Reprogramming Mouse Embryonic Fibroblasts with Transcription Factors to Induce a Hemogenic Program
Summary
The protocol described here details the induction of a hemogenic program in mouse embryonic fibroblasts via overexpression of a minimal set of transcription factors. This technology may be translated to the human system to provide platforms for future study of hematopoiesis, hematologic disease, and hematopoietic stem cell transplant.
Abstract
This protocol details the induction of a hemogenic program in mouse embryonic fibroblasts (MEFs) via overexpression of transcription factors (TFs). We first designed a reporter screen using MEFs from human CD34-tTA/TetO-H2BGFP (34/H2BGFP) double transgenic mice. CD34+ cells from these mice label H2B histones with GFP, and cease labeling upon addition of doxycycline (DOX). MEFS were transduced with candidate TFs and then observed for the emergence of GFP+ cells that would indicate the acquisition of a hematopoietic or endothelial cell fate. Starting with 18 candidate TFs, and through a process of combinatorial elimination, we obtained a minimal set of factors that would induce the highest percentage of GFP+ cells. We found that Gata2, Gfi1b, and cFos were necessary and the addition of Etv6 provided the optimal induction. A series of gene expression analyses done at different time points during the reprogramming process revealed that these cells appeared to go through a precursor cell that underwent an endothelial to hematopoietic transition (EHT). As such, this reprogramming process mimics developmental hematopoiesis “in a dish,” allowing study of hematopoiesis in vitro and a platform to identify the mechanisms that underlie this specification. This methodology also provides a framework for translation of this work to the human system in the hopes of generating an alternative source of patient-specific hematopoietic stem cells (HSCs) for a number of applications in the treatment and study of hematologic diseases.
Introduction
Hematopoiesis is a complex developmental process where hematopoietic stem cells (HSCs) bud off hemogenic endothelium present in a variety of embryonic hematopoietic sites such as the Aorta-Gonad-Mesonephros and the placenta1,2. The inability to culture HSCs in vitro prevents the in depth analysis of this process as well as the clinical application of these studies. To circumvent this limitation, previous studies have attempted to derive HSCs de novo either via differentiation of pluripotent stem cells (PSCs)3, or induced plasticity in somatic cells and directed differentiation using reprogramming media4,5. These studies, however, do not generate clinically safe engraftable cells or allow study of definitive developmental hematopoiesis "in a dish."
The novel work established by Yamanaka and colleagues to generate induced pluripotent stem cells (iPSCs) from somatic fibroblasts provides a framework for transcription factor (TF) based overexpression strategies in reprogramming cell fate6,7. This work has prompted investigators in several fields to generate cell types of choice via TF reprogramming of easily obtainable somatic cells. The goal of the reprogramming strategy described here is to induce a hemogenic process from mouse somatic cells using a TF based reprogramming approach with the goal of translating these findings to the human system to reprogram patient-specific fibroblasts in order to study human hematopoiesis in vitro and generate patient-specific blood products for disease modeling, drug testing, and stem cell transplant.
The first step to ensure proper reprogramming in this mouse system was to develop a reporter line that served as a read-out for CD34 expression, a known marker in endothelial progenitor cells and HSCs. To do this, the huCD34-tTA and TetO-H2BGFP transgenic mouse lines were used to generate double transgenic mouse embryonic fibroblasts (MEFs), now denoted 34/H2BGFP, that fluoresce green upon activation of the CD34 promoter8. This allowed screening of a variety of TFs known to be required at different points during hematopoietic specification and development. Beginning with 18 TFs in pMX retrovial vectors (determined through literature mining and profiling of GFP label retaining HSCs from the previously described 34/H2BGFP mice), 34/H2BGFP MEFs were transduced with all factors and cultured on AFT024 HSC-supporting stromal cells. After detection of 34/H2BGFP activation, TFs were subsequently removed from the reprogramming cocktail until the optimal set of TFs for reporter activation was identified. After this initial screen, the factors were transferred to a DOX inducible pFUW vector system to allow controllable expression of the TFs. Since these two DOX controllable systems are incompatible (the 34/H2BGFP cells and the pFUW inducible vectors), MEFs from wild-type C57BL/6 mice were required. It was also necessary to provide an appropriate microenvironment to allow hemogenesis to proceed and create multilineage clonogenic progenitors.
Current studies attempting to reprogram somatic cells into hematopoietic stem and progenitor cells (HSPCs) have met varied levels of success9-11. To date, the generation of both mouse and human transplantable HSPCs with long term and self-renewing repopulating ability has not been achieved using the same set of TFs. In this protocol, we provide a detailed description of the previously established strategy to reproducibly induce hemogenesis in MEFs. We demonstrate that introduction of a minimal set of TFs (Gata2, Gfi1b, cFos, and Etv6) is capable of instigating a complex developmental program in vitro that provides a platform by which developmental hematopoiesis and clinical application of hematopoietic reprogramming can be further studied12.
Protocol
Ethics statement: Mouse cell lines are derived following the animal care guidelines of the Icahn School of Medicine at Mount Sinai, and should be done in compliance with any host institution.
1. Mouse Embryonic Fibroblast (MEF) Isolation of C57BL/6 Mice
- Set up timed mating13. Once a vaginal plug is visualized, consider this Day 0.5.
- Separate the plugged females on the plug date and check on them on Day 10-11 to confirm pregnancy.
- On Day 13.5-14.5 euthanize pregnant female mice via CO2 inhalation followed by cervical dislocation.
- Soak pregnant female abdomen with 70% ethanol, dissect the abdominal cavity with sterile scissors and forceps down the midline, and surgically remove the uterine horns containing the embryos14. Take out the embryos gently while cutting off the remaining abdominal tissue.
NOTE: Recover about 8 embryos with 4 on each side. - Place the uterine horns containing the embryos in a 10 cm dish with 5-10 ml of sterile chilled phosphate buffered saline (PBS).
- With sterile scissors and forceps, make an incision along the uterine horns to isolate the sacs carrying the embryos. Transfer the sacs to a new 10 cm dish with 5-10 ml of sterile chilled PBS and place on ice.
- Place a few of the sacs into a new 10 cm dish with 5-10 ml of sterile chilled PBS. Using sterile scissors and forceps gently cut open the sacs (without cutting too deep) in order to avoid piercing the embryos.
- Separate the embryos from the placenta by cutting the umbilical cord.
- Transfer the embryos to a new 10 cm dish containing 5-10 ml of chilled PBS and leave on ice while completing the remaining dissections.
- Decapitate the embryos using sterile forceps. Carefully cut down the midline of the embryo using sterile scissors and forceps, starting from the decapitated area to open up the abdomen. Position forceps underneath the visceral organs (including the heart, liver, and lungs) and scrape them out15.
- Cut the remaining tissue into small pieces using scissors and forceps and transfer to a 50 ml conical tube containing 10 ml of trypsin.
- Pipet up and down with a 10 ml pipet 20-30 times to mix and dissociate the tissue.
- Incubate the tissue and trypsin mix in a 37 °C water bath for 45 min, swirling occasionally.
- Pipet up and down with a 10 ml pipet for about 5 minutes to mix the contents.
- Add trypsinized cells to 3x volume of standard Dulbecco's Modified Eagle Medium (DMEM) with 10% fetal bovine serum (FBS), 10 µg/ml Penicillin/Streptomycin (P/S), and 1 mM L-Glutamine (L-GLUT) in 10 cm dishes (~10 ml) and culture at 37 °C until confluent (about 2-4 days). Culture about 1-2 embryos per 10 cm dish.
NOTE: Freeze cells once plates are confluent. Frozen cells can be thawed and passaged 2 more times. Cells can alternatively be split 2 times prior to freezing, and then once more after being thawed.
2. Viral Production
- Expand 293T cells in 10 cm plates with 10 ml DMEM with 10% FBS, 1% P/S, and 1% L-GLUT for transfection.
- Trypsinize 293T cells with 2 ml trypsin, incubate at 37 °C for 5 min, collect the cells into 15 ml tubes, spin at 300 x g, and plate appropriately (10 ml per 10 cm dish).
- 24 hr prior to transfection, split confluent 10 cm plate of 293T cells 1:6 and seed in 10 cm gelatin coated (0.1% gelatin in PBS) plates.
NOTE: 4 plates per virus will be required.- To coat plates in gelatin, add at least 2 ml of the 0.1% gelatin solution to coat the bottom of 10 cm plates. Incubate at 37 °C for at least 20 min. Aspirate off gelatin prior to adding cells to the plates.
- In a 15 ml tube, add 84 µg plasmid (e.g. Gata2, Gfi1b, cFos, or Etv6 (sub-cloned into the pFUW-TetO vector16) or FUW-M2rtTA17), 84 µg psPAX2, and 42 µg pMD2.G. Add water (H2O) to make up the volume to 2 ml. Prepare a tube for each factor (e.g. Gata2, Gfi1b, cFos, Etv6 and FUW-M2rtTA).
- Add 250 µl of 2M CaCl2 to each tube containing the 2 ml mixture composed of 84 µg of the chosen gene (Gata2, Gfi1b, cFos, Etv6 or FUW-M2rtTA) + 84 µg psPAX2 + 42 µg pMD2.G + H2O.
- Using a Pasteur pipet inserted into the pipet-aid, release bubbles into the 2.25 ml DNA + H2O + 2 M CaCl2 mixture. As the bubbles are being produced, place the tip of a P1000 against the glass pipet and slowly eject 2 ml of 100 mM N,N-bis (2-hydroxyethyl)-2-aminoethane sulfonic acid (BES) saline down the Pasteur pipet 1 ml at a time.
- Incubate all tubes in the culture hood at room temperature for at least 15 min.
NOTE: The 4.25 ml mixture (now DNA + H2O + 2M CaCl2 + BES saline) will look slightly cloudy. - As the mixture incubates, aspirate media off 293T plates and add 10 ml DMEM + 10% FBS + 1 mM L-Glutamine + 25 nM chloroquine.
NOTE: This media does not contain P/S. - After at least 15 min of incubation (or until changing media on 293T cells), add the 4.25 ml DNA + H2O + 2 M CaCl2 + BES saline mixture drop wise to the 293T cells, distributing evenly across 4 cell plates per tube (i.e. slightly more than 1 ml of the mixture per plate).
- Incubate plates overnight (O/N) at 37 °C.
- 24 hr post incubation, replace media on all plates with 4 ml standard DMEM (see step 1.15).
- Keep cells in a 32 °C incubator from now on.
- 24 hr after step 2.10, collect media into separate 50 ml tubes. Replace the collected media from each plate with 4 ml of standard DMEM.
NOTE: 4 plates per initial tube mixture results in 16 ml of collected media.- After 12 hr of incubation, collect media again into the same 50 ml tubes and replace with another 4 ml of standard DMEM.
- After 12 hr of incubation, collect the media and add to the same 50 ml tubes.
NOTE: each tube should now contain about 48 ml of media containing virus.
- To concentrate the collected virus, first filter the collected media through 0.45 µM low-protein binding filters.
- Pour the filtered media containing virus into viral concentration centrifuge tubes (about 16 ml at a time).
- Spin at 4,000 x g at 4 °C for 25 min and remove flow-through.
NOTE: A small volume of concentrated media and virus will be visible in the filter. - Add another 16 ml of filtered virus-containing media to the concentrated media + virus volume remaining in the filter and mix by inverting the tube.
- Spin tube again at 4,000 x g at 4 °C for 25 min and discard flow-through.
- Add remaining filtered collection to the concentrated media + virus volume left in the filter and mix by inverting the tube.
- Spin once more at 4,000 x g at 4 °C for 10-20 min (depending on the volume in the filter) and ensure that the remaining volume of concentrated media + virus left in the filter is about 1 ml. If greater than 1 ml of concentrated media + virus remains in the filter, spin again at 4,000 x g for 1 min and repeat until 1 ml is left in the filter.
- Aliquot 200 µl of the concentrated virus into 1.5 ml tubes and freeze at -80 °C or use immediately.
3. MEF Reprogramming
- Pre-coat 6 well-plates with 0.5 ml of 0.1% gelatin in PBS per well, ensuring that the gelatin covers the entire area of the well. Place in a 37 °C incubator and incubate for at least 20 min. After incubation, remove the plates and aspirate the gelatin.
- Plate MEFs with standard DMEM at a density of 25,000 cells per well (150,000 cells per 6-well plate) on the gelatinized 6-well plate(s) from step 3.1 and allow to settle O/N in a 37 °C incubator.
- Prepare a 100 µl virus cocktail by mixing 50 µl M2rtTA and the remaining 50 µl a mix of Gata2, Gfi1b, cFos, and Etv6 (12.5 µl each).
- Aspirate media from MEFs and add 2 mL standard DMEM with 8 µg/ml hexadimethrine bromide.
- Transduce each well of the MEF plates with 15 µl of the virus cocktail and incubate O/N at 37 °C.
NOTE: This is Day 0 of the reprogramming process. - On Day 1, after 16-20 hr of incubation, replace media with 2 ml fresh standard DMEM supplemented with 1 µg/ml DOX per well.
- Prepare hematopoietic culture media supplemented with hydrocortisone (10-6 M), 100 ng/ml stem cell factor (SCF), 100 ng/ml Fms-related tyrosine kinase 3 ligand (Flt3L), 20 ng/ml interleukin-3 (IL-3), 20 ng/ml interleukin-6 (IL-6), and 1 µg/ml DOX.
- On Day 4 aspirate media from each well and wash cells with 1 ml of PBS per well.
- Aspirate PBS, dissociate cells using 1 ml of 0.05% trypsin per well and collect the cells.
- Count cells with a hemocytometer, spin at 300 x g for 5 min, resuspend in 12 ml of hematopoietic media described in step 3.7 and plate 60,000 cells per 6-well plate on 0.1% gelatin coated plates (2 ml per well, 10,000 cells per well).
- Replace media with fresh supplemented hematopoietic culture media every 6 days for the duration of culture (35 to 36 days).
- Analyze either intermediate cells or emerging hematopoietic-like cells via immuno-staining12, FACS analysis8,12, qRT-PCR12, and mRNA sequencing12 to confirm the acquisition of hemogenic endothelial or HSC-like markers and gene expression.
4. Placental Aggregation and Colony Forming Unit (CFU) Assays in Methylcellulose
- Dissect placentas from pregnant C57BL/6 mice as previously described18 at E12.5 and keep tissue on ice.
- To start placenta dissociation19, wash placental tissue in 2-5 ml of 0.2% collagenase type I in PBS with 20% FBS and mechanically dissociate with an 18G needle fitted into a 5 ml syringe by passaging the placenta and PBS through the needle at least 3 times.
- After mechanical dissociation, keep placental cells in 2-5 ml of collagenase solution at 37 °C for 1.5 hr.
- Passage cells through 20-25 G needles fitted onto 5 ml syringes several times to further mechanically dissociate the placental cells.
- Filter single cell suspensions through 70 µm cell strainers.
- Count cells with a hemocytometer and irradiate at 2,000 cGy for mitotic inactivation20.
- Add 10 ml of hematopoietic culture media supplemented with 100 ng/ml SCF, 100 ng/ml Flt3L, 20 ng/ml IL-3, 20 ng/ml IL-6, and 10 ng/ml thrombopoietin (TPO) to a 10 cm dish.
NOTE: DOX is not required at this stage. - Place a 0.65 µm filter directly on hematopoietic culture media in the dish, allowing it to float to create a liquid-gas interface and set the dish aside.
- Dissociate day 25 transduced MEFs (derived from step 3.11) as described in steps 3.8-3.10 and mix 33,000 of the transduced MEFs with 167,000 cells of the placental aggregates from step 4.6 (1:6 ratio).
- To generate an aggregate18,21, first spin down the MEF and placental cell mix from step 4.9 for 5 min at 300 x g. Aspirate the media and resuspend the pelleted cells in 30-50 µl of standard DMEM using a P200 pipette, drawing the single cell suspension into the 200 µl nonbevelled pipette tip.
- Occlude the pipette tip with paraffin film. Place the blocked tip into a 15 ml centrifuge tube and spin down for 5 min at 300 x g so a pellet forms at the covered end of the tip.
- Remove the tip from the 15 ml tube carefully and place the tip onto the end of the P200 pipette. Discard the paraffin film and extrude the cell pellet onto the floating filter from step 4.8.
NOTE: Plate at most 3 aggregates per filter. - Culture the aggregates on the floating 0.65 µm filters for 4-5 days in 37 °C with 5% CO2.
- Collect the aggregates (at most 3 per filter) with a cell scraper, combine them, and digest with 0.2% collagenase, following the same protocol as done with the whole placentas to dissociate the cells (steps 4.2-4.5).
- After dissociation, wash the aggregates with 2-5 ml of PBS supplemented with 5% FBS and spin down cells at 300 x g for 5 min.
- Resuspend the aggregates in 500 µl of DMEM without FBS, P/S, or L-GLUT. Take this 500 µl resuspension and add it to 3 ml 1% methylcellulose media supplemented with 10 ng/ml of TPO, carefully avoiding the formation of bubbles. Plate equal volumes of this mixture in 3 35 x 10 mm non-treated culture dishes for CFU assays12.
- As a control, culture irradiated placental aggregates without mixing in reprogrammed MEFs 4-5 days and place in CFU assays as described above in steps 4.7-4.16.
NOTE: These aggregates alone do not form colonies. - Count hematopoietic colonies manually under the microscope after 10-14 days of culture at 37 °C with 5% CO2.
- Cytospin22 picked colonies to show morphological phenotype of single cells.
Representative Results
Hematopoiesis is a complex developmental process that begins in various embryonic sites. Hemogenic endothelial cells reside in these sites and give rise to HSCs via cell budding23. This process currently cannot be reproduced by placing HSCs or hematopoietic precursors in culture, necessitating a methodology to somehow obtain these cells in vitro, either by HSPC expansion ex vivo or generation de novo. This protocol demonstrates our novel technology that attempts to obtain these cells via TF overexpression in MEFs.
Figure 1 illustrates the overall reprogramming process. After MEF generation and expansion, cells are transduced with Gata2, Gfi1b, cFos, Etv6 and FUW-M2rtTA. After 3 days of expansion and exposure to DOX to begin transgene activation, cells are dissociated and split on Day 4 onto gelatin coated plates and grown in supplemented hematopoietic media.
Following prolonged culture post transduction with reprogramming media, cells adopt clear morphological changes. At Day 20 cells adopt endothelial morphology distinct from MEFs. Further culture to Day 35 results in several round hematopoietic-like cells emerging from the endothelial-like intermediates that are GFP+ (when using the 34/H2BGFP MEFs) and stain positive for the hematopoietic markers Sca1 and CD45 (Figure 2). This staining demonstrates that these cells gain phenotypic markers suggestive of hemogenic induction. Further culture results in cells expressing CD45 while maintaining expression of the huCD34 reporter. Upon placental aggregation culture cells adopt clonogenic potential and generate colonies in methylcellulose containing various hematopoietic morphologies and blast-like cells (Figure 3).
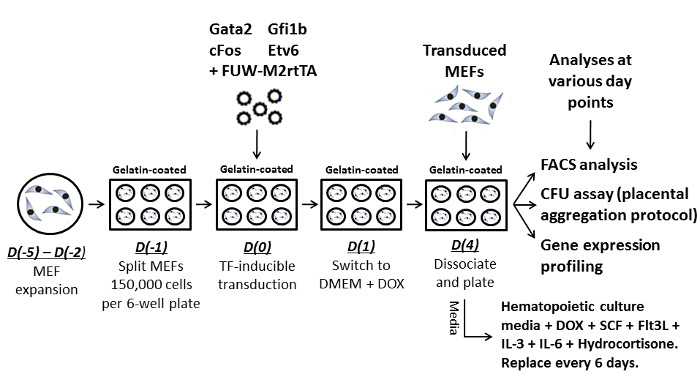
Figure 1: Strategy for hemogenic induction in MEFs. Diagram illustrating the reprogramming process and each step within it. The general reprogramming process first involves MEF expansion, followed by splitting at the appropriate density into gelatin-coated 6-well plates. Cells are then transduced with the cocktail of Gata2, Gfi1b, cFos, Etv6, and FUW-M2rtTA. Cells are further cultured with standard DMEM supplemented with DOX. At Day 4 cells are trypsinized and split into 6-well plates. Every 6 days media is changed and at chosen time points cells can be analyzed by FACS, CFU, or gene expression assays. Please click here to view a larger version of this figure.
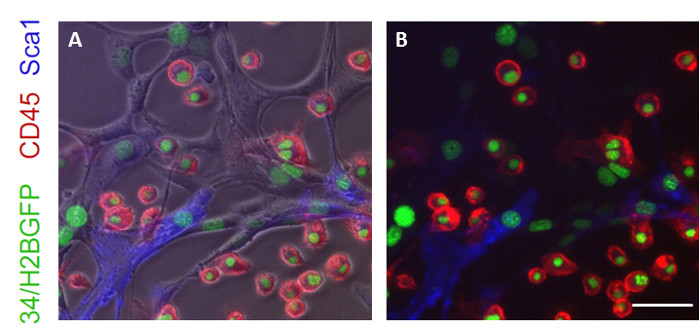
Figure 2: Induction of hematopoietic cells emerging from precursors. Cells positive for 34/H2BGFP stained for Sca1 and CD45 demonstrate induction of a hemogenic program. (A) This image shows a merge of cells stained for CD45 and Sca1 while fluorescing GFP with a underlying brightfield image of the reprogrammed cells. Only a subset of the flat cell population expresses Sca1, an endothelial/hemogenic marker, and only rounded hematopoietic-like cells emerging from these cells stain red for CD45, a pan-hematopoietic marker. (B) This image shows the stained and GFP fluorescent cells without the brightfield image, clearly showing close association of CD45+ cells with the Sca1+ cells. Scale bar = 100 µM. Please click here to view a larger version of this figure.
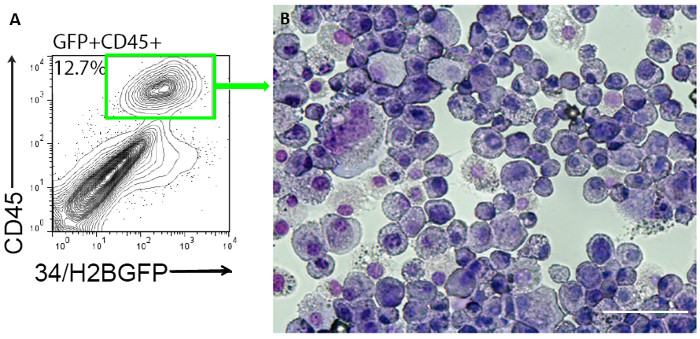
Figure 3: Generation of CD45+ hematopoietic cells. (A) About 12.7% of 34/H2BGFP MEFs transduced with Gata2, Gfi1b, cFos, and Etv6 are GFP+CD45+ after 35 days of reprogramming. Clear myeloid morphologies and blast-like cells can be seen following H&E staining (B) after cytospin of CD45+ sorted cells plated in methylcellulose for 10-14 days. This demonstrates the clonal multilineage potential of the reprogrammed cells that adopt hematopoietic function after transduction with this minimal set of TFs. Scale bar = 100 µM. Please click here to view a larger version of this figure.
Discussion
Generating HSPCs de novo from easily attainable somatic cells offers a unique method to study hematopoiesis in vitro, and the opportunity to potentially apply this technology to the human system. This translation would generate a new tool to study human hematologic disease in a dish, as well as provide drug testing platforms and gene targeting opportunities to treat numerous disorders with novel therapeutics or HSC transplants. In the field, recent studies have expanded on the ability to generate HSPCs de novo, demonstrating the importance of several features of the reprogramming process. These include the choice of the starting cell populations and TF cocktails, as well as how culture conditions (co-culture niche) and supplementation (cytokines, small molecules, etc.) significantly impact the efficiency of reprogramming24-26. Several studies are applying reprogramming technology to the human system without traveling through a pluripotent intermediate,27,28 an undesirable step in these processes.
Here is a protocol that was the first to generate HSC-like cells from somatic cells via a developmental process while avoiding the use of the pluripotency factors and the formation of pluripotent intermediates. The generated HSC-like cells appear to develop via a developmental process, first requiring cells to travel through a hemogenic endothelial intermediate similar to an EHT. At Day 20 of reprogramming endothelial-like intermediates form that contain endothelial and hematopoietic gene expression profiles. HSC-like cells emerge from these intermediate cells at Day 35 that contain cell surface immuno-phenotypes and gene expression profiles highly similar to HSCs and can produce erythroid and myeloid colonies in CFU assays. This suggests that, unlike most other studies, this reprogramming protocol recapitulates a complex developmental process in vitro, and can permit further study of hematopoiesis and hematologic disease processes that involve embryonic hematopoiesis. These studies allow further research on how to treat and cure the multitude of hematologic disorders that exist, and how to manipulate hematopoiesis to this end. This can be done by inducing a hemogenic program in patient-specific fibroblasts to establish in vitro disease models. With these models, normal and diseased hematopoiesis can be finely dissected and studied, as well as subjected to drug screens and gene editing to treat/correct hematopoietic defects associated with the disease of interest.
Using mouse fibroblasts supports various beneficial qualities of this reprogramming process. The fibroblasts themselves are easy to obtain from donor mice, making cell acquisition simple. Translating this technology to humans would thus only necessitate patient skin samples, making acquisition of patient-specific blood products and subsequent cell testing a viable option for hematologic disease treatment. Additionally, fibroblasts are very epigenetically distinct from hematopoietic cells, demonstrating the ability of the chosen TFs in this reprogramming protocol to both epigenetically repress fibroblast identity, and also to instigate a hemogenic program by altering the epigenetic memory of the starting cells29,30. Although transplantation studies do not yet demonstrate full multilineage engraftment function of these derived cells, seeing if these factors induce a hemogenic program that generates fully functional hematopoietic cells in the human system will be of great interest.
Several steps within this protocol can be modified in case of difficulty during the reprogramming, such as the number of cells plated prior to transduction and the amount of virus used for transduction. Optimal results were seen using 150,000 cells per 6-well plate (step 3.2) with the previously described volume of virus (steps 3.3 and 3.5), but more cells can be used in case of cell death upon exposure to the virus. Similarly, smaller volumes of virus can be used should cell death be an issue, as long as reprogramming proceeds as described (that can be checked via FACS analysis, CFU assays, and gene profiling). Ensuring that steps 2.13-2.19 yield proper amounts of virus is required for the success of the rest of the protocol. Steps 3.5-3.7 are also critical, as successful transduction of cells is crucial for inducing this hemogenic program. The appropriate amount of DOX per well of transduced cells must be kept consistent and fresh to ensure expression of the transgenes (thus necessitating the frequent media changes). Though not absolutely necessary, cell culture in the dark when using DOX may help prevent loss of DOX function. After transduction, cells should be closely monitored under the microscope by morphology and assayed by various analytical methods (such as FACS and CFU assays) to ensure successful viral transduction and reprogramming. Reprogrammed cells may not adopt as many morphological changes as expected, requiring the previously mentioned analytical methods to serve as the best indicators of proper reprogramming.
Recent studies have utilized different features of this reprogramming protocol, such as Gata2 in the TF cocktail or fibroblasts as the starting cell population31-33. These methodologies also appear to induce developmental programs during the reprogramming process. Other strategies have shown the importance of the hematopoietic niche during reprogramming9,10,25,26. Identifying all the factors that assist in reprogramming will be essential to develop the protocol that generates bona fide HSCs from easily attainable patient-specific cells. In summary, here we describe a strategy to generate HSC-like cells from an induced developmental process in mouse fibroblasts via inducible Gata2, Gfi1b, cFos, and Etv6 overexpression. This technology will be translated to the human system, where, in combination with other published studies, will allow generation of patient-specific blood products suitable for a variety of clinical applications.
Divulgaciones
The authors have nothing to disclose.
Acknowledgements
This work was supported by an NHLBI grant to K.M. and I.R.L. (1RO1HL119404). Carlos-Filipe Pereira was the recipient of a Revson Senior Biomedical Fellowship. We gratefully acknowledge the Mount Sinai hESC/iPSC Shared Resource Facility and S. D’Souza for assistance with materials and protocols. We also thank the Mount Sinai Flow Cytometry, Genomics, and Mouse facilities.
Materials
| DMEM | Invitrogen | 11965-092 | |
| 0.45uM filters | Corning | 430625 | |
| Amicon Ultra centrifugal filters | Millipore | UFC900324 | |
| Penicillin/Streptomycin | Invitrogen | 15140-122 | |
| L-Glutamine | Invitrogen | 25030-081 | |
| FBS | Gemini's Benchmark | 100-106 | |
| PBS | Life Technologies | 14190-144 | |
| 18G needles | BD | 305195 | |
| 20G needles | BD | 305175 | |
| 25G needles | BD | 305125 | |
| Collagenase Type I | Sigma | C0130-100MG | |
| TrypLE Express | Invitrogen | 12605-010 | |
| Myelocult media | Stem Cell Technologies | M5300 | |
| SCF | R & D Systems | 455-MC | |
| Flt3L | R & D Systems | 427-FL | |
| IL-3 | R & D Systems | 403-ML | |
| IL-6 | R & D Systems | 406-ML | |
| TPO | R & D Systems | 488-TO | |
| Doxycycline | Sigma | D9891-1G | |
| Polybrene (hexadimethrine bromide) | Sigma | AL-118 | |
| Durapore 0.65uM membrane filters | Millipore | DVPP14250 | |
| Methylcellulose media | Stem Cell Technologies | Methocult M3434 | |
| Hydrocortisone | Stem Cell Technologies | 07904 | |
| C57BL/6 mice | The Jackson Laboratory | 000664 | |
| Gelatin | Sigma | G-1890 100g | |
| pFUW-tetO | Addgene | Plasmid #20321 | |
| Gata2 | Origene | MR226728 | |
| Gfi1b | Origene | MR204861 | |
| cFos | Addgene | Plasmid #19259 | |
| Etv6 | Origene | MR207053 | |
| psPAX2 | Addgene | Plasmid #12260 | |
| pMD2.G | Addgene | Plasmid #12259 | |
| CaCl2 | Sigma | C5670-100g | |
| FUW-M2rtTA | Addgene | Plasmid #20342 | |
| 35 x 10 mm culture dishes | Thermo Scientific | 171099 |
Referencias
- Zovein, A. C., et al. Fate tracing reveals the endothelial origin of hematopoietic stem cells. Cell Stem Cell. 3 (6), 625-636 (2008).
- Bertrand, J. Y., et al. Haematopoietic stem cells derive directly from aortic endothelium during development. Nature. 464 (7285), 108-111 (2010).
- Papapetrou, E. P., Sadelain, M. Reconstructing blood from induced pluripotent stem cells. F1000 Med Rep. 2, (2010).
- Szabo, E., et al. Direct conversion of human fibroblasts to multilineage blood progenitors. Nature. 468 (7323), 521-526 (2010).
- Mitchell, R., et al. Molecular evidence for OCT4-induced plasticity in adult human fibroblasts required for direct cell fate conversion to lineage specific progenitors. Stem Cells. 32 (8), 2178-2187 (2014).
- Takahashi, K., Yamanaka, S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 126 (4), 663-676 (2006).
- Takahashi, K., et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 131 (5), 861-872 (2007).
- Qiu, J., Papatsenko, D., Niu, X., Schaniel, C., Moore, K. Divisional history and hematopoietic stem cell function during homeostasis. Stem Cell Reports. 2 (4), 473-490 (2014).
- Riddell, J., et al. Reprogramming committed murine blood cells to induced hematopoietic stem cells with defined factors. Cell. 157 (3), 549-564 (2014).
- Sandler, V. M., et al. Reprogramming human endothelial cells to haematopoietic cells requires vascular induction. Nature. 511 (7509), 312-318 (2014).
- Daniel, M. G., Pereira, C. F., Lemischka, I. R., Moore, K. A. Making a Hematopoietic Stem Cell. Trends Cell Biol. , (2015).
- Pereira, C. F., et al. Induction of a hemogenic program in mouse fibroblasts. Cell Stem Cell. 13 (2), 205-218 (2013).
- Mader, S. L., Libal, N. L., Pritchett-Corning, K., Yang, R., Murphy, S. J. Refining timed pregnancies in two strains of genetically engineered mice. Lab Anim (NY. 38 (9), 305-310 (2009).
- Jain, K., Verma, P. J., Liu, J. Isolation and handling of mouse embryonic fibroblasts). Methods Mol Biol. 1194, 247-252 (2014).
- Bryja, V., Bonilla, S., Arenas, E. Derivation of mouse embryonic stem cells. Nat Protoc. 1 (4), 2082-2087 (2006).
- Carey, B. W., et al. Reprogramming of murine and human somatic cells using a single polycistronic vector. Proc Natl Acad Sci U S A. 106 (1), 157-162 (2009).
- Hockemeyer, D., et al. A drug-inducible system for direct reprogramming of human somatic cells to pluripotency. Cell Stem Cell. 3 (3), 346-353 (2008).
- Taoudi, S., et al. Extensive hematopoietic stem cell generation in the AGM region via maturation of VE-cadherin+CD45+ pre-definitive HSCs. Cell Stem Cell. 3 (1), 99-108 (2008).
- Gekas, C., K, E. R., H, K. A. M. Isolation and analysis of hematopoietic stem cells from the placenta. J Vis Exp. (16), (2008).
- Lewis, I. D., et al. Umbilical cord blood cells capable of engrafting in primary, secondary, and tertiary xenogeneic hosts are preserved after ex vivo culture in a noncontact system. Blood. 97 (11), 3441-3449 (2001).
- Sheridan, J. M., Taoudi, S., Medvinsky, A., Blackburn, C. C. A novel method for the generation of reaggregated organotypic cultures that permits juxtaposition of defined cell populations. Genesis. 47 (5), 346-351 (2009).
- Koh, C. M. Preparation of cells for microscopy using cytospin. Methods Enzymol. 533, 235-240 (2013).
- Orkin, S. H., Zon, L. I. Hematopoiesis: an evolving paradigm for stem cell biology. Cell. 132 (4), 631-644 (2008).
- Martinez-Agosto, J. A., Mikkola, H. K., Hartenstein, V., Banerjee, U. The hematopoietic stem cell and its niche: a comparative view. Genes Dev. 21 (23), 3044-3060 (2007).
- Butler, J. M., et al. Development of a vascular niche platform for expansion of repopulating human cord blood stem and progenitor cells. Blood. 120 (6), 1344-1347 (2012).
- Gori, J. L., et al. Vascular niche promotes hematopoietic multipotent progenitor formation from pluripotent stem cells. J Clin Invest. 125 (3), 1243-1254 (2015).
- Bar-Nur, O., et al. Lineage conversion induced by pluripotency factors involves transient passage through an iPSC stage. Nat Biotechnol. 33 (7), 761-768 (2015).
- Maza, I., et al. Transient acquisition of pluripotency during somatic cell transdifferentiation with iPSC reprogramming factors. Nat Biotechnol. 33 (7), 769-774 (2015).
- Kim, K., et al. Epigenetic memory in induced pluripotent stem cells. Nature. 467 (7313), 285-290 (2010).
- Vaskova, E. A., Stekleneva, A. E., Medvedev, S. P., Zakian, S. M. “Epigenetic memory: phenomenon in induced pluripotent stem cells. Acta Naturae. 5 (4), 15-21 (2013).
- Elcheva, I., et al. Direct induction of haematoendothelial programs in human pluripotent stem cells by transcriptional regulators. Nat Commun. 5, 4372 (2014).
- Vereide, D. T., et al. An expandable, inducible hemangioblast state regulated by fibroblast growth factor. Stem Cell Reports. 3 (6), 1043-1057 (2014).
- Batta, K., Florkowska, M., Kouskoff, V., Lacaud, G. Direct reprogramming of murine fibroblasts to hematopoietic progenitor cells. Cell Rep. 9 (5), 1871-1884 (2014).

