Tea Aroma Analysis Based on Solvent-Assisted Flavor Evaporation Enrichment
Summary
Presented here is a method for enriching and analyzing the volatile components of tea extracts using solvent-assisted flavor evaporation and solvent extraction followed by gas chromatography-mass spectrometry, which can be applied to all types of tea samples.
Abstract
Tea aroma is an important factor in tea quality, but it is challenging to analyze due to the complexity, low concentration, diversity, and lability of the volatile components of tea extract. This study presents a method for obtaining and analyzing the volatile components of tea extract with odor preservation using solvent-assisted flavor evaporation (SAFE) and solvent extraction followed by gas chromatography-mass spectrometry (GC-MS). SAFE is a high-vacuum distillation technique that can isolate volatile compounds from complex food matrices without any non-volatile interference. A complete step-by-step procedure for tea aroma analysis is presented in this article, including the tea infusion preparation, solvent extraction, SAFE distillation, extract concentration, and analysis by GC-MS. This procedure was applied to two tea samples (green tea and black tea), and qualitative as well as quantitative results on the volatile composition of the tea samples were obtained. This method can not only be used for the aroma analysis of various types of tea samples but also for molecular sensory studies on them.
Introduction
Tea is a preferred beverage of many people all over the world1,2. The aroma of the tea is a quality criterion as well as a price-determining factor for tea leaves3,4. Thus, the analysis of the aroma composition and content of tea is of great significance for molecular sensory studies and the quality control of tea. As a result, aroma composition analysis has been an important topic in tea research in recent years5,6,7.
The content of aroma components in tea is very low, as they generally only account for 0.01%-0.05% of the dry weight of the tea leaves8. Furthermore, the large amount of non-volatile components in the sample matrix significantly interferes with analysis by gas chromatography9,10. Therefore, a sample preparation procedure is essential to isolate the volatile compounds in tea. The key consideration for the isolation and enrichment method is minimizing the matrix interference and, at the same time, maximizing the preservation of the original odor profile of the sample.
Solvent-assisted flavor evaporation (SAFE), originally developed by Engel, Bahr, and Schieberle, is an improved high-vacuum distillation technique used to isolate volatile compounds from complex food matrixes11,12. A compact glass assembly connected to a high-vacuum pump (under a typical operating pressure of 5 x 10−3 Pa) can efficiently collect volatile compounds from solvent extracts, oily foods, and aqueous samples.
This article described a method that combines the SAFE technique with solvent extraction to isolate volatile substances from a black tea infusion, followed by analysis using GC-MS.
Protocol
1. Preparation of the internal standard and tea infusion
- Stock solution: Dissolve 10.0 mg of paraxylene-d10 (see Table of Materials) in 10.0 mL of anhydrous ethanol to prepare a 1,000 ppm stock solution of the internal standard.
- Working solution: Dilute 1 mL of the stock solution (step 1.1) to 100 mL with pure water to prepare a 10 ppm working solution of the internal standard.
NOTE: The working solution must be prepared on the same day as the analysis. - Place 3 g of tea leaves (both for green tea and black tea, see Table of Materials) into an Erlenmeyer flask, and add 150 mL boiling water. Cover the flask with a glass stopper.
- After 5 min, quickly filter out the tea infusion through a 300-mesh sieve.
- Wash the spent tea leaves twice with 30 mL of water, and combine the wash solution with the tea infusion.
- Cool the tea infusion to room temperature quickly in an ice water bath.
- Add 1.00 mL of working solution (step 1.2) into the tea infusion, and mix them well.
2. Distillation of the tea infusion by SAFE and liquid-liquid extraction of the distillate
- Prepare the SAFE assembly following the steps below.
- Install the SAFE assembly (Figure 1), and connect the distillation bottle at the lower left (Figure 1[3]) and the collection bottle at the lower right (Figure 1[4]). Connect the circulating water tube at the rear of the SAFE glass assembly. Install the cold trap (Figure 1[5]), and connect the tube to the vacuum pump (see Table of Materials) at the upper right of the glass assembly.
NOTE: Check the connection of the circulating water tube; ensure that the inlet enters the top and the outlet exits from the bottom. Use deionized water for the circulation to prevent the scale from blocking the white tube in the SAFE assembly, which would result in poor circulation of the circulating water and the eventual explosion of the SAFE assembly. The distillation bottom (Figure 1[3]) can be stirred by a stirring bar to facilitate the evaporation of the sample. - Set the temperature of the circulating water to 50 °C and that of the water bath for the sample flask to 40 °C. Close the vacuum valve (Figure 1[2]).
- Install the SAFE assembly (Figure 1), and connect the distillation bottle at the lower left (Figure 1[3]) and the collection bottle at the lower right (Figure 1[4]). Connect the circulating water tube at the rear of the SAFE glass assembly. Install the cold trap (Figure 1[5]), and connect the tube to the vacuum pump (see Table of Materials) at the upper right of the glass assembly.
- Perform the vacuum pump operation.
- Power on the vacuum pump.
- Gradually increase the speed to the maximum speed of 100%.
NOTE: If the speed does not reach 100%, check whether the system is airtight and whether there is solvent residue inside the system. - After reaching a high vacuum (preferably 10-3 Pa)
NOTE: The vacuum will improve when the liquid nitrogen is added to the cold trap.
- Perform sample distillation.
- Start the water circulation.
- Add liquid nitrogen to the cold trap to cover the outside of the collecting bottle.
- Pour the tea infusion into the sample funnel at the top left (Figure 1[1]), and then cover it with a glass stopper.
- Introduce the sample into the distillation flask dropwise. Control the sample drop speed so that the vacuum is kept in the proper range of around 10−3 Pa.
NOTE: Add liquid nitrogen during the process to ensure that the right collecting bottle is always submerged in liquid nitrogen. Try to avoid condensate formation in the cold trap.
- Turn off the vacuum pump after the distillation is completed.
- Press the power switch. When "STOP" flashes, press the Enter key to confirm.
- Unplug the power cord when the speed of the molecular pump decreases to "0".
NOTE: Restart only when the speed decreases to "0".
- Restore the system to atmospheric pressure.
- Remove the grinding plug above the sampling bottle.
- Unscrew the knob of the vacuum valve slowly to restore the system to atmospheric pressure.
- Take down the collecting bottle with the sample.
- Remove the liquid nitrogen outside the collection bottle after recovering the system to atmospheric pressure.
- Unscrew the collection bottle slowly. Take down the collecting bottle with the sample carefully.
- Close the circulating water.
- Perform liquid-liquid extraction of the SAFE distillate.
- Let the SAFE distillate in the bottle warm to room temperature.
- Extract the SAFE distillate thrice with 50 mL of dichloromethane (see Table of Materials).
- Combine the dichloromethane layers. Dry the extract with anhydrous sodium sulfate (see the Table of Materials).
NOTE: The anhydrous sodium sulfate in the solvent is considered dry enough when it is no longer cemented and can flow freely. - Concentrate the extract to about 2 mL using a gentle nitrogen stream.
- Transfer to a sample vial of 1-2 mL, and further concentrate to 200 µL using a gentle nitrogen stream.
3. GC-MS analysis and data processing
- Analyze the aroma concentrates prepared in protocol section 2 using a GC-MS system (Figure 2) equipped with fused silica capillary columns (see the Table of Materials).
- Use helium as carrier gas with a linear velocity of 40 cm/s.
- Inject 3 µL of the concentrate in splitless injection mode.
- Set the GC oven temperature program: (1) hold at 40 °C for 5 min; (2) increase to 200 °C at 5 °C/min; (3) increase to 280 °C at 10 °C/min; (4) hold at 280 °C for 10 min.
- Operate the mass selective detector in positive EI mode13 with a mass scan range from 30 m/z to 350 m/z at 70 eV.
- Deconvolute the GC-MS data using the Automated Mass Spectral Deconvolution and Identification System (AMDIS, see the Table of Materials).
- Match and qualify the data after deconvolution using the NIST (National Institute of Standards and Technology) 17 mass spectrometer search program3.
- Calculate the retention index of the compounds14 based on the result of a set of n-alkanes (C5-C25, see the Table of Materials) under the same GC conditions.
- Identify the GC peaks using the NIST mass spectrometry library and the retention index database based on the simultaneous matching of the mass and retention indexes.
- Calculate the concentration of each volatile component in the SAFE sample relative to the internal standard using the TIC (total ion chromatography) peak area.
- Repeat the analysis three times, starting from the tea infusion preparation.
Representative Results
The analytical procedure described above is illustrated in this section using the example of the aroma analysis of black tea and green tea samples.
A representative GC-MS chromatogram is shown in Figure 3. Figure 3A shows a set of n-alkanes, and Figure 3B shows the profile of an internal standard. The evaluation results for the extracts from the green tea and black tea samples are shown in Figure 3C and Figure 3D, respectively. By analyzing the internal standards, one definitive peak with a stable baseline can be detected (Figure 3B). The GC chromatogram shows the complete GC profiles obtained from the green tea and black tea infusion extracts after the total ion counting.
A total of 104 aroma compounds were identified in the green tea and black tea samples by mass spectrometry matching combined with the retention index. The relative quantification was calculated by the peak area of the compound relative to the internal standard. The heat map, drawn according to the qualitative and quantitative results, shows the aroma compound contents relative to the internal standard for the green tea and black tea samples (Figure 4).
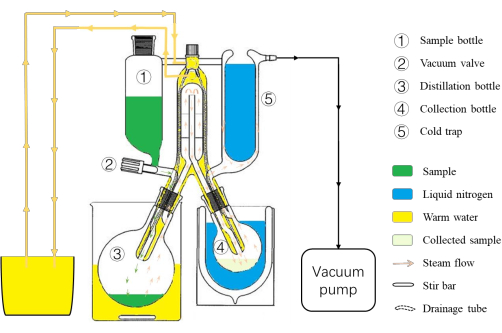
Figure 1: Schematic diagram of the SAFE system. (1) The sample bottle for the sample collection. (2) The vacuum valve; the system must be kept closed before adding samples, and the drop flow of the sample should be adjusted appropriately. (3) The distillation bottle for the sample distillation. (4) The collection bottle for the collection of the distilled sample. (5) The cold trap for the recovery of samples not collected by the collection bottle and for preventing the solvent from entering the vacuum pump. Please click here to view a larger version of this figure.
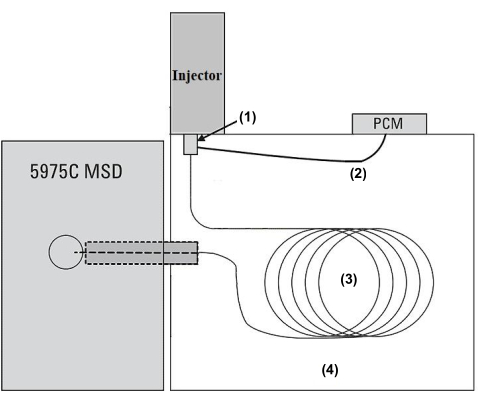
Figure 2: Schematic diagram of the GC-MSD system. The GC/MS system is equipped with (1) a multi-mode injection port, (2) a flow control module (PCM) that controls the helium carrier flow, (3) a 60 m x 0.25 m x 0.25 m 5 ms capillary column, and (4) a GC column oven. The tea extracts in the injected sample are separated in the GC column, through which the carrier gas flows and the oven temperature increases. The components are ionized by an EI ion source and then analyzed in a mass analyzer. Please click here to view a larger version of this figure.
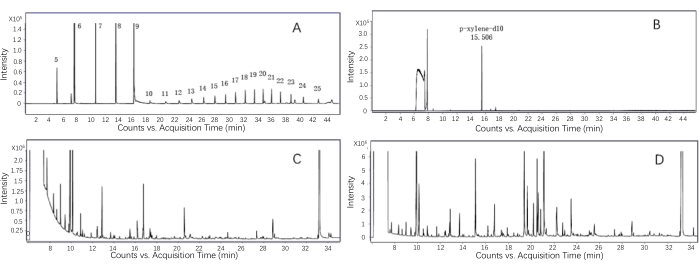
Figure 3: Typical total ion chromatogram from a successful GC-MS analysis. (A) The chromatogram of the n-alkanes. All the n-alkane peaks are assigned to the corresponding carbon number. (B) The chromatogram of the internal standard (paraxylene-d10). (C) Representative aroma profile of the green tea infusion. (D) Representative aroma profile of the black tea infusion. Please click here to view a larger version of this figure.
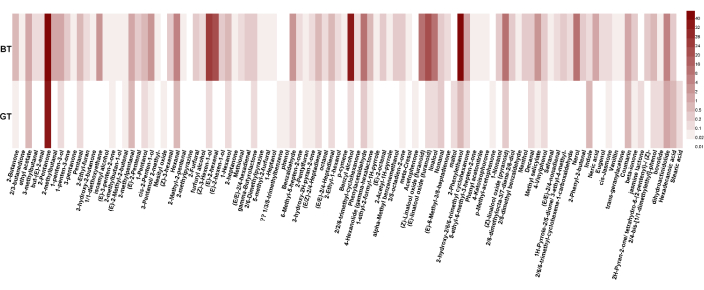
Figure 4: Heatmap of 104 aroma compounds identified in the black tea (BT) and green tea (GT) samples. The number next to the color note on the right side of the heat map indicates the content of the compound (relative to the internal standard). The color depth indicates the level of matter content; the deeper the color, the higher the relative content. Please click here to view a larger version of this figure.
Discussion
This article describes an efficient method for analyzing volatile compounds in tea infusions using SAFE and GC-MS analysis.
Tea infusions have a complex matrix with a high content of non-volatile components. Several methods have been described in the literature for isolating the volatile components from tea infusions. A common method is simultaneous distillation extraction (SDE)15,16. However, it is not suitable for the analysis of tea aromas because the tea leaves must be boiled with water for the entire distillation/extraction process, which results in the tea components undergoing a chemical reaction and, thus, yields an odor profile very different from the original sample17. SAFE distillates the tea infusion at a low temperature under a high vacuum, thus minimizing changes in the analytes and allowing the original aroma composition to be preserved.
Solid-phase microextraction (SPME) is another method commonly used for the aroma analysis of tea18,19. Its advantages lie in the simple and solvent-free procedure. However, the selectivity of the fiber adsorption of the aroma components makes it difficult to obtain a quantitative profile reflecting the aroma characteristics of the sample, which limits the application of this method for tea aroma analysis20.
The high-vacuum transfer (HVT) technique was developed to reduce the chance of artifacts being formed in aroma analyses21. However, HVT has a low extraction yield for substances with high boiling points and strong polarity, which limits its scope of use.
Unlike with the above custom methods, the SAFE distillate of a tea infusion is free of any non-volatile components22,23,24. The aroma in the distillate can be quantitatively extracted using organic solvents, meaning that an extract with an odor profile close to the original sample can be obtained. Engel et al.11 distilled mixtures of n-alkanes using HVT or SAFE distillation to check the efficiency. The distillate yields using the SAFE system were found to be significantly higher than those of HVT for each alkane. Additionally, alkanes with boiling points below 285 °C could be completely recovered by SAFE.
Close attention needs to be paid to the experimental details for further successful analyses. (1) The vacuum pressure during the SAFE distillation can affect the recovery of the volatile components and must be maintained at a high level, such as by slowing down the sample addition. (2) It is necessary to ensure that the collection bottle is immersed in liquid nitrogen before the system returns to atmospheric pressure to avoid solvent volatiles being condensed by the upper-right cold trap or entering the vacuum pump. (3) One should ensure that the circulating water is turned on first and turned off last. The circulating water should only be turned off after the liquid nitrogen has been removed; otherwise, it will freeze the device. (4) The water bath should be stirred with a magnet to aid the heat transfer.
In this study, SAFE distillation was performed before the solvent extraction. A reversed procedure is also feasible, and this would be especially advantageous if a large volume of tea infusion is extracted first and the obtained extract is then distilled by SAFE. The challenge of infusion extraction using an organic solvent is the possible formation of an emulsion. In this case, additional steps are needed to recover the organic layer, such as centrifugation or choosing different solvents. After the experiment, the SAFE glass assembly must be cleaned. Ethanol or acetone can be used as the cleaning solvent. The parts should be dried before use.
In summary, this protocol proposes a method to obtain an aroma concentrate with an odor profile close to the original tea sample using SAFE distillation followed by solvent extraction. This method can be applied to all types of tea samples, including, for example, instant tea powders and tea concentrates, and is well suited for molecular sensory studies of tea.
Disclosures
The authors have nothing to disclose.
Acknowledgements
This research was supported by the National Natural Science Foundation of China (32002094, 32102444), the China Agriculture Research System of MOF and MARA (CARS-19), and the Innovation Project for Chinese Academy of Agricultural Sciences (CAAS-ASTIP-TRI).
Materials
| Alkane mix (C10-C25) | ANPEL | CDAA-M-690035 | |
| Alkane mix (C5-C10) | ANPEL | CDAA-M-690037 | |
| AMDIS | National Institute of Standards and Technology | version 2.72 | Gaithersburg, MD |
| Analytical balance | OHAUS | EX125DH | |
| Anhydrous ethanol | Sinopharm | ||
| Anhydrous sodium sulfate | aladdin | ||
| Black tea | Qianhe Tea | Huangshan, Anhui province, China | |
| Concentrator | Biotage | TurboVap | |
| Data processor | Agilent | MassHunter | |
| Dichloromethane | TEDIA | ||
| GC | Agilent | 7890B | |
| GC column | Agilent | DB-5MS | |
| Green tea | Qianhe Tea | Huangshan, Anhui province, China | |
| MS | Agilent | 5977B | |
| p-Xylene-d10 | Sigma-Aldrich | ||
| SAFE | Glasbläserei Bahr | ||
| Ultra-pure deionized water | Milipore | Milli-Q | |
| Vacuum pump | Edwards | T-Station 85H |
References
- Liang, S., et al. Processing technologies for manufacturing tea beverages: From traditional to advanced hybrid processes. Trends in Food Science & Technology. 118, 431-446 (2021).
- Guo, X. Y., Ho, C. T., Schwab, W., Wan, X. C. Aroma profiles of green tea made with fresh tea leaves plucked in summer). Food Chemistry. 363, 130328 (2021).
- Feng, Z. H., Li, M., Li, Y. F., Wan, X. C., Yang, X. G. Characterization of the orchid-like aroma contributors in selected premium tea leaves. Food Research International. 129, 108841 (2020).
- Hong, X., et al. Characterization of the key aroma compounds in different aroma types of Chinese yellow tea. Foods. 12 (1), 27 (2023).
- Flaig, M., Qi, S. C., Wei, G., Yang, X., Schieberle, P. Characterisation of the key aroma compounds in aLongjinggreen tea infusion (Camellia sinensis) by the sensomics approach and their quantitative changes during processing of the tea leaves. European Food Research and Technology. 246 (12), 2411-2425 (2020).
- Feng, Z., et al. Tea aroma formation from six model manufacturing processes. Food Chemistry. 285, 347-354 (2019).
- Wang, J. -. Q., et al. Effects of baking treatment on the sensory quality and physicochemical properties of green tea with different processing methods. Food Chemistry. 380, 132217 (2022).
- Zhai, X., Zhang, L., Granvogl, M., Ho, C. -. T., Wan, X. Flavor of tea (Camellia sinensis): A review on odorants and analytical techniques. Comprehensive Reviews in Food Science and Food Safety. 21 (5), 3867-3909 (2022).
- Chaturvedula, V. S. P., Prakash, I. The aroma, taste, color and bioactive constituents of tea. Journal of Medicinal Plants Research. 5 (11), 2110-2124 (2011).
- Ridgway, K., Lalljie, S. P. D., Smith, R. M. Sample preparation techniques for the determination of trace residues and contaminants in foods. Journal of Chromatography A. 1153 (1-2), 36-53 (2007).
- Engel, W., Bahr, W., Schieberle, P. Solvent assisted flavour evaporation – A new and versatile technique for the careful and direct isolation of aroma compounds from complex food matrices. European Food Research and Technology. 209 (3-4), 237-241 (1999).
- Wang, B., et al. Characterization of aroma compounds of Pu-erh ripen tea using solvent assisted flavor evaporation coupled with gas chromatography-mass spectrometry and gas chromatography-olfactometry. Food Science and Human Wellness. 11 (3), 618-626 (2022).
- Zou, C., et al. Zijuan tea- based kombucha: Physicochemical, sensorial, and antioxidant profile. Food Chemistry. 363, 130322 (2021).
- Vandendool, H., Kratz, P. D. A generalization of the retention index system including linear temperature programmed gas-liquid partition chromatography. Journal of Chromatography. 11, 463-471 (1963).
- Khvalbota, L., Virba, M., Furdikova, K., Spanik, I. Simultaneous distillation-solvent extraction gas chromatography-mass spectrometry analysis of Tokaj Muscat Yellow wines. Separation Science Plus. 5 (8), 393-406 (2022).
- Ayalew, Y., et al. Volatile organic compounds of anchote tuber and leaf extracted using simultaneous steam distillation and solvent extraction. International Journal of Food Science. 2022, 3265488 (2022).
- Zhu, M., Li, E., He, H. Determination of volatile chemical constitutes in tea by simultaneous distillation extraction, vacuum hydrodistillation and thermal desorption. Chromatographia. 68 (7-8), 603-610 (2008).
- Lau, H., et al. Characterising volatiles in tea (Camellia sinensis). Part I: Comparison of headspace-solid phase microextraction and solvent assisted flavour evaporation. Lwt-Food Science and Technology. 94, 178-189 (2018).
- Li, Z. W., Wang, J. H. Analysis of volatile aroma compounds from five types of Fenghuang Dancong tea using headspace-solid phase microextraction combined with GC-MS and GC-olfactometry. International Food Research Journal. 28 (3), 612-626 (2021).
- Dong, F., et al. Herbivore-induced volatiles from tea (Camellia sinensis) plants and their involvement in intraplant communication and changes in endogenous nonvolatile metabolites. Journal of Agricultural and Food Chemistry. 59 (24), 13131-13135 (2011).
- Acena, L., Vera, L., Guasch, J., Busto, O., Mestres, M. Comparative study of two extraction techniques to obtain representative aroma extracts for being analysed by gas chromatography-olfactometry: Application to roasted pistachio aroma. Journal of Chromatography A. 1217 (49), 7781-7787 (2010).
- Kumazawa, K., Wada, Y., Masuda, H. Characterization of epoxydecenal isomers as potent odorants in black tea (Dimbula) infusion. Journal of Agricultural and Food Chemistry. 54 (13), 4795-4801 (2006).
- Wu, H. T., et al. Effects of three different withering treatments on the aroma of white tea. Foods. 11 (16), 2502 (2022).
- Wang, J., et al. Decoding the specific roasty aroma Wuyi rock tea (Camellia sinensis: Dahongpao) by the sensomics approach. Journal of Agricultural and Food Chemistry. 70 (34), 10571-10583 (2022).

