Role of CD4 T Cell in Relapsing-Remitting Experimental Autoimmune Encephalomyelitis
Summary
An experimental autoimmune encephalomyelitis (EAE) model in mice was employed to investigate the role of CD4 T cells in the initial and relapse of EAE from the perspective of the activation phase and immune effect function.
Abstract
Multiple sclerosis (MS) is an autoimmune disease characterized by the infiltration of immune cells and demyelination in the central nervous system (CNS). Experimental autoimmune encephalomyelitis (EAE) serves as a prototypic animal model for studying MS. In this study, we aimed to investigate the role of CD4 T cells in the initiation and relapse of EAE, focusing on the activation phase and immune response. To create the EAE mice model, female mice were immunized with myelin oligodendrocyte glycoprotein (MOG)35-55 emulsified with complete Freund's adjuvant (CFA). Clinical scores were assessed daily, and results demonstrated that mice in the EAE group exhibited a classic relapsing-remitting pattern. Hematoxylin-eosin (H&E) and luxol fast blue (LFB) staining analysis revealed significant infiltration of inflammatory cells in the CNS and demyelination in EAE mice. Regarding the activation phase, both CD4+CD69+ effector T (Teff) cells and CD4+CD44+CD62L– effector memory T (Tem) cells may contribute to the initiation of EAE, however, the relapse stage was probably dominated by CD4+CD44+CD62L– Tem cells. Additionally, in terms of immune function, helper T (Th)1 cells are primarily involved in initiating the EAE. However, both Th1 and Th17 cells contribute to the relapse stage, and the immunosuppressive function of regulatory T (Treg) cells was inhibited during the EAE pathological process.
Introduction
Multiple sclerosis (MS) is an autoimmune disease that is characterized by the infiltration of the central nervous system (CNS) with immune cells and demyelination1,2. A recent study has shown it to be the most common disabling disease affecting young people, with its incidence continuously increasing worldwide3. Experimental autoimmune encephalomyelitis (EAE) is a prototypic animal model for MS that simulates many aspects of the inflammatory phase of the human MS4. From the perspective of immune functions, the initial CD4 T cells that have not yet encountered antigens are referred to as helper T(Th) 0 cells. These cells undergo maturation and activation processes to exhibit various functions. CD4 T cells can be categorized into several subsets according to their specific functions, which include Th1, Th2, regulatory T (Treg), follicular helper T (Tfh), Th17, Th9, and Th22 cells5. It is a common consensus that Th1 and Th17 cell subtypes are crucial pathogenesis factors of EAE6. Th1 cells could secrete interferon γ (IFN-γ), and Th17 cells secrete interleukin (IL)-17 and other inflammatory factors, which could activate other immune cells like microglial cells and astrocytes. These cells produce inflammatory cytokines7, such as IL-18, IL-12, IL-23, and IL-1β, which could further induce Th1/Th17 cells' immune response, resulting in progressive demyelination and axonal damage. From the activation phase perspective, CD4 T cells possess a predetermined destiny to develop into distinct cell subsets and differentiated states, including naive, effector, and memory T cells8. The initial CD4 T cells proliferate and differentiate into a variety of effector subsets with different functions in different environments9. Most effector cells are short-lived, with a small population of T cells developing into memory T cells that exhibit rapid effector functions when re-encountering the same antigens and provide the host with highly potent and long-term protection10,11.
Although current research suggests that CD4 T cells play a significant role in the development of EAE, it is still unclear how different CD4 T cell subsets, classified based on their activation phase and immune effect function, contribute to the initiation and relapse of EAE. In the present study, we established the EAE model in C57BL6/J mice by immunizing myelin oligodendrocyte glycoprotein (MOG)35-55 peptide and investigated the initiation and relapse of EAE, focusing on the activation phase and immune function of CD4 T cells.
Protocol
A total of 18 female C57BL/6J mice aged six to eight weeks were randomly divided into a control group (n = 6) and an EAE group (n = 12) for this study. Mice were purchased from the experiment animal center of Ningxia Medical University. Mice were housed in a temperature- and humidity-controlled room with a 12 h light/dark cycle, and free access to fresh water and food. Ethics approval was obtained from the Experimental Animal Ethics Committee of Ningxia Medical University before the study began.
1. Development of relapsing remitting EAE in C57BL/6 mice
- Reagent dissolution
- Dissolve 5.4 mg MOG35-55 (Table of Materials) in 1.8 mL of phosphate buffered saline (PBS) solution. Weigh 12.6 mg Mycobacterium tuberculosis H37RA (Table of Materials) and add it into 1.8 mL of complete Freund's adjuvant (CFA) solution (Table of Materials). Dissolve 360 µL of pertussis toxin (PTX; Table of Materials) into 7.2 mL of PBS using a pipette (Table of Materials).
- Preparation of the emulsion of MOG35-55 and CFA
- Pipette MOG35-55 solution and CFA solution using two 5 mL syringes prepared in step 1.1 and connect them with a tee valve. Mix continuously on ice for 1 h until a milky water-in-oil emulsion is obtained.
NOTE: The emulsion is successfully prepared when it is floating on the water surface without dispersing after dropping it into the water.
- Pipette MOG35-55 solution and CFA solution using two 5 mL syringes prepared in step 1.1 and connect them with a tee valve. Mix continuously on ice for 1 h until a milky water-in-oil emulsion is obtained.
- Mouse anesthetization
- Unscrew the filling seal cap at the front end of the anesthesia machine evaporator. Slowly pour 20 mL of isoflurane along the sealing screw in the center and lock the filling seal cap. Switch the tee valve switch to ensure that the airflow from the anesthesia machine evaporator is communicated with the anesthesia induction box (Table of Materials).
- Connect the air pump power supply, turn on the air pump switch, and rotate and adjust the air source valve at the front end of the oxygen flow meter so that the output gas flow is 400 mL/min. Open the evaporator, adjust the induction concentration to 3%, and wait for the anesthetic to fill the induction box.
- About 1 min later, put the mice into the induction box, then close the induction box, and wait for the mice to be in deep sleep, muscle relaxation, and steady breathing; then, the mice could be determined as the ideal state of anesthesia (Table of Materials).
NOTE: Switch the tee valve switch to ensure that the airflow from the anesthesia machine evaporator is communicated with the anesthesia mask until the mice are fully anesthetized.
- Mouse immunization
- Subcutaneously inject 200 µL of emulsion prepared in step 1.2 using a 1 mL syringe with the needle at four sites on the mouse's back, about 0.5 cm on both sides of the spine in the axilla and groin plane12 in the EAE group mice. Use 50 µL of emulsion at each site (Table of Materials).
- PTX injection
- Take the mice out of the cage by catching the tail and place it on the cage wire. Hold the tail of mice with the 4th and 5th fingers and gently pull it up to maintain the trend of the body and limbs forward. Grasp the head of the mice and capture the skin on the back of the mice using the middle and ring fingers.
- At 0 h and 48 h after immunization in step 1.4, inject 200 µL of PTX intraperitoneally at the position of 0.5 cm along either side of the midabdominal line with the penetration depth of 1-2 cm using 1 mL syringe with needle (Table of Materials).
NOTE: Before injecting PTX at 48 h after immunization of MOG35-55, it is necessary to anesthetize mice using the procedures described in step 1.3. The mice should be attended to until it has regained enough consciousness to maintain sternal recumbency. - For the mice in control group, treat with saline using the same method and assess the clinical score daily.
NOTE: Mice were assessed for signs of EAE according to the following scale13: 0 = no disease symptoms, 1 = complete paralysis of the tail, 2 = partial hind paralysis/weakness, 3 = complete hind limb paralysis, 4 = front and hind limb paralysis, and 5 = moribund state.
2. Histological staining analysis
- Brain collection
- Anesthetize the mice following step 1.3. Euthanize the mice by cervical dislocation and open the chest to expose the heart fully. Cut the auricula dextra and perfuse slowly with a pre-cooled saline solution using a 20 mL syringe with needle at the left ventricle until the liver becomes gray and white.
- Perfuse slowly with 4% paraformaldehyde until the tail tip turns up, the body becomes stiff, and the liver becomes tough. Decapitate the head, cut the skull, collect the brain using tweezers, and fix it in 4% paraformaldehyde solution for 24 h (Table of Materials).
- Preparation of paraffin sections
- Gradient dehydration: Immerse the brain tissues from step 2.1 successively in 70% ethanol for 1 h, 80% ethanol for 1 h, 95% ethanol for 1 h, anhydrous ethanol for 1 h, replace with fresh anhydrous ethanol, continue immersing for 1 h (Table of Materials).
- Transparency: Immerse the brain tissues from step 2.2.1 in xylene for 30 min, replace them with fresh xylene, and continue immersing until the tissues are transparent (Table of Materials).
- Embedding: Embed the brain tissues from step 2.2.2 in paraffin for 1 h, replace with fresh paraffin, continue embedding for 1 h, cut into 6 µm slices after mold release, place the slide in hot water, dry at 37 °C, store at room temperature (Table of Materials).
- Dewaxing: Immerse brain tissues from step 2.2.3 in xylene for 20 min, replace with fresh xylene, continue immersing for 20 min, place in anhydrous ethanol for 5 min, replace with fresh anhydrous ethanol, immerse for 5 min, 75% ethanol for 5 min, rinse with tap water (Table of Materials).
- Hematoxylin-eosin (H&E) staining: Stain tissues from step 2.2.4 with hematoxylin solution for 3-5 min and then rinse with tap water. Treat the tissues with a hematoxylin differentiation solution, briefly and rinse thoroughly with tap water. Treat the tissues with hematoxylin Scott tap bluing solution, and rinse with tap water. Immerse in 85% ethanol for 5 min and 95% ethanol for 5 min, and stain steps with eosin dye for 5 min (Table of Materials).
- Dehydrate: Immerse tissues from step 2.2.5 successively in anhydrous ethanol for 5 min, replace with fresh anhydrous ethanol, continue immersing for 5 min, replace with fresh anhydrous ethanol, immerse for 5 min, xylene for 5 min, replace with fresh xylene, immerse for 5 min, seal with neutral gum (Table of Materials).
- Observe using a microscope the brain tissues and perform image acquisition and analysis (Table of Materials).
3. LFB staining analysis
- Myelin sheath collection: Cut the mice spine from the head terminal up 2 segments to the tail terminal down 2 segments, blow out the spinal cord to the tail terminal, and fix the spinal cord in 4% paraformaldehyde solution for 24 h.
NOTE: The myelin sheath was harvested from the same mice in step 2.1, and the steps for heart perfusion are the same as in 2.1. - Paraffin sections deparaffinization and rehydration: Put the slides from step 3.1 successively into xylene for 20 min, replace with fresh xylene, continue immersing for 20 min, anhydrous ethanol for 5 min, replace with fresh anhydrous ethanol, immerse for 5 min, 75% alcohol for 5 min, wash with tap water.
- Fast blue staining: Place luxol fast blue staining solution A in a 60 °C oven and preheat for 30 min. Put the slides from step 3.2 in solution A and cover them with a plastic membrane for 1 h to prevent slices from drying out, then take out the slides and quickly wash them with tap water.
- Background differentiation: Put the slides from step 3.3 into luxol fast blue staining solution B for 2 s (while the dye is hot), and directly immerse in luxol fast blue staining solution C for differentiation for 15 s, wash with water until the myelin sheath is blue and the background is almost colorless.
- Eosin counter staining: Put the slides from step 3.4 in the oven at 65 °C for drying. Once done, remove them from the oven and allow them to cool. Put the slides into 95% ethanol and eosin counterstaining for 1 min.
- Dehydration and sealing: Put the slides from step 3.5 successively into anhydrous ethanol 5 min, replace with fresh anhydrous ethanol, continue immersing for 5 min, replace with fresh anhydrous ethanol again, continue immersing for 5 min, xylene 5 min, replace with fresh xylene, immerse for 5 min. Wash and dry the samples and seal them with neutral gum.
- Microscope examination: Collect and analyze images using an upright optical microscope (Table of Materials).
4. Flow cytometry analysis
- Open the abdominal cavity of mice. Extract the spleens using tweezers and mince into small pieces of 1.5-2.5 cm in PBS on ice using scissors. Grind the spleen in 10 mL of RPMI-1640 containing 2% fetal bovine serum (FBS) using a 2.5 mL syringe piston and push it through a 70 µm cell strainer to obtain single-cell suspension (Table of Materials).
NOTE: The spleens were harvested from the same mice in step 2.1. - Transfer the suspension obtained in step 4.1 into 15 mL tubes and lyse using 5 mL of red blood cell lysis buffer on ice for 10 min. Wash with RPMI-1640 containing 2% FBS, centrifuge at 350 x g for 5 min, discard the supernatant, and resuspend cells with RPMI-1640 containing 2% FBS.
- Count the cells with an automatic cell counter (Table of Materials) and adjust the concentration to 1 x 106 cells/mL.
- Pipette 100 µL of cell suspension into 1.5 mL tubes and incubate with 1 µL of anti-mouse CD16/32 antibody14 (0.5 mg/mL) for 30 min to block non-specific staining. Wash with RPMI-1640 and centrifuge at 350 x g for 5 min, discard the supernatant. Resuspend the cells in 100 µL of RPMI-1640 containing 2% FBS.
- Stain with antibodies specific for CD45 (Alexa Fluor 700), CD3 (FITC), CD4 (APC), and CD69 (BV674) for Teff cells15 for 30 min, 2 µL for each antibody. Stain with antibodies specific for CD45 (Alexa Fluor 700), CD3 (FITC), CD4 (APC), CD44 (BV421), and CD62L (PE) for Tem cells16 for 30 min, 2 µL for each antibody. Wash with RPMI-1640 and centrifuge at 350 x g for 5 min, discard the supernatant and resuspend cells in 350 µL of PBS. Analyze cell proportion using a flow cytometer and the associated software (Table of Materials).
5. Analysis of cytokines by cytometric bead array
- Use the cytometric bead array (Table of Materials) to measure IL-2, IL-4, IL-6, IL-10, IL-17A, tumor necrosis factor (TNF)-α and IFN-γ in mice brains, methods as described below.
- Collect mouse brain as described in step 2.1.
- Extraction of total protein: Cut and weigh 50 mg of brain tissue (three brains in the control group, EAE group in the peak stage, and RR stage). Place brains in 1.5 mL tubes and add 500 µL of lysis buffer containing PMSF, protease inhibitor, and phosphatase inhibitors.
- Cut brains into small pieces around 1 mm3, extract by ultrasonic homogenizer at 400 W, 5 s ON and 5 s OFF; repeat for 5x. Place the tube on ice for 10 min, centrifuge at 12000 x g for 5 min, and collect the supernatant (Table of Materials).
- Prepare mouse Th1/Th2/Th17 cytokine standards as described below.
- Open a vial of lyophilized mouse Th1/Th2/Th17 standards. Transfer the standards into a 15 mL conical polypropylene tube. Label the tube top as standard.
- Reconstitute the standards in 2.0 mL of assay diluent. Allow the reconstituted standard to equilibrate for 15 min at room temperature. Gently mix the reconstituted protein by pipetting.
- Label 12 mm x 75 mm tubes and arrange them in the following order of dilution: 1:2, 1:4, 1:8, 1:16, 1:32, 1:64, 1:128, and 1:256. Pipette 300 µL of assay diluent in each labeled tube.
- Perform serial dilutions: Transfer 300 µL of the top standard to the 1:2 dilution tube and mix thoroughly by pipetting. Continue serial dilutions by transferring 300 µL from the 1:2 tube to the 1:4 tube and so on till the 1:256 tube. Prepare a 12 mm x 75 mm tube containing only the assay diluent to serve as the 0 pg/mL negative control.
NOTE: Mix thoroughly by pipetting. Do not vortex to prevent the antibody coupling on the microbeads from falling off. - To mix the mouse Th1/Th2/Th17 cytokine capture beads, prepare 19 tubes, including standards with 3 samples in each group (control group, EAE group in peak stage, and RR stage) for the experiment. Vortex the capture bead suspension vigorously for 5 s before mixing. Add 190 µL of each capture bead into a single tube and label it as mixed capture beads. Vortex the bead mixture thoroughly.
NOTE: The antibody-conjugated beads will settle out of suspension over time. It is necessary to vortex the vial before taking a bead-suspension aliquot. - To perform the assay, vortex the mix capture beads. Add 50 µL of beads to all assay tubes. Add 50 µL of mouse Th1/Th2/Th17 cytokine standard dilutions to the control tubes as in Table 1.
- Add 50 µL of the mouse Th1/Th2/Th17 PE detection reagent to all assay tubes. Incubate the assay tubes for 2 h at room temperature and protect them from light. Add 1 mL of wash buffer to each assay tube and centrifuge at 200 x g for 5 min. Aspirate and discard the supernatant from each assay tube carefully.
- Add 300 µL of wash buffer to each assay tube to resuspend the bead pellet. Analyze mouse Th1/Th2/Th17 cytokine data using FCAP Array software (Table of Materials).
Representative Results
Clinical scores assessment
As shown in Figure 1, the mice in the control group did not exhibit any clinical symptoms. The mice in the EAE group, which were immunized with MOG35-55, showed tail paralysis approximately 12 days after immunization. By day 16, the symptoms reached complete hind limb paralysis (defined as peak stage, Peak). After that, the symptoms gradually were remitted. Mice's clinical symptoms were aggravated at around day 25 and reached complete hind limb paralysis at approximately day 30 (defined as a relapsing stage, RR). Notably, the symptoms lasted longer than the previous time, which developed a classical relapsing-remitting feature. The principal manifestation of relapsing-remitting EAE mice experience different stages of outbreak, remission, and relapse during the progression of the disease, which have a certain periodicity and fluctuation.
Histological analysis
The control group mice displayed intact brain tissue structure with evenly distributed and clearly defined blood vessels, without any inflammatory cell infiltration. (Figure 2A). At the peak stage of the EAE group mice, a significant infiltration of inflammatory cells was observed in the brain (Figure 2B), forming cuff-like changes around blood vessels (indicated by red arrow). Similarly, in EAE group mice at the RR stage, there was evident infiltration of inflammatory cells (Figure 2C), with a predominant aggregation around blood vessels (indicated by red arrow).
LFB staining analysis
In the control group, the myelin sheath of the spinal white matter appeared blue, indicating that its structure was intact and tightly packed (Figure 3A). At the peak stage of the EAE group, the spinal white matter exhibited a light blue or white color; the structure appeared to be loose and discontinuous, with a significant presence of vacuolar degenerations indicated by a red arrow (Figure 3B). In the RR stage of the EAE model group, extensive loss of white matter myelin in the spinal cord was observed. The structure appeared to be loose, and a significant number of vacuolar degenerations could be observed (indicated by red arrows; Figure 3C).
Flow cytometry assay
The results depicted in Figure 4A,B demonstrated a significant increase in the proportion of CD4+CD69+ Teff cells in EAE mice at the peak stage when compared to the control group (P < 0.0001). Additionally, the proportion of these cells also increased during the RR stage, although the difference was not statistically significant (P > 0.05). Figure 4C,D revealed a notable rise in the proportion of CD4+CD44+CD62L– Tem cells in EAE mice at both the peak and RR stages, as compared to the control group (P < 0.0001).
Cytokine measured by cytometric bead array
The results depicted in Figure 5 demonstrate that, when compared to the control group, the levels of IL-4 (P < 0.0001), IL-6 (P < 0.001), IFN-γ (P < 0.0001), and TNF-α (P < 0.0001) were significantly elevated in the EAE group at the peak stage. While the expression of IL-17A also increased, the difference was not statistically significant (P > 0.05). Moreover, the level of IL-2 exhibited no change during the peak stage of EAE mice (P > 0.05); however, it significantly increased during the RR stage (P < 0.01). Furthermore, both TNF-α (P < 0.001) and IL-17A (P < 0.01) expressions in the RR stage were significantly higher than those observed in the control group. Conversely, the expression of IL-10 in the EAE group was significantly lower than that in the control group, both at the peak and RR stages (P < 0.0001).
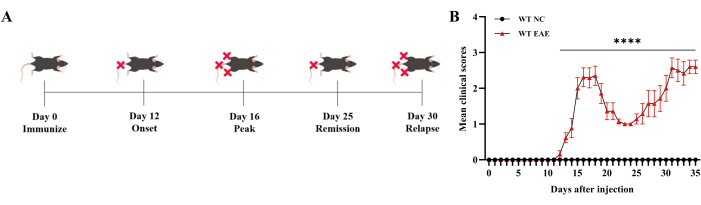
Figure 1: Clinical score assessment. The clinical score was assessed daily and shows that mice developed a classical relapsing-remitting EAE immunized by MOG35-55. (A) The diagram of symptom progression of the EAE mice. (B) The assessment of EAE mice through mean clinical scores. Data are expressed as mean ± SEM, n = 6. The data were analyzed using one-way ANOVA with post-hoc comparisons to assess statistically significant differences. * P < 0.05, ** P < 0.01, *** P < 0.001, **** P < 0.0001. Please click here to view a larger version of this figure.
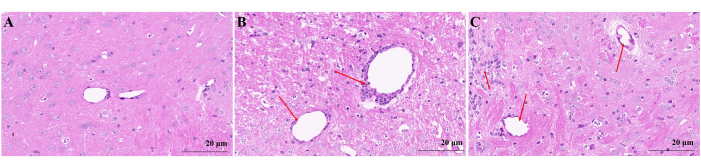
Figure 2: Microscopic map of brain H&E staining. (A) Mice brain in the control group. (B) EAE mice brain in peak stage. Inflammatory cells were infiltrated in the brain at the peak stage in EAE group mice and distributed around blood vessels to form cuff-like change (red arrow). (C) EAE mice brain in RR stage. Inflammatory cells in EAE group mice of RR stage aggregated around blood vessels (red arrow). Please click here to view a larger version of this figure.
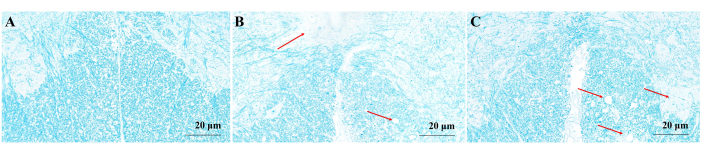
Figure 3: Microscopic map of myelin sheath LFB staining. (A) Mice myelin sheath in the control group. (B) EAE mice myelin sheath in peak stage. The spinal white matter was light blue or white, the structure was loose and discontinuous, and a large number of vacuolar degenerations appeared (red arrow). (C) EAE mice myelin sheath in RR stage. The white matter myelin of the spinal cord was widely lost, the structure was loose, and a large number of vacuolar degenerations appeared (red arrow). Please click here to view a larger version of this figure.
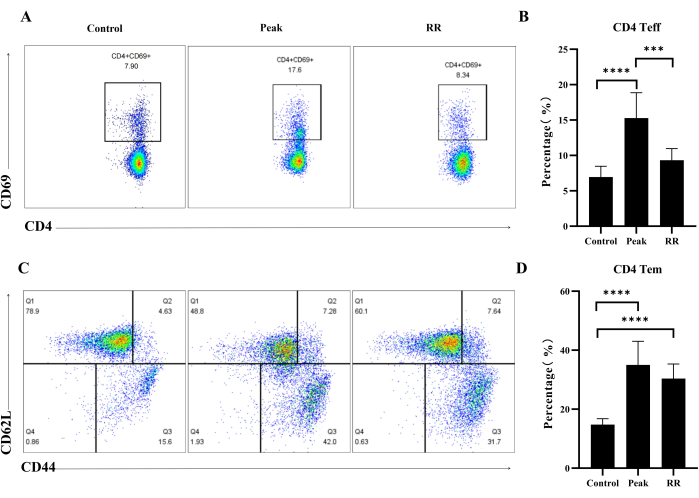
Figure 4: Immunization by MOG35-55 induced the up-regulation of CD4 Teff and Tem cells proportion in both peak and RR stage in EAE mice. (A) Illustrative flow plot displaying the immunostaining of CD4+CD69+ cells. (B) Percentage of CD4+CD69+ Teff cells. (C) Illustrative flow plot displaying the immunostaining of CD44+CD62L+ cells (gate on CD4 T cells). (D) Percentage of CD44+CD62L+ Tm cells. Data are expressed as mean ± SEM, n = 5. The data were analyzed using one-way ANOVA with post-hoc comparisons to assess statistically significant differences. * P < 0.05, ** P < 0.01, *** P < 0.001, **** P < 0.0001. Please click here to view a larger version of this figure.
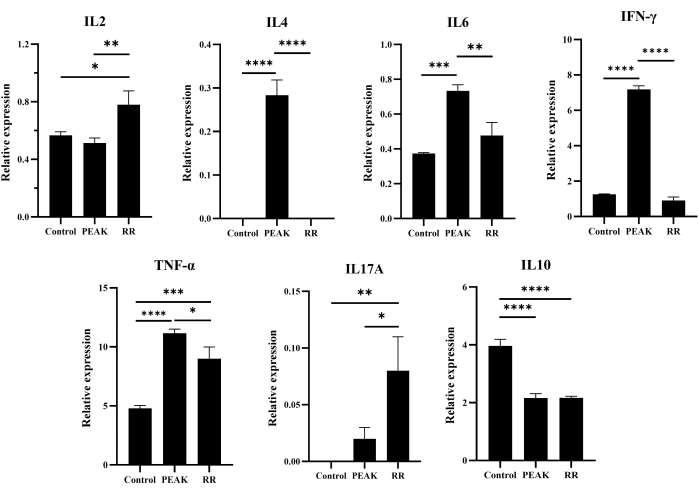
Figure 5: Level of cytokines in mice brain analyzed by cytometric bead array. Data are expressed as mean ± SEM, n = 3. The data were analyzed using one-way ANOVA with post-hoc comparisons to assess statistically significant differences. * P < 0.05, ** P < 0.01, *** P < 0.001, **** P < 0.0001. Please click here to view a larger version of this figure.
| Tube label | Concentration(pg/mL) | Cytokine Standard dilution |
| 1 | 0 (negative control) | no standard dilution(Assay Diluent only) |
| 2 | 20 | 1:256 |
| 3 | 40 | 1:128 |
| 4 | 80 | 1:64 |
| 5 | 156 | 1:32 |
| 6 | 312.6 | 1:16 |
| 7 | 625 | 1:8 |
| 8 | 1250 | 1:4 |
| 9 | 2500 | 1:2 |
| 10 | 5000 | Top standard |
Table 1: List of cytokine standard dilutions to the control tubes.
Discussion
MS is the most prevalent autoimmune demyelinating disease of the CNS, which affects millions of people worldwide17. EAE is the typical animal model for simulating the clinical pathological features of MS18. Studies have demonstrated that individuals with MS experience cognitive impairment and other disabilities due to the degeneration of axons and neurons in the CNS19,20,21. At the peak and RR stage of the EAE model, mice exhibited paralysis in their tails and hindlimbs. Additionally, inflammatory cells were observed to infiltrate into the CNS, leading to varying degrees of myelin sheath loss. These findings indicated the successful establishment of the EAE model in this experiment.
The emulsification process of MOG35-55 and CFA needs to be conducted on ice to prevent the degradation of MOG35-55 caused by heat generation during the homogenization process. PTX is administered intraperitoneally to enhance vascular permeability, allowing T cells to easily cross the blood-brain barrier and reach the myelin sheath surrounding neurons22. What calls for special attention is that in cases where mice become paralyzed and cannot stand to eat or drink by themselves, it is important to provide food and water trays in their cage or manually feed them to prevent excessive thirst and hunger.
Flow cytometry was used to analyze the proportion of CD4+CD69+ Teff cells and CD4+CD44+CD62L– Tem cells. The proportion of CD4+CD69+ Teff cells in the spleen of EAE mice was remarkably up regulated in the peak stage. Additionally, it was higher than that of the control group in the relapse stage, although this difference did not reach statistical significance. On the other hand, the proportion of CD4+CD44+CD62L– Tem cells were significantly higher in the EAE mice compared to the control group at both the peak and RR stage. This suggested that the initiation of EAE might involve both CD4+CD69+ Teff and CD4+CD44+CD62L– Tem cells in the peak stage, while the RR stage is perhaps primarily dominated by CD4+CD44+CD62L– Tem cells. CD69 is the earliest membrane surface molecule expressed during lymphocyte activation. It plays a crucial role in modulating the adhesion and migration of T cells, thereby aiding immune cells in identifying and localizing infection or inflammation sites within the body23. Resting T cells do not express CD69, but when they are stimulated and activated by anti-CD3/TCR activators can be inductively expressed24. After the effector stage, a significant portion of T cells undergo apoptosis since they are unable to differentiate any further. This reduction in numbers is primarily caused by the absence of pro-survival cytokines, including IL-2, which are produced during antigen stimulation. Additionally, the downregulation of receptors for these cytokines further contributes to this clonal contraction phenomenon25. In addition, clearance of the infectious agent through an adaptive immune response leads to clonal contraction of T cells, ultimately leaving fewer long-lived memory cells to provide immune response to the host in the event of reinfection with the pathogen26. In the present study, the proportion of CD4+CD69+ T cells was remarkably elevated at peak stage however declined at the RR stage, which indicated that CD4 Teff cells are unable to survive for a extent period and ultimately undergo apoptosis, only a small fraction of antigen-specific T cells develop into long-lived memory T cells. Tem cells have a low expression of CD62L. However, they are capable of expressing some chemokine receptors and adhesion molecules that facilitate their migration to inflamed tissues. After antigen reactivation, the proliferation rate is relatively slow, but cytokines are rapidly released to initiate effector function27. In the present study, the antigens in mice released from the emulsion and attacked the immune system again, Tem cells can be reactivated and release a large number of cytokines, such as IL-6 and IFN-γ, which may result in the relapse of EAE mice.
The primary pro-inflammatory CD4 T cell populations associated with autoimmune diseases (including MS) are Th1 cells that secrete IL-2, IL-6, IFN-γ, TNF-α, and Th17 cells that secrete IL-17. It has been demonstrated that Th1 cell-associated IFN-γ can have a direct detrimental effect on oligodendrocytes, resulting in damage to the patient's CNS neurons28. Th17 cells also play a significant role in several autoimmune diseases, including MS. Research has observed elevated levels of Th17 cells and IL-17 mRNA in brain lesions of MS patients when compared to individuals without the disease29. Treg cells, which are characterized by the production of IL-10 and TGF-β30, have an immunosuppressive effect and play a crucial role in maintaining immune system tolerance to self-components, thereby enabling the body to maintain immune homeostasis. Here we observed significant up-regulation of IL-4, IL-6, IFN-γ, and TNF-α expression in the EAE group at the peak stage. Additionally, the expression of IL-17A was also increased, although there was no significance. In contrast, the expression levels of TNF-α and IL-17A in the RR stage were significantly higher compared to the control group. This suggests that the peak stage of EAE is primarily driven by Th1 cells rather than Th17 cells, whereas the RR stage of EAE was initiated by both the Th1 and Th17 cells. It is worth noting that the expression of IL-10 in the EAE group at both the peak and RR period was markedly lower than that in the control group, suggesting that the immunosuppressive function of Treg cells was hindered during the pathological procession of EAE.
In conclusion, our studies suggest the successful establishment of a mice relapsing-remitting EAE model through immunization with MOG35-55 peptide. From the perspective of active phase, the peak stage of EAE was probably initiated by CD4+CD69+ Teff and CD4+CD44+CD62L– Tem cells, while the relapse of EAE RR stage might be mainly dominated by CD4+CD44+CD62L– Tem cells. From the perspective of immune effect function, the peak stage of EAE was mainly initiated not by Th17 cells but by Th1 cells. However, the RR stage of EAE was initiated by Th1 and Th17 cells. The immunosuppressive function of Treg cells was inhibited during the EAE pathological process. Nevertheless, the molecular mechanisms underlying the activation of Th1/Th17 cells and the suppression of Treg cells require further investigation.
Divulgaciones
The authors have nothing to disclose.
Acknowledgements
This work was supported by the Natural Science Foundation of Ningxia (2022AAC03601 and 2023AAC02087) and Research Foundation of Ningxia Medical University (XM2019052). Thanks for the support of the Medical Science and Technology Research Center of Ningxia Medical University.
Materials
| Anesthesia machine evaporator | Norvap | 20-17368 | |
| Isoflurane | Sigma-Aldrich (Shanghai) Trading Co.Ltd. | 792632 | |
| 70 μm cell strainer | XIYAN Co.,Ltd. | 15-1070 | |
| Alexa Fluor 700 anti-mouse CD45 Antibody | Biolegend | 103128 | |
| APC anti-mouse CD4 Antibody | Biolegend | 100616 | |
| Automatic cell counter | Jiangsu JIMBIO technology Co., LTD | JIMBIO iCyta S2 | |
| BD* Cytometric Bead Array (CBA) Mouse Th1/Th2/Th17 CBA Kit | BD Biosciences | 560485 | |
| Biotin anti-mouse CD16/32 Antibody | Biolegend | 101303 | |
| Brilliant Violet 510 anti-mouse CD69 Antibody | Biolegend | 104532 | |
| BV786 Rat Anti-Mouse CD62L(MEL-14) | BD Pharmingen | 563109 | |
| Column Tissue&Cell Protein Extraction Kit | Shanghai Epizyme Biomedical Technology Co., Ltd | PC201Plus | |
| Complete Freund's Adjuvant | Sigma-Aldrich (Shanghai) Trading Co.Ltd. | SLCH4887 | |
| Dehydrator | DIAPATH | Donatello | |
| Disposable sterilized syringe (1 mL) | Yikang Group | 210820 | |
| Disposable sterilized syringe (2.5 mL) | Yikang Group | 210820 | |
| Disposable sterilized syringe (5 mL) | Yikang Group | 210820 | |
| Dyeing machine | DIAPATH | Giotto | |
| Embedding machine | Wuhan Junjie Electronics Co., Ltd | JB-P5 | |
| Ethanol | SCRC | 100092683 | |
| Fetal Bovine Serum | Procell Life Science&Technology Co.,Ltd. | FSP500 | |
| FITC Hamster Anti-Mouse CD3e(145-2C11) | BD Pharmingen | 553062 | |
| Flow cytometer | Becton,Dickinson and Company | FACSCelesta | |
| Flow cytometer | Becton,Dickinson and Company | Accuri C6 | |
| Flow Jo software | BD Biosciences | 10.8.1 | |
| Frozen platform | Wuhan Junjie Electronics Co., Ltd | JB-L5 | |
| Glass slide | Servicebio | G6004 | |
| HE dye solution set | Servicebio | G1003 | |
| Hematoxylin-Eosin solution | Servicebio | G1002 | |
| High speed refrigerated centrifuge | Thermo Fisher Scientific | Mogafugo8R | |
| Imaging system | Nikon | NIKON DS-U3 | |
| Luxol fast blue staining kit | Servicebio | G1030 | |
| Ms CD44 BV421 IM7 | BD Pharmingen | 564970 | |
| Mycobacterium tuberculosis H37RA | BD Pharmingen | 231141 | |
| Myelin oligodendrocyte glycoprotein (MOG35-55) | AnaSpec | AS-60130-1 | |
| Neutral gum | SCRC | 10004160 | |
| Organizer | KEDEE | KD-P | |
| Oven | Labotery | GFL-230 | |
| Pathology slicer | Leica | RM2016 | |
| Pertussis Toxin from Bordetella pertussis | Sigma-Aldrich (Shanghai) Trading Co.Ltd. | P7208 | |
| Phosphate buffered saline | Servicebio | G4202 | |
| Pipette 0.5-10 μL | DLAB Scientific | 7010101004 | |
| Pipette 100-1000 μL | DLAB Scientific | 7010101014 | |
| Pipette 20-200 μL | DLAB Scientific | 7010101009 | |
| RPMI-1640 | Procell Life Science&Technology Co.,Ltd. | PM150110 | |
| Tee valve | Guangdong Kanghua Medical Co., LTD | A06 | |
| Tissue spreader | Zhejiang Kehua Instrument Co.,Ltd | KD-P | |
| Upright optical microscope | Nikon | NIKON ECLIPSE E100 | |
| Xylene | SCRC | 10023418 |
Referencias
- Swanberg, K., et al. Multiple sclerosis diagnosis and phenotype identification by multivariate classification of in vivo frontal cortex metabolite profiles. Sci Rep. 12 (1), 13888 (2022).
- Zahoor, I., et al. An emerging potential of metabolomics in multiple sclerosis: a comprehensive overview. Cell Mol Life Sci. 78 (7), 3181-3203 (2021).
- Vitturi, B., et al. Spatial and temporal distribution of the prevalence of unemployment and early retirement in people with multiple sclerosis: A systematic review with meta-analysis. PloS one. 17 (7), 0272156 (2022).
- Brambilla, R. The contribution of astrocytes to the neuroinflammatory response in multiple sclerosis and experimental autoimmune encephalomyelitis. Acta neuropathologica. 137 (5), 757-783 (2019).
- Niedźwiedzka-Rystwej, P., Tokarz-Deptuła, B., Deptuła, W. Characteristics of T lymphocyte subpopulations. Postepy Hig Med Dosw. 67, 371-379 (2013).
- Loos, J., et al. Functional characteristics of Th1, Th17, and ex-Th17 cells in EAE revealed by intravital two-photon microscopy. J neuroinflammation. 17 (1), 357 (2020).
- Prajeeth, C., et al. Effectors of Th1 and Th17 cells act on astrocytes and augment their neuroinflammatory properties. J neuroinflammation. 14 (1), 204 (2017).
- Nielsen Birgitte, R., et al. Characterization of naïve, memory and effector T cells in progressive multiple sclerosis. J Neuroimmunol. 310, 17-25 (2017).
- Xie, L., et al. The role of CD4+ T cells in tumor and chronic viral immune responses. Med Comm. 4 (5), e390 (2023).
- Sun, L., et al. T cells in health and disease. Signal Transduct Target Ther. 8 (1), 235 (2023).
- Kawabe, T., et al. Memory-phenotype CD4+ T cells spontaneously generated under steady-state conditions exert innate TH1-like effector function. Sci Immunol. 2 (12), (2017).
- Moon, J., et al. A study of experimental autoimmune encephalomyelitis in dogs as a disease model for canine necrotizing encephalitis. J Vet Sci. 16 (2), 203-211 (2015).
- Pérez-Nievas, B., et al. Chronic immobilisation stress ameliorates clinical score and neuroinflammation in a MOG-induced EAE in Dark Agouti rats: mechanisms implicated. J neuroinflammation. 7, 60 (2010).
- Zheng, J., et al. Prostaglandin D2 signaling in dendritic cells is critical for the development of EAE. J Autoimmun. 114, 102508 (2020).
- Adamczyk, M., et al. The expression of activation markers CD25 and CD69 increases during biologic treatment of Psoriasis. J Clin Med. 12 (20), 6573 (2023).
- Meng, R., et al. Echinococcus multilocularisIndoleamine 2,3-dioxygenase 1 signaling orchestrates immune tolerance in -infected mice. Front Immunol. 13, 1032280 (2022).
- Sheinin, M., et al. Suppression of experimental autoimmune Encephalomyelitis in mice by β-Hydroxy β-Methylbutyrate, a body-building supplement in humans. J Immunol. 211 (2), 187-198 (2023).
- Ghareghani, M., et al. Hormones in experimental autoimmune encephalomyelitis (EAE) animal models. Transl Neurosci. 12 (1), 164-189 (2021).
- Pérez-Miralles, F., et al. Clinical impact of early brain atrophy in clinically isolated syndromes. Mult Scler. 19 (14), 1878-1886 (2013).
- Nygaard, G., et al. Cortical thickness and surface area relate to specific symptoms in early relapsing-remitting multiple sclerosis. Mult Scler. 21 (4), 402-414 (2015).
- Biberacher, V., et al. Atrophy and structural variability of the upper cervical cord in early multiple sclerosis. Mult Scler. 21 (7), 875-884 (2015).
- Ma, Y., et al. Epsilon toxin-producing Clostridium perfringens colonize the multiple sclerosis gut microbiome overcoming CNS immune privilege. J Clin Invest. 133 (9), e163239 (2023).
- Cibrián, D., Sánchez, M. CD69: from activation marker to metabolic gatekeeper. Eur J Immunol. 47 (6), 946-953 (2017).
- Clarkson Benjamin, D., et al. Preservation of antigen-specific responses in cryopreserved CD4 and CD8 T cells expanded with IL-2 and IL-7. J Transl Autoimmun. 5, 100173 (2022).
- Raeber Miro, E., et al. The role of cytokines in T-cell memory in health and disease. Immunol Rev. 283 (1), 176-193 (2018).
- Ville, S., et al. Co-stimulatory blockade of the CD28/CD80-86/CTLA-4 balance in transplantation: Impact on memory T cells. Front Immunol. 6, 411 (2015).
- Natalini, A., et al. OMIP-079: Cell cycle of CD4+ and CD8+ naïve/memory T cell subsets, and of Treg cells from mouse spleen. Cytometry A. 99 (12), 1171-1175 (2021).
- Joffre, O., et al. Cross-presentation by dendritic cells. Nat Rev Immunol. 12 (8), 557-569 (2012).
- Montes, M., et al. Oligoclonal myelin-reactive T-cell infiltrates derived from multiple sclerosis lesions are enriched in Th17 cells. Clin Immunol. 130 (2), 133-144 (2009).
- Feruglio, S., Kvale, D., Dyrhol, R. T. Cell responses and regulation and the impact of in vitro IL-10 and TGF-β modulation during treatment of active tuberculosis. Scand J Immunol. 85 (2), 138-146 (2017).

.
