In Vitro Culture of Epicardial Cells From Mouse Embryonic Heart
Summary
The epicardium is an essential source of multipotent cardiovascular progenitor cells and paracrine factors that are required for cardiovascular development and regeneration. We describe here a method to culture mouse embryonic epicardial cells.
Abstract
During embryogenesis, the epicardial contribution to coronary vasculature development has been very well established. Cells derived from the epicardium differentiate into smooth muscle cells, fibroblasts and endothelial cells that contribute to the formation of coronary vessels. Here we have established an in vitro culture method for embryonic epicardial cells. Using genetic labelling, we have demonstrated that the majority of the migrating cells in our explant culture are of epicardial origin. Epicardial explant cells also retain the expression of epicardial markers (Wt1 and Tbx18). Furthermore, we provide evidence that epicardial explant cells undergo epithelial to mesenchymal transition (EMT), migrate and differentiate into smooth muscle cells after Transforming growth factor beta 1 (TGF-β1) treatment in a manner indistinguishable from that of epicardial cells in vivo. In conclusion, we provide a novel method for the culture of embryonic epicardial cells, which will help to explore the role of specific genes in epicardial cell biology.
Introduction
A wealth of experimental data have illustrated that the epicardium influences critical steps in cardiac development. During development, the septum transversum gives rise to a clump of mesothelial cells known as the proepicardium1-4. Cells from the proepicardium then migrate and envelope the myocardium forming the epicardium. Following this, a subset of epicardial cells undergo EMT giving rise to a migratory population of epicardium-derived cells (EPDCs) that subsequently invade the myocardium. Genetic as well as retroviral lineage tracing experiments have demonstrated that EPDCs differentiate into various lineages including smooth muscle cells, fibroblasts, endothelial cells and cardiomyocytes (if any). Therefore EPDCs contribute significantly to the development of the coronary vasculature and myocardial architecture1,2,4-9. Furthermore, the epicardium is essential for the development of the ventricular compact layer10-12. For example Gittenberger-de Groot et al. demonstrated that inhibiting the outgrowth of the proepicardium leads to an array of defects such as a thin myocardium, deficient looping of the heart and abnormal interventricular septum formation and as a result, embryonic lethality13. Paracrine factors secreted from the embryonic epicardium modulate cardiomyocyte proliferation and differentiation. Consistent with this, epicardium-specific deletion of signaling pathways such as retinoic acid (RA), fibroblast growth factors (FGFs) and Wnt/β-catenin resulted in defective myocardial growth and embryonic lethality14-16.
Though the epicardium was believed to be quiescent in adult hearts, recent studies have shown that the developmental program is reactivated in the epicardium following cardiac injury17,18. Upon activation, the cells undergo rapid proliferation and EMT that result in EPDC formation. These cells exhibit the capacity to differentiate into fibroblast and smooth muscle cells but not cardiomyocytes or endothelial cells18. In addition, the EPDCs secrete proangiogenic factors that aid in the vascularization of the injured area and thus facilitate improved cardiac function by reducing the infarct size. Due to these findings, the epicardium has gained interest in the study of cardiovascular development, disease and regeneration.
Transgenic technology has revolutionized medical research in the 21st century. With the aid of transgenic technologies, diseased mouse models mimicking the human condition metabolically and pathophysiologically have been successfully developed. However, studying the epicardial cell behavior in these mutants has been a challenge mainly due to early embryonic lethality. Considering the significant role that the epicardium plays in cardiac development and regeneration, we have established an in vitro culture system for mouse epicardial cells. This method allows the long term culture of the epicardial cells and facilitates the detailed study of the two important properties of the epicardium: its ability to migrate and differentiate. The excised ventricles from the mouse could be cultured on collagen gels which can be used to conduct migration assays. Being cultured in a 3D matrix that replicates the collagen-rich extracellular matrix of the subepicardial layer better recapitulates the in vivo cell physiology. Alternatively they can be cultured on chamber slides in order to establish an epicardial monolayer which can then be used for a variety of downstream applications. This monolayer can be used to stain for tight junction proteins which will provide insights on the ability of the epicardium to undergo EMT which is crucial for migration. In addition, differentiation experiments can also be carried out on these cells. Furthermore, gene expression profile can be analyzed by extracting RNA from the cells and performing quantitative polymerase chain reaction (qPCR). Lastly, the monolayers could also be treated with agents followed by a molecular analysis to test for potential therapeutics. Put together, this epicardial culture system provides us with the opportunity to visualize and gather molecular data which furthers our understanding about epicardial development.
Another desirable feature of this method is that it is straightforward and no elaborate setup is required. In brief, the embryos are harvested at E11.5 or E12.5 following which the heart is excised. The ventricles are then cultured on either collagen gel or chamber slides. Subsequently, these cells can be used to conduct downstream experiments.
Protocol
All experiments were approved by the Duke-NUS Graduate Medical School Institutional Animal Care and Use Committee.
1. Retrieving the Embryonic Ventricles
- Sacrifice a timed pregnant mouse at the desired embryonic stage (E11.5 or E12.5) using a euthanasia chamber with carbon dioxide gas supply or another approved euthanasia method.
- Place the mouse on its back on a dissecting table. Disinfect the abdomen of the female with 70% ethanol. Lift the skin over the belly and make a small incision (1-2 cm) with a blade, then hold the skin with both hands and pull away to expose the abdominal wall.
- Now cut the abdominal wall to expose the uterine horn. Use sterile forceps to lift up the uterine horn and carefully cut away the fat tissues and blood vessels attached to the uterine horn. Cut at the cervix to retrieve the uterus.
- Place the uterine horn in a petri dish containing sterile cold 1x Phosphate Buffered Saline (PBS) and rinse gently. Place the petri dish on ice.
- Use scissors to cut through the midline of the uterine horn (opposite to the site where the placenta is located) to expose the embryos that are still inside the yolk sac and attached to the uterine wall through the placenta.
- Using sterile forceps, cut open the embryonic yolk sac to free the embryo.
- Place the embryo in a new petri dish containing sterile cold 1x PBS. Decapitate the embryo and place it on its back. Cut open the chest wall to expose the heart.
- Use sterile forceps to lift the heart and cut away the vessels around it to free the heart from the chest wall. Pay special attention not to damage the epicardium of the heart with the surgical tools.
- Trim off the outflow tract and both atria. Transfer the ventricle to another dish with cold 1x PBS. Place the dish on ice.
- Repeat steps 1.6-1.9 till all the ventricles are retrieved.
2. Epicardial Explant Culture
Note: Culture these epicardial explants either on glass chamber slides or on collagen gel for downstream applications.
- Glass Chamber Slides
- To prepare the culture media, add 10% fetal bovine serum (FBS), 1% penicillin/streptomycin and 2 ng/ml recombinant fibroblast growth factor 2 (FGF2) to Dulbecco's modified Eagle's medium (DMEM). Perform all the steps in the laminar flow hood.
- Add 500 µl culture media to each well of an 8-well chamber.
- Place each excised ventricle in the center of a well. Orient the ventricle to keep the dissected side down on the bottom surface of the well.
- Gently put the plate in a 37 °C, 5% CO2 cell culture incubator.
- Maintain the culture with minimum disturbance to allow the explants to adhere. Formation of the epicardial monolayer can be observed after 48-72 hr.
- For differentiation of epicardial cells into smooth muscle cells, culture the epicardial monolayer cells in differentiation media for another 6 days. To prepare the differentiation media, add 10% FBS, 1% penicillin/streptomycin and 50 ng/ml recombinant transforming growth factor beta 1 (TGF-β1) to DMEM.
- Change the culture media on alternate days.
- Collagen Gel
- Prepare the collagen gel in a 96-well plate using a commercial 3D Collagen Culture kit according to manufacturer's protocol.
- To prepare the desired volume of collagen gel, mix the appropriate amount of collagen solution with 5x of DMEM and pipette up and down. The solution should turn yellow.
- Add a corresponding volume of neutralization solution and mix well immediately by pipetting up and down. The solution will turn pink.
- Pipette 100 µl of this mixture into each well of a 96-well plate. Place the plate in a 37 °C, 5% CO2 incubator for 30-60 min to allow the gel to polymerize.
- Add 200 µl of culture media described above to each well.
- Place each excised ventricle in the center of a well. Orient the ventricle to keep the dissected side down on the collagen gel (apex of the ventricle should face the experimenter). Gently put the plate back in the cell culture incubator.
- After 3 days, remove the ventricles. Put the plate back into the cell culture incubator for another 2 days.
- Change the culture media on alternate days.
3. Fixation and Visualization
Note: Epicardial cells can be visualized on either glass chamber slides or collagen gel.
- Aspirate out the culture media and add an equal volume of sterile 1x PBS to wash the cells.
- Add just enough 4% paraformaldehyde (PFA) to cover the cells. Fix the cells for 10 min at 4 °C.
- Remove PFA and wash explants with 1x PBST (1x PBS with 0.1% triton X-100) to permeabilize the cells.
- Immunostain with desired antibody (e.g. Alexa Fluor 488-phalloidin; ZO-1, α-Tubulin and α-smooth muscle actin)7.
Representative Results
Using this culture protocol, primary epicardial cells can be isolated with high purity for downstream applications. The cultured cells are able to undergo EMT, migrate and differentiate just as epicardial cells do in vivo.
To determine the purity of our primary epicardial cell culture, we analyzed the epicardial explants generated from Sema3dGFPCre/+;R26Tom/+ embryos. Sema3d is expressed in epicardial cells during early cardiac development, thus epicardial cells derived from these embryos will be RFP labelled. We isolated ventricles from Sema3dGFPCre/+;R26Tom/+ embryos for epicardial explants. After 48-72 hr, we were able to see a monolayer of epicardial cells. Superimposing the RFP immunofluorescence and brightfield images showed that a majority of the cells that migrated from the ventricles are of epicardial origin (Figure 1A–C). We further validated our results by isolating RNA from primary epicardial cells and performing qPCR which showed a strong expression of epicardial specific genes (Tbx18 and Wt1) as opposed to a cardiomyocyte marker gene (Nkx2.5) (Figure 1C).
Next we examined the ability of the cells to undergo EMT by analyzing the cell polarity and cell-cell contact of epicardial cells. EMT is a biological process that allows an epithelial cell to lose its cell polarity and cell-cell contact in order to transform into a migratory mesenchymal cell. The first step of EMT is the detachment of cell-cell contacts. We immunostained epicardial cells with ZO-1 (also known as Tjp1, Tight junction protein 1) which showed localization of ZO-1 to the plasma membrane indicating that cells have yet to undergo EMT (Figure 2A). Next we performed immunostaining for α-Tubulin (Figure 2B) so as to observe the organization of the microtubules in epicardial explants, which showed a polarized alignment that facilitates directional migration of epicardial cells. In addition, to determine the differentiation potential of the epicardial cells, we cultured them for 6 days in a differentiation media containing recombinant TGF-β1. Immunostaining for SMA demonstrated successful differentiation of epicardial cells into smooth muscle cells (Figure 2C).
Lastly, to analyze epicardium-derived cell migration and epicardial EMT, we also performed a collagen gel invasion assay. Epicardium-derived cells were visualized by phalloidin immunostaining. Successful migration of epicardium-derived cells can be seen all around the explant (Figure 3A). To determine the depth of cell penetration into the collagen matrix, 3D-reconstruction was generated from confocal images. Penetration of epicardium-derived cells can be seen in z-stacks (Figure 3B).
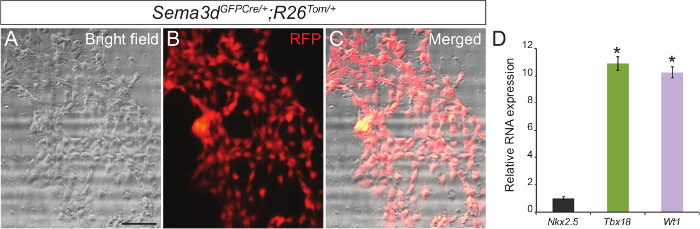
Figure 1: Primary Culture of Epicardial Cells from Embryonic Mouse Hearts. Representative brightfield (A) and RFP immunofluorescence (B) images of primary epicardial cells generated from E12.5 Sema3dGFPCre/+;R26Tom/+ hearts. Merged brightfield and immunofluorescence image (C). Relative mRNA expression levels of epicardial markers (Tbx18 and Wt1) and cardiomyocyte marker (Nkx2.5) in primary epicardial cells (D). The results were normalized to Gapdh expression, and the relative expression levels are given as a fold difference compared to Nkx2.5 expression (n = 3). The data are presented as the mean ± SD. Scale bar = 200 µm. *, p <0.05. Please click here to view a larger version of this figure.
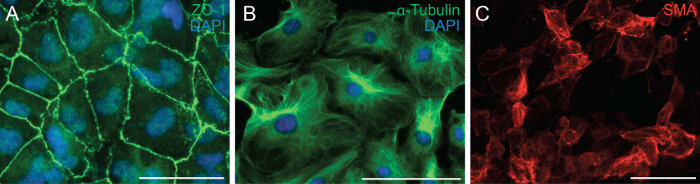
Figure 2: Primary Epicardial Cells Retain their Ability to Undergo EMT and Differentiation. Immunostaining on epicardial explants for ZO-1 (A, green) or α-Tubulin (B, green). DAPI was used to visualize nuclei (blue). Epicardial cells were differentiated into smooth muscle cells for 6 days and stained with α-smooth muscle actin (SMA) (C, red). Scale bar = 100 µm. Please click here to view a larger version of this figure.
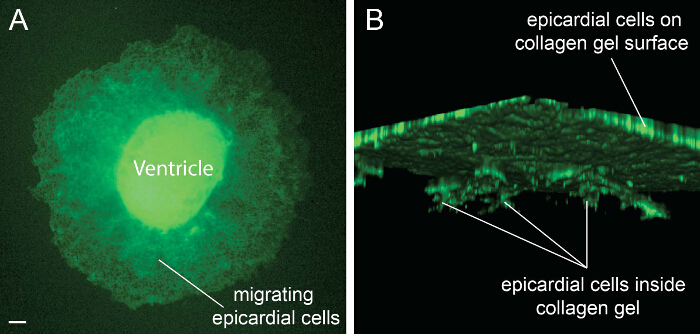
Figure 3: Epicardial Cell Migration on the Collagen Gel Surface. Ventricles from E12.5 embryos were cultured on a three-dimensional collagen gel matrix and stained with Alexa Fluor 488-phalloidin to visualize epicardium-derived cell migration and penetration (A). Confocal images were used to construct a three-dimensional image to determine cell penetration along the z-axes (B). Scale bar = 50 µm. Please click here to view a larger version of this figure.
Discussion
It is pivotal to develop techniques that facilitate the study of the epicardium to cater to the increasing significance of the epicardium in cardiac development and regeneration. The epicardial culture system poses significant advantages for epicardial research.
An alternative way to isolate epicardial cells is to use fluorescence-activated cell sorting (FACS). This method relies on the use of epicardial markers (or epicardium-cell-specific transgenic expression of a fluorescent protein) to separate epicardial cells from other lineages. However, an increasing amount of evidence has shown that epicardial markers are not exclusively expressed in the epicardium. For example, Tbx18 is expressed in the myocardium of interventricular septum (IVS) while Wt1 is expressed in the endothelium19-21. Hence, the purity of the population of epicardial cells isolated is questionable. In contrast, the culture method described herein takes advantage of the migratory nature of the epicardial cells. This enables the isolation of a highly pure population of epicardial cells as shown by our genetic labelling experiments. Another way to obtain epicardial cells would be the differentiation of human pluripotent stem cells (hPSCs) into epicardial cells by modulating primarily the BMP, WNT and retinoic acid signaling pathways. These differentiated cells closely resemble the physical and molecular characteristics of embryonic epicardial cells. They are not only able to undergo EMT but are also able to differentiate into fibroblasts and smooth muscle cells22,23. Though this method is very promising, it is significantly more complicated and time consuming than the epicardial culture described in this paper.
In addition, this culture technique does not require additional specialized equipment and an existing tissue culture setup is sufficient. While following this protocol, it is crucial to pay special attention to not damage the epicardium with surgical tools when retrieving the heart. In addition, it is critical to orient the excised ventricle with the dissected side facing either the surface of the collagen gel or chamber slide in order to facilitate epicardial cell migration. Lastly, the culture should be maintained with minimal disturbance so as to allow the explant to adhere to the culture dish.
The population of cells obtained can then be used to perform a variety of downstream experiments which will further our understanding of epicardial cell biology. Culturing the explants on chamber slides provides epicardial monolayers for various experiments including epicardial cell proliferation, survival, migration, EMT and differentiation into cardiac lineages. It also provides an opportunity to test the effect of drugs on the cellular properties of the epicardium as well as gene expression changes associated with the drug treatment. In addition, culturing the epicardium on collagen gels enables the analysis of the cells in a 3D matrix instead of the traditional 2D matrix, which provides a more accurate illustration of the in vivo physiology.
However, a drawback of this technique would be that this method can only be used to culture epicardial cells from embryonic hearts between E11.5-E12.5. During later gestation or in the neonatal period the epicardium loses the ability to proliferate and migrate. Thus it is difficult to use this culture method to isolate epicardial cells from later embryonic stages or adult mice.
Despite this limitation, this technique uses basic culturing skills and setup to facilitate the growth of epicardial cells ex vivo which would be very beneficial for the study of the epicardium and its roles in cardiac development and regeneration.
Divulgaciones
The authors have nothing to disclose.
Acknowledgements
This work was supported by funds from DUKE-NUS Graduate Medical School Singapore, Goh foundation and Singapore NRF fellowship (NRF-NRFF2016-01) to Manvendra K. Singh.
Materials
| Dulbecco’s modified Eagle’s medium (DMEM) | Life tech invitrogen | 11995065 |
| Penicillin/streptomycin solution | Life tech invitrogen | 15140122 |
| Fetal bovine serum (FBS) | Life tech invitrogen | 10500064 |
| Paraformaldehyde | Sigma | P6148-5KG |
| Recombinant fibroblast growth factor 2 (FGF2) | PeproTech | 450-33 |
| Recombinant transforming growth factor beta 1 (TGF-β1) | PeproTech | 100-21 |
| ZO-1 antibody | Life tech invitrogen | 40-2200 |
| α-Tubulin antibody | Sigma | T 6074 |
| α-smooth muscle actin (SMA) antibody | Sigma | A 2547 |
| Phalloidin antibody | Life tech invitrogen | A12379 |
| 3D Collagen Culture kit | Millipore | ECM 675 |
| 8-well chamber slide | Fisher Scientific | NNU 154534-PK |
| Trizol reagent | Life Technologies | 15596-018 |
| ViiA 7 Real-Time PCR System | Life Technologies | 4453536 |
| Superscript First Strand Synthesis kit | Life Technologies | 11904-018 |
Referencias
- Mikawa, T., Fischman, D. A. Retroviral analysis of cardiac morphogenesis: discontinuous formation of coronary vessels. Proc Natl Acad Sci U S A. 89, 9504-9508 (1992).
- Mikawa, T., Gourdie, R. G. Pericardial mesoderm generates a population of coronary smooth muscle cells migrating into the heart along with ingrowth of the epicardial organ. Dev Biol. 174, 221-232 (1996).
- Manner, J., Perez-Pomares, J. M., Macias, D., Munoz-Chapuli, R. The origin, formation and developmental significance of the epicardium: a review. Cells Tissues Organs. 169, 89-103 (2001).
- Gittenberger-de Groot, A. C., Vrancken Peeters, M. P., Mentink, M. M., Gourdie, R. G., Poelmann, R. E. Epicardium-derived cells contribute a novel population to the myocardial wall and the atrioventricular cushions. Circ Res. 82, 1043-1052 (1998).
- von Gise, A., Pu, W. T. Endocardial and epicardial epithelial to mesenchymal transitions in heart development and disease. Circ Res. 110, 1628-1645 (2012).
- Katz, T. C., et al. Distinct compartments of the proepicardial organ give rise to coronary vascular endothelial cells. Dev Cell. 22, 639-650 (2012).
- Singh, M. K., Lu, M. M., Massera, D., Epstein, J. A. MicroRNA-processing enzyme Dicer is required in epicardium for coronary vasculature development. J Biol Chem. 286, 41036-41045 (2011).
- Degenhardt, K., Singh, M. K., Epstein, J. A. New approaches under development: cardiovascular embryology applied to heart disease. J Clin Invest. 123, 71-74 (2013).
- Singh, M. K., Epstein, J. A. Epicardium-derived cardiac mesenchymal stem cells: expanding the outer limit of heart repair. Circ Res. 110, 904-906 (2012).
- Manner, J. Experimental study on the formation of the epicardium in chick embryos. Anat Embryol (Berl). 187, 281-289 (1993).
- Manner, J., Schlueter, J., Brand, T. Experimental analyses of the function of the proepicardium using a new microsurgical procedure to induce loss-of-proepicardial-function in chick embryos. Dev Dyn. 233, 1454-1463 (2005).
- Pennisi, D. J., Ballard, V. L., Mikawa, T. Epicardium is required for the full rate of myocyte proliferation and levels of expression of myocyte mitogenic factors FGF2 and its receptor, FGFR-1, but not for transmural myocardial patterning in the embryonic chick heart. Dev Dyn. 228, 161-172 (2003).
- Gittenberger-de Groot, A. C., Vrancken Peeters, M. P., Bergwerff, M., Mentink, M. M., Poelmann, R. E. Epicardial outgrowth inhibition leads to compensatory mesothelial outflow tract collar and abnormal cardiac septation and coronary formation. Circ Res. 87, 969-971 (2000).
- Lavine, K. J., et al. Endocardial and epicardial derived FGF signals regulate myocardial proliferation and differentiation in vivo. Dev Cell. 8, 85-95 (2005).
- Merki, E., et al. Epicardial retinoid X receptor alpha is required for myocardial growth and coronary artery formation. Proc Natl Acad Sci U S A. 102, 18455-18460 (2005).
- Stuckmann, I., Evans, S., Lassar, A. B. Erythropoietin and retinoic acid, secreted from the epicardium, are required for cardiac myocyte proliferation. Dev Biol. 255, 334-349 (2003).
- Huang, G. N., et al. C/EBP transcription factors mediate epicardial activation during heart development and injury. Science. 338, 1599-1603 (2012).
- Zhou, B., et al. Adult mouse epicardium modulates myocardial injury by secreting paracrine factors. J Clin Invest. 121, 1894-1904 (2011).
- Christoffels, V. M., et al. Tbx18 and the fate of epicardial progenitors. Nature. 458, 8-9 (2009).
- Rudat, C., Kispert, A. Wt1 and epicardial fate mapping. Circ Res. 111, 165-169 (2012).
- Duim, S. N., Kurakula, K., Goumans, M. J., Kruithof, B. P. Cardiac endothelial cells express Wilms’ tumor-1: Wt1 expression in the developing, adult and infarcted heart. J Mol Cell Cardiol. 81, 127-135 (2015).
- Iyer, D., et al. Robust derivation of epicardium and its differentiated smooth muscle cell progeny from human pluripotent stem cells. Development. 142, 1528-1541 (2015).
- Witty, A. D., et al. Generation of the epicardial lineage from human pluripotent stem cells. Nat Biotechnol. 32, 1026-1035 (2014).

