An In Vitro Method to Identify Innate and Adaptive Immune Cells Residing in Murine Lungs
Abstract
Source: Christofides, A., et al. Flow Cytometric Analysis for Identification of the Innate and Adaptive Immune Cells of Murine Lung. J. Vis. Exp. (2021).
This video demonstrates an in vitro technique to identify immune cells residing in murine lungs. After isolating target immune cells from murine lung tissue, they are surface-labeled using a cocktail of fluorophore-tagged antibodies against specific markers on innate and adaptive immune cells. Additionally, monocytes, macrophages, and dendritic cells are labeled using an intracellular marker. The various immune cell types are then identified using flow cytometry.
Protocol
All procedures involving animal models have been reviewed by the local institutional animal care committee and the JoVE veterinary review board.
1. Surgical excision and tissue preparation
- Euthanize the mouse by intraperitoneally injecting 1 mL of tribromoethanol (prepared according to standard protocol; Table of Materials).
NOTE: CO2 asphyxiation should be avoided in lung studies as it might cause lung injury and alter the features and properties of lung immune cells. Cervical dislocation should also be avoided as it might cause mechanical injury of the lung. - Transfer the mouse to a clean and dedicated area for surgical operation.
- Stabilize the mouse dorsal side down by using needles or tape on the four extremities. Use 70% ethanol to sanitize the skin of the ventral area.
- Perform an incision in the skin, from the neck to the abdomen. Carefully remove the skin from the thoracic area.
- Carefully remove the sternum and ribs.
- Flush the lungs by injecting 10 mL of cold phosphate-buffered saline (PBS) directly in the right ventricle, using an 18-21 G needle, until the lungs become completely white.
- Carefully remove the thymus and heart without touching the lungs.
- Gently detach the lungs from the surrounding tissues and transfer them to a tube with cold bovine serum albumin (BSA) buffer (Table 1).
NOTE: Effort should be made to remove all adjacent fat from the lungs before further preparing the single-cell suspension, as this could bias the readouts.
2. Preparation of single-cell suspension
- Transfer the lungs to an empty Petri dish and mince them with two fine scalpels. Transfer all the pieces of the minced lung to a new 50 mL conical tube. Use 5 mL of digestion buffer to wash the plate and add it to the 50 mL tube containing the minced lung (Table 1).
NOTE: Digestion buffer should be prepared immediately before use. Use 5 mg/mL of collagenase. Combining 1 or 5 mg of collagenase with BSA buffer or protein-free PBS did not improve results (Figure 1). - Secure the lid of the tube and digest the lung for 30 min on an orbital shaker at a speed of 150 rpm at 37 °C. Stop the reaction by adding 10 mL of cold BSA buffer.
- After digestion, use an 18 G needle to mix and dissolve the lung pieces. Place a 70 µm filter strainer at the top of a new 50 mL conical tube.
NOTE: Usage of a smaller micron filter might result in the loss of major myeloid populations. - Slowly transfer the digested lung mixture directly on the strainer. Use the rubber side of a 10 mL syringe plunger to smash the remaining lung pieces on the filter. Wash the processed material on the filter with BSA buffer.
- Centrifuge the single-cell suspension at 350 × g for 8 min at 4 °C.
- Carefully discard the supernatant and resuspend the cells in 1 mL of ammonium–chloride–potassium (ACK) lysis buffer. Mix well using a 1 mL pipet, and incubate for 90 s at room temperature.
- Add 10 mL of cold BSA buffer to stop the reaction and centrifuge at 350 × g for 7 min at 4 °C.Carefully discard the supernatant and resuspend the pellet in Staining Buffer to count the cells using a hemocytometer.
- Resuspend the cells at a concentration of 5 × 106 cells/mL and use them for surface staining (see section 2).
NOTE: For this purpose, plate the cells in a 96-well round-bottom plate followed by antibody staining and washes. If a plate centrifuge is not available, use flow tubes instead of plates. With this protocol, ~15-20 × 106 cells per lung can be obtained from a 6-10-week-old C57BL/6 mouse of average size.
3. Surface antibody staining
- Transfer 1 × 106 cells in 200 µL per well in a 96-well plate. Centrifuge the plate at 350 × g for 7 min at 4 °C. In the meantime, prepare the Fc-block solution by diluting anti-16/32 antibody (1:100) in staining buffer (Table 1).
- Resuspend the cells in 50 µL of the pre-prepared Fc-blocking solution (Table of Materials) and incubate for 15-20 min at 4 °C or on ice.
- Add 150 µL of staining buffer and centrifuge the plate at 350 × g for 5 min at 4 °C. Meanwhile, prepare the surface antibody cocktail by diluting surface antibodies (1:100; Table 2) in staining buffer.
NOTE: (i) Anti-16/32 antibody for Fc-blocking can be used with the surface antibodies in the same mixture. (ii) If fixable viability dye is used, add it to the surface antibody cocktail at a dilution of 1:1,000. - Resuspend the cells in 50 µL of the pre-prepared surface antibody cocktail and incubate for 30-40 min at 4 °C in the dark. Wash the cells with staining buffer twice.
NOTE: If no intracellular staining is required, resuspend the cells in 200 µL of staining buffer and proceed directly to the acquisition of data on the flow cytometer. Alternatively, cells might be fixed and stored at 4 °C for acquisition later. We recommend using the cells for flow cytometry within 24 h.
4. Cell fixation and intracellular staining
- Prepare the fixation/permeabilization buffer (Fix/Perm Buffer) by mixing three parts of fixation/permeabilization concentrate and 1 part of fixation/permeabilization diluent of the FoxP3/Transcription Factor Staining Buffer Set (Table 1).
- Resuspend the cells in 50 µL of the pre-prepared Fix/Perm Buffer per well of the 96-well plate, where cells were plated as described in section 3, and incubate them for 20-25 min at 4 °C in the dark.
- Dilute the 10x permeabilization buffer as 1: 10 in purified deionized water to prepare 1x permeabilization buffer.
- Wash the cells once with 1x permeabilization buffer. Meanwhile, prepare the intracellular antibody cocktail by diluting intracellular antibodies (1:100) in 1 mL of permeabilization buffer.
- Resuspend the cells using 50 µL of the pre-prepared surface antibody cocktail per cell of the 96-well plate and incubate for 40 min at 4 °C in the dark.
- Wash the cells once with permeabilization buffer and once with staining buffer. After the final wash, resuspend the cells in 200 µL of staining buffer.
NOTE: If no flow cytometer with plate reader is available, transfer the cells into flow cytometry tubes. - Acquire a minimum of 1.5 × 106 cells per sample on the flow cytometer.
NOTE: For single colors and unstained control samples, 0.5-1 × 106 cells per sample will be sufficient. It is recommended to titer the individual antibodies used to achieve optimal staining and reduce costs. The present protocol has been optimized using Fix/Perm Buffer prepared using the FoxP3 staining buffer set. Because CD68 is a cytoplasmic and not a nuclear marker, other permeabilization solutions such as a low concentration of paraformaldehyde or cytofix/ cytoperm kits from various vendors might be sufficient.
Table 1: Buffers.
| BSA buffer | PBS + 0.5% Bovine serum albumin |
| Digestion buffer | Prewarmed (37 °C) BSA buffer + 5 mg/mL collagenase type 1 + 0.2 mg/mL DNase I |
| Staining buffer | PBS + 2.5% FBS |
| Fix/Perm Buffer | Three parts of fixation/permeabilization diluent and 1 part of fixation/permeabilization diluent of the Foxp3/Transcription Factor Staining Buffer Set |
| Permeabilization buffer | 10x permeabilization buffer from the Foxp3/Transcription Factor staining Buffer Set diluted 10 times in purified deionized water |
Table 2: Usage of monoclonal antibodies.
| Antigen | Clone | Fluorochrome | Dilution | Surface/intracellular |
| CD45 | 30-F11 | APC/CY7 | 1:100 | surface |
| Gr-1 | RB6-8C5 | BV421 | 1:100 | surface |
| CD68 | FA-11 | PerCPCy5.5 | 1:100 | intracellular |
| CD11b | M1/70 | PECy7 | 1:100 | surface |
| Siglec F | S17007L | FITC | 1:100 | surface |
| CD11c | N418 | BV650 or BV510 | 1:100 | surface |
| CD64 | X54-5/7.1 | PE/Dazzle 594 | 1:100 | surface |
| MHC-II | M5/114.15.2 | AF700 | 1:100 | surface |
| CD103 | 2.00E+07 | PE / FITC | 1:100 | surface |
| Live/Dead Fixable Far Read Dead Cell Stain Kit | FarRed (APC) or Aqua (BV510) | 1:1000 | surface | |
| CD3 | 17A2 | PE | 1:100 | surface |
| B220 | RA3-6B2 | AF488 | 1:100 | surface |
| NK1.1 | PK136 | FITC | 1:100 | surface |
| CD24 | 30-F1 | PE | 1:100 | surface |
| MERTK | 2B10C42 | PE | 1:100 | surface |
| F4/80 | BM8 | BV605 | 1:100 | surface |
| CX3CR1 | SA011F11 | PE | 1:100 | surface |
| FcBlock (CD16/32) | 93 | 1:100 | surface |
Representative Results
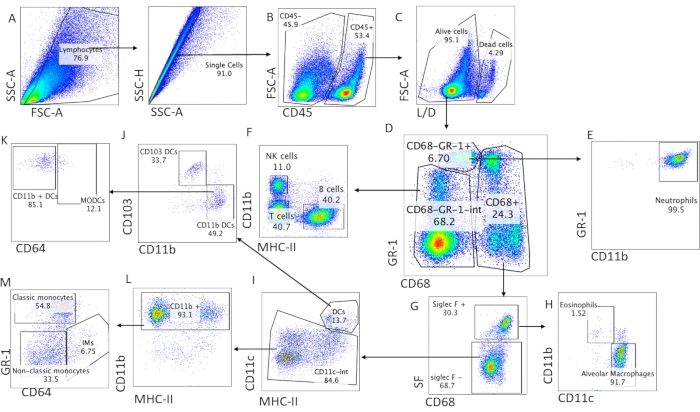
Figure 1: The best cellular dissociation is achieved by 5 mg/mL of collagenase 1 in prewarmed PBS + 0.5% BSA. In all different conditions, 0.2 mg of DNAse I was also included. Abbreviations: BSA = bovine serum albumin; FSC-A = peak area of forward-scattered light; L/D = live/dead staining; SF = Siglec F.
Divulgaciones
The authors have nothing to disclose.
Materials
| 10 mL syringe plunger | EXELINT | 26265 | |
| 18 G needles | BD Precision Glide Needle | 305165 | |
| 21 G needles | BD Precision Glide Needle | 305195 | |
| 50 mL conical tubes | Falcon | 3520 | |
| 70 μm cell strainer | ThermoFisher | 22363548 | |
| 96-well plates | Falcon/corning | 3799 | |
| ACK Lysing Buffer | ThermoFisher | A10492-01 | |
| anti-mouse CD11b | Biolegend | 101215 | For details see Table 2 |
| anti-mouse CD11c | Biolegend | 117339 / 117337 | For details see Table 2 |
| anti-mouse CD45 | Biolegend | 103115 | For details see Table 2 |
| anti-mouse CD64 | Biolegend | 139319 | For details see Table 2 |
| anti-mouse CD68 | Biolegend | 137009 | For details see Table 2 |
| anti-mouse GR-1 | Biolegend | 108433 | For details see Table 2 |
| anti-mouse Siglec F | Biolegend | 155503 | For details see Table 2 |
| AVERTIN | Sigma-Aldrich | 240486 | |
| B220 | Biolegend | 103228 | For details see Table 2 |
| Bovine Serum Albumin (BSA) | Sigma-Aldrich | 9048-46-8 | |
| CD103 | Biolegend | 121405 / 121419 | For details see Table 2 |
| CD24 | Biolegend | 138503 | For details see Table 2 |
| CD3 | Biolegend | 100205 | For details see Table 2 |
| Centrifuge | |||
| Collagenase Type 1 | Worthington Biochemical Corp | LS004196 | |
| CX3CR1 | Biolegend | 149005 | For details see Table 2 |
| DNase I | Millipore Sigma | 10104159001 | |
| Ethanol | |||
| F4/80 | Biolegend | 123133 | For details see Table 2 |
| FcBlock (CD16/32) | Biolegend | 101301 | For details see Table 2 |
| Fetal Bovine Serum | R&D Systems | ||
| Fine Serrated Forceps | Roboz Surgical Instrument Co | ||
| Foxp3 / Transcription Factor Staining Buffer Set | ThermoFisher | 00-5523-00 | |
| Futura Safety Scalpel | Merit Medical Systems | SMS210 | |
| Live/Dead Fixable Far Read Dead Cell Stain Kit | ThermoFisher | L34973 | For details see Table 2 |
| MERTK | Biolegend | 151505 | For details see Table 2 |
| MHC-II | Biolegend | 107621 | For details see Table 2 |
| NK1.1 | Biolegend | 108705 | For details see Table 2 |
| Orbital Shaker | VWR | Model 200 | |
| Petri dish | Falcon | 351029 | |
| Refrigerated benchtop centrifuge | SORVAL ST 16R | ||
| Small curved scissor | Roboz Surgical Instrument Co |

