO2 and N2O8. The goal was to expand on previous systematic assessments of these closed-loop systems that represent an alternative analytical platform to conventional gas chromatography in GHG concentration measurement studies.
Similar to gas chromatography, in this approach, it is critical to use check standards to ensure quality control and assessment in the analysis process. The standards analyzed with the method were comparable with those of standard chromatography (Figure 4). However, the main perceived advantages include the lack of carrier gases or frequent calibration routines. Users can also run samples immediately after sampling in the field, reducing the time allowed for microbial oxidation of reactive species like CH4 in water samples. Even when GHG-free gas to create the headspace needed in sample preparation is unavailable, users could still create headspaces using ambient air, assuming that the concentration in the water of interest is above that of air20. There are also perceived disadvantages, such as the time required to measure large sample sets. Although single sample analysis can take a similar time to that of GC-FID, there are no automated ways to conduct injections in optical analyzers, and more operation time is required for this approach than for more established GC-autosampler systems.
The main difference between this approach and other closed-loop systems with in-line injection ports (e.g.,8) is the addition of an injection chamber. In it, high-concentration samples can be diluted as part of the regular operation during analysis, avoiding additional steps to dilute the samples before their injection in the loop. In practice, the operator can proceed with injections from the headspace into the chamber instead of creating dilutions for samples with suspected high concentrations. The chamber volume can be modified and made larger to accommodate samples of high concentration or smaller for samples with low concentrations. Another advantage of this method compared to other closed-loop systems with in-line injections in terms of efficiency in analyzing multiple samples is the possibility of stack injections without flushing the chamber right after each injection. The dilution in the injection chamber allowed methane concentration in the loop to remain consistently below 100 ppm CH4 during the 20-stacked injection approach we tested, which previous work identified as the loop concentration threshold at which accuracy in CH4 analysis starts dropping3.
Overall, this method is suitable for sample analysis in remote locations or cases when chromatographs, including portable ones21, are not feasible or available. For example, scientists in field expeditions in remote locations will be able to determine actual GHG concentrations in water bodies and aqueous systems after some simple steps to create the headspace and make decisions regarding sampling priorities and needs in the spot instead of having to bring the samples back to a lab or field station equipped with chromatography.
Divulgaciones
The authors have nothing to disclose.
Acknowledgements
This work was funded through DOE awards DE-SC0021067, DE-SC0023084, and DE-SC0022972. The porewater concentration data of the sampled sites at the marsh is publicly available at ESS-DIVE Data Archive (https://data.ess-dive.lbl.gov/view/doi:10.15485/1997524 , accessed on June 21, 2024)
Materials
| 1/4 in. I.D. x 3/8 in. O.D. Clear Vinyl Tubing | Home Depot | SKU # 702098 | Use to couple stopcocks and tubing connected to the instrument. Two short pieces (~4 cm). |
| 5/16 – 5/8 in. Stainless Steel Hose Clamp | Everbilt | 6260294 | Use to secure tubing connecting the stopcock valves and tubing connected to the instrument. |
| Crack-Resistant Teflon PFA Semi-Clear Tube for chemicals, 5/32" ID, 1/4" OD | McMaster-Carr | 51805K86 | Use to connect the injection chamber to the inlet and outlet ports of the instrument. We used two 0.68 m-long tubing in our experiment. |
| Drill with titanium step drillbit | Multiple companies | Use to drill the holes for septum and stopcocks in the jar's metallic lid. | |
| Gay butyl septum (stopper) | Weathon Microliter | 20-0025-B | Use as injection port and as vial septum (if compatible). |
| Headspace vials 20ml (23x75mm), Clear, Crimp Rounded Bottom | Restek | 21162 | Use to store the headspace sample. |
| Heavy Duty Steel Bond Epoxy GorillaWeld | Gorilla | 4330101 | Use to glue stopcock valves and septum to the jar's metallic lid. |
| Hypodermic Needles | Air-Tite Products Co. | N221 | Use to extract water from field vials, inject heaspace sample in vial and inject subsample to the injection chamber. |
| Mason jar (12 oz) | Ball, Kerr, Jarden | Larger or smaller chamber volumes can be chosen depending on sample concentrations. | |
| Optical spectroscopy-based gas analyzer | Multiple companies | Picarro G4301, Licor 7810, Licor 7820, ABB GLA131-GGA | These are some specific examples of analyzers that could be coupled to the injection chamber. We recognize that it is not an extensive list and other optical spectroscopy analyzers may also be suitable for the method. |
| Stopcock valve | DWK Life Sciences | 420163-0001 | Keep the valves open during normal operation. |
| Syringe (2.5 mL) | Air-Tite Products Co. | R2 | Use to extract subsamples from the headspace vials and inject them in the injecion chamber for analysis. |
| Syringe (30 mL) | Air-Tite Products Co. | R30HJ | Use to create headspace for gas analysis. |
Referencias
- Bastviken, D., Tranvik, L. J., Downing, J. A., Crill, P. M., Enrich-Prast, A. Freshwater methane emissions offset the continental carbon sink. Science. 331 (6013), 50 (2011).
- Quick, A. M., et al. Nitrous oxide from streams and rivers: A review of primary biogeochemical pathways and environmental variables. Earth-Sci Rev. 191, 224-262 (2019).
- Bridgham, S. D., Cadillo-Quiroz, H., Keller, J. K., Zhuang, Q. Methane emissions from wetlands: biogeochemical, microbial, and modeling perspectives from local to global scales. Glob Chang Biol. 19 (5), 1325-1346 (2013).
- Keller, J. K., Wolf, A. A., Weisenhorn, P. B., Drake, B. G., Megonigal, J. P. Elevated CO2 affects porewater chemistry in a brackish marsh. Biogeochemistry. 96 (1), 101-117 (2009).
- McAuliffe, C. Gas chromatographic determination of solutes by multiple phase equilibrium. Chem Technol. 1, 46-51 (1971).
- Hoyos Ossa, D. E., Gallego Rios, S. E., Rodríguez Loaiza, D. C., Peñuela, G. A. Implementation of an analytical method for the simultaneous determination of greenhouse gases in a reservoir using FID/µECD gas chromatography. Int J Environ Anal Chem. 103 (12), 2915-2929 (2023).
- Kittler, F., et al. Long-term drainage reduces CO2 uptake and CH4 emissions in a Siberian permafrost ecosystem. Glob Biogeochem Cycles. 31 (12), 1704-1717 (2017).
- Wilkinson, J., Bors, C., Burgis, F., Lorke, A., Bodmer, P. Measuring CO2 and CH4 with a portable gas analyzer: Closed-loop operation, optimization and assessment. PLoS One. 13 (4), e0193973 (2018).
- Villa, J. A., et al. Ebullition dominates methane fluxes from the water surface across different ecohydrological patches in a temperate freshwater marsh at the end of the growing season. Sci Tot Environ. 767, 144498 (2021).
- Holland, E. A., et al. Soil CO2, N2O and CH4 exchange. Std Soil Meth Long-Term Ecol Res. , 185-201 (1999).
- MacDonald, L. H., Paull, J. S., Jaffé, P. R. Enhanced semipermanent dialysis samplers for long-term environmental monitoring in saturated sediments. Environ Monitor Assess. 185 (5), 3613-3624 (2013).
- Villa, J. A., et al. Plant-mediated methane transport in emergent and floating-leaved species of a temperate freshwater mineral-soil wetland. Limnol Oceanography. 65, 1635-1650 (2020).
- Villa, J. A., et al. Methane and nitrous oxide porewater concentrations and surface fluxes of a regulated river. Sci Tot Environ. 715, 136920 (2020).
- Kampbell, D. H., Wilson, J. T., Vandegrift, S. A. Dissolved oxygen and methane in water by a GC headspace equilibration technique. Int J Environ Anal Chem. 36 (4), 249-257 (1989).
- Rochette, P., Bertrand, N. Soil air sample storage and handling using polypropylene syringes and glass vials. Canadian J Soil Sci. 83 (5), 631-637 (2003).
- Sander, R. Compilation of Henry’s law constants (version 4.0) for water as solvent. Atmos Chem Phys. 15 (8), 4399-4981 (2015).
- Keller, J. K., Wolf, A. A., Weisenhorn, P. B., Drake, B. G., Megonigal, J. P. Elevated CO2 affects porewater chemistry in a brackish marsh. Biogeochemistry. 96 (1), 101-117 (2009).
- Comer-Warner, S. A., et al. Seasonal variability of sediment controls of carbon cycling in an agricultural stream. Sci Total Environ. 688, 732-741 (2019).
- . . Title 42 – The Public Health and Wefare. , (2009).
- Magen, C., et al. A simple headspace equilibration method for measuring dissolved methane. Limnol Oceanography Meth. 12 (9), 637-650 (2014).
- Regmi, B. P., Agah, M. Micro gas chromatography: An overview of critical components and their integration. Anal Chem. 90 (22), 13133-13150 (2018).

.
