Tracing Gene Expression Through Detection of β-galactosidase Activity in Whole Mouse Embryos
Summary
Here we describe the standard protocol for the detection of β-galactosidase activity in early whole mouse embryos and the method for paraffin sectioning and counterstaining. This is an easy and quick procedure to monitor gene expression during development that can also be applied to tissue sections, organs or cultured cells.
Abstract
The Escherichia coli LacZ gene, encoding β-galactosidase, is largely used as a reporter for gene expression and as a tracer in cell lineage studies. The classical histochemical reaction is based on the hydrolysis of the substrate X-gal in combination with ferric and ferrous ions, which produces an insoluble blue precipitate that is easy to visualize. Therefore, β-galactosidase activity serves as a marker for the expression pattern of the gene of interest as the development proceeds. Here we describe the standard protocol for the detection of β-galactosidase activity in early whole mouse embryos and the subsequent method for paraffin sectioning and counterstaining. Additionally, a procedure for clarifying whole embryos is provided to better visualize X-gal staining in deeper regions of the embryo. Consistent results are obtained by performing this procedure, although optimization of reaction conditions is needed to minimize background activity. Limitations in the assay should be also considered, particularly regarding the size of the embryo in whole mount staining. Our protocol provides a sensitive and a reliable method for β-galactosidase detection during the mouse development that can be further applied to the cryostat sections as well as whole organs. Thus, the dynamic gene expression patterns throughout development can be easily analyzed by using this protocol in whole embryos, but also detailed expression at the cellular level can be assessed after paraffin sectioning.
Introduction
In order to describe specific gene expression patterns, the use of reporter genes as markers has been paramount from Drosophila to mammals. In experiments involving transgenic and knockout animals, the bacterial β-galactosidase gene (LacZ) of Escherichia coli (E. coli) is one of the most widely used1,2,3,4. β-galactosidase (β-gal) catalyzes the hydrolysis of β-galactosides (such as lactose) into its monosaccharides (glucose and galactose)5. Its most commonly used substrate is X-gal (5-bromo-4-chloro-3-indolyl-β-D-galactopyranoside), a glycoside that is hydrolyzed by β-galactosidase giving rise to 5-bromo-4-chloro-3-hydroxyindole and galactose. The first is oxidized into a dimer that, when used combined with potassium ferri-and ferro-cyanide, produces a characteristic insoluble, blue color precipitate (Figure 1)6.
The LacZ gene started to be used as a reporter gene over thirty years ago7,8. Usually, LacZ is inserted downstream of an endogenous promoter in the place of the open reading frame, so it can be used in bacterial and cell culture to visualize cells containing a particular insert, as well as in transgenic animals as a tracer of endogenous gene expression patterns during development9. In this regard, the visualization of β-galactosidase activity has been extensively used in Drosophila to understand the developmental and cellular processes from single cells to whole tissues. Drosophila genetics favor the generation of stable lines in which a modified P-element construct containing the reporter gene LacZ is inserted at random locations in the genome. Thus, when placed under the influence of enhancer elements it may drive its expression in a tissue specific manner, which has allowed the systematic analysis of the expression patterns of many genes during the past two decades10. In addition, the use of transgenic mice to monitor LacZ gene expression also allows detection of gene recombination events by Cre-loxP mediated recombination, and localization of the mutant embryonic stem cell derivatives in chimeric analyses11, which facilitates the control of LacZ expression in specific tissues as well as temporally. Also, in whole embryos, detection of the β-galactosidase activity may produce differential staining patterns at different intensities that can be conveniently observed across different developmental stages to analyze temporal changes in gene expression8,12.
In this article, we present a protocol to visualize gene expression through X-gal staining in the whole mount tissue at early developmental stages of mouse embryos. We present this histochemical method as a highly sensitive and inexpensive technique that favors accurate detection of the labeled cells either in whole mount specimens or at the cellular level after paraffin embedded tissues or embryos. The method allows for the direct visualization of staining in the mouse tissue with the minimum background when compared with other methods13.
Protocol
All experimental procedures were approved by the Committee on the Ethics of Animal Experiments of the CNIC (Centro Nacional de Investigaciones Cardiovasculares) and the Comunidad Autónoma de Madrid to ensure minimal animal suffering.
1. Collection of Embryos from Pregnant Mice (from E8.5 to E12.5)
- Sacrifice pregnant mice by either cervical dislocation or CO2 inhalation. The day of the first observed vaginal plug was considered embryonic day 0.5 (E0.5).
- Lay the animal in the supine position on the absorbent pad and clean the abdominal skin of the mouse with 70% ethanol.
NOTE: Sterility is not required in the following steps. - Pinch and lift the abdominal skin using mouse-tooth forceps.
- Make an incision through the skin and the abdominal wall, 2 to 4 cm vertically across the midline of the abdomen using surgical scissors.
- Expose the abdomen and remove the uterine horns from the mother with forceps cutting blood vessels along the inner curvature of the uterus with surgical scissors.
- Remove the string of embryos and transfer it to a Petri dish on ice containing 10 mL ice-cold 0.01 M phosphate buffer saline pH 7.4 (PBS).
- Carefully dissect the embryos out from the uterus under a stereomicroscope in a Petri dish with 10 mL fresh ice-cold PBS. Grasp the muscle wall with watchmakers' forceps to expose the amniotic sac, and then tear the amniotic membrane to remove the enclosed embryo and the yolk sac using the tips of the forceps.
- Collect the littermate embryos from pregnant mice at early stages (from E8.5 to E12.5) (Figure 2).
- Transfer the embryos to a fresh dish with 10 mL cold 1x PBS using curved forceps or, for very small embryos (E8.5-E9.5), use plastic transfer pipettes to collect the samples without embryo damage.
- Before starting the fixation, take an embryonic sample in a microcentrifuge tube for genotyping by cutting a small piece of the embryo or taking the amniotic membrane in the smallest embryos (from E8.5 to E9.5) with watchmakers' forceps.
NOTE: LacZ genotyping is determined by polymerase chain reaction (PCR) primers: forward, 5´-ATCGTGCGGTGGTTGAACTG-3´; reverse, 5´-CGAGGCGTTAACCGTCAGCA -3´.
2. Fixation of Mouse Embryos
- Transfer each embryo into a 2 mL microcentrifuge tube with a rounded base containing 1 mL 1x PBS.
NOTE: Perform all steps in the protocol in these tubes with agitation using a rolling shaker. - Wash the embryos in 1x PBS for 1 min at room temperature to eliminate the remaining blood. Use plastic transfer pipettes to change liquids in the tubes.
- Fix the embryo in 1 mL of 0.125% glutaraldehyde on ice.
NOTE: The time of fixation depends on the size of the embryo: 20 min for E8.5-E9.5 embryos, 30 min for E10.5 embryos and 1 h for E12.5 embryos. - Remove the fixative and wash the embryos in 1x PBS twice for 10 min at room temperature before processing the sample for X-gal staining.
3. Whole Mount β-galactosidase Histochemistry of Mouse Embryos
- Prepare X-Gal Rinse buffer and X-Gal Staining Solution before starting the procedure (see Table 1 and Table of Materials). Use a wild-type (WT) littermate embryos as negative controls for the enzymatic reaction.
- Incubate the embryos in X-Gal Rinse buffer for 10 min at room temperature (Figure 2).
- Incubate the embryos in X-Gal Staining Solution from 2 h to overnight at 37 °C protected from light. Monitor the color reaction periodically via a dissecting microscope until sufficient intensity of blue staining is observed without the background in the embryo. Keep the pH of the solution between 8 and 9 to reduce background during the whole staining. If the staining solution begins to turn light, replace it with fresh substrate solution to extend the enzymatic reaction.
- Stop the reaction when the desired signal is obtained by washing embryos in a microcentrifuge tube with 1 mL 1x PBS twice for 10 min each at room temperature.
- Transfer stained embryos to 1 mL 4% paraformaldehyde (PFA) to re-fix them, for 1 h to overnight at 4 °C.
NOTE: Embryos can be stored in 4% PFA/PBS for longer times at 4 °C or can be immediately processed for sectioning. This final fixation step is crucial for the long-term conservation of the staining pattern. - Wash the embryos in 1 mL 1x PBS twice for 10 min at room temperature.
- Option 1: Clear embryos by immersion in a series of solutions with increasing concentrations of glycerol (20%, 40%, 60% and 80% glycerol in 1x PBS) for 1 h at room temperature in each solution.
NOTE: Wash embryos in 80% glycerol for longer times, even days, until they are cleared, and then store them in 80% glycerol at 4 °C at this step (Figure 2). Adding a very small amount of sodium azide ora crystal of thymol to the embryos stored at 4 °C either in glycerol or 1x PBS is recommended to prevent mold growth. After clearing, whole mount embryos can be used for photographing (step 4). - Option 2: Process embryos for paraffin embedding and sectioning using a microtome (Figure 2). Document the expression pattern in whole stained embryos before sectioning (steps 4 and 5).
4. Photography of Whole Mount Embryos
- To photograph whole mount stained embryos prior to sectioning, transfer them to a Petri dish prepared with a base of solidified 1-2% agarose in 1x PBS.
- Cover the embryo completely with 10 mL 1x PBS in the agarose-coated dishes to prevent dehydration and reflection while photographing through the liquid.
- Orientate the embryo immersed in 1x PBS using forceps. For older embryos (E10.5-E12.5), make a small hole in the agarose with forceps to place and position the specimen within it at different angles and to avoid the free-floating during photography.
- Place the dish on the stereomicroscope stage, using the transmitted (under-stage) light. Change between the 'dark-field' and 'bright-field' adjustments to set the desired illumination during photography and to optimize the translucency of the sample.
NOTE: Use fiber optic illumination for later-stage embryos to avoid reflection at the surface of the embryo. When photographing cleared embryos, follow the same procedure but transfer them to a Petri dish containing 100% glycerol and fully immerse them.
5. Paraffin Embedding and Sectioning of X-gal Stained Embryos
- Paraffin wax embedding
- Remove the X-gal stained embryos from 4 °C (step 3.5) and wash them with 1 mL 1x PBS three times to eliminate the excess of fixative.
- Transfer each embryo to a histology cassette using forceps.
- Place the cassettes containing the embryos in a beaker and then dehydrate by passing them through a graded ethanol series at room temperature: 70% ethanol, once for 30 min; 96% ethanol, twice for 30 min each; 100% ethanol, twice for 30 min each.
NOTE: Embryos can be stored in 70% ethanol for an undetermined time at 4 °C until starting the dehydration process. - Incubate the whole cassette in isopropanol for 30 min at room temperature.
- By using forceps, transfer the cassettes to a glass staining trough containing pre-warmed isopropanol and leave in an oven at 60 °C for 30 min.
- Place the cassettes in a new glass staining trough containing a mixture of 50% isopropanol/ 50% melted paraffin wax and leave for 4 h in a 60 °C oven.
- Incubate the cassettes with the embryos with two changes of molten paraffin wax, 30 min at 60 °C each.
- At the end of the final paraffin wash, open the cassette and transfer each embryo to a separate tissue embedding mold to view the sample under a stereomicroscope.
- Fill the well with molten paraffin wax at 60 °C. Use heated forceps to keep the wax molten and carefully orientate the embryo in the mold.
NOTE: The proper orientation of the sample is a critical step for sectioning the embryo in the desired plane. - Before paraffin solidifies in the mold, place a cassette without the lid on the top of the block and fill it with molten paraffin wax. Let the remaining wax cool until solidified overnight at room temperature.
- Once the paraffin is completely solidified, remove the solid block from the mold and store the paraffin blocks either at room temperature or at 4 °C until sectioning14.
- Paraffin Sectioning
- Orient the blade on the microtome and set up 5-7 µm of thickness. 5.2.2. Cool the paraffin block on the ice and trim it to produce a cube or a pyramid.
- Attach the block to the microtome holder by using a small amount of molten wax.
- Orient the block for optimal cutting. Section the paraffin blocks into 5-7 µm thick slices at room temperature using standard microtome.
- Using a fine paintbrush, place the sections in a water bath at room temperature for manipulating and selecting slices.
- Pick up sections from the water bath with a microscope slide and transfer them into a water bath at 42 °C for stretching.
- Remove sections from the water bath and gently place them onto adhesion microscope slides.
- Dry the slides well in an oven at 37 °C overnight to allow the sections to completely adhere, otherwise, they may get lost during the staining.
NOTE: Store the slides at 4 °C until starting staining procedures.
- Deparaffinization and Counterstaining of X-Gal-stained Paraffin Sections
- Put the slides on a microscope-slide tray in an oven at 60 °C for at least 45 min to make the paraffin wax more fluid.
- Transfer the slides onto a rack and use glass staining dishes to perform the following steps: submerge the rack in the staining jars containing alcohols of decreasing concentrations for complete paraffin removal and hydration of the tissues: isopropanol for 5 min at 60 °C; isopropanol for 3 min; 100% ethanol for 2 min; 96% ethanol for 1 min; 70% ethanol for 1 min at room temperature.
- Rinse sections in distilled water twice to make sure that there are no alcohol residues.
- Counterstain sections with a stain (e.g. Nuclear-Fast Red solution) for 5 min (usually between 3-8 min) at room temperature (Figure 2).
NOTE: This staining helps to reveal overall tissue structure in the sections. - After staining, wash the slides in distilled water for 1 min and check section staining in the microscope.
NOTE: If the desired staining is too weak, counterstain sections again for a longer time. - Dehydrate sections in the following series with increasing concentrations of ethanol: two washes in 95% ethanol for 2 min each, two washes in 100% ethanol for 2 min each, and, to avoid dissolving the blue color precipitates, just one quick wash in xylene.
- Remove the slides from the rack one by one and place them on a piece of paper. Then mount and coverslip each slide using a synthetic, permanent mounting medium.
NOTE: Xylene is a flammable product whose vapors are irritating and harmful to human health and to aquatic organisms. It needs to be manipulated within a hood and requires the use of proper protective equipment.
Representative Results
Here we show the results from applying the standard protocol for the β-galactosidase histochemical reaction using X-gal as the substrate in whole mouse embryos (Figure 1 and Figure 2). By using this protocol, we examine Membrane type 4-matrix metalloproteinase (Mt4-mmp) expression at different embryonic developmental stages (E9.5, E11.5, and E12.5) using Mt4-mmp mutant mice that express the LacZ reporter under the control of the endogenous Mt4-mmp promoter (Figure 3, Figure 4, and Figure 5)9,15. Mt4-mmp is an endopeptidase that is tethered to the cell membrane through a glycophosphatidyl inositol anchor (GPI). Although Mt4-mmp has been detected in several human cancers and has been related to the progression of tumor growth, information about its proteolytic activity against extracellular matrix components is very limited to date. Additionally, almost nothing has been reported on the role and expression of Mt4-mmp during embryonic development9,16.
Wild type (WT), Mt4-mmpLacZ/+ heterozygous (HT) and knockout (KO) littermate embryos are processed in parallel for the X-gal staining protocol (Figure 2). Several controls must be included during the staining process to validate the specificity of the procedure. Thus, WT embryos are used as negative controls for the histochemical reaction and serve to monitor non-specific X-gal labeling (see Figure 3A-D and Figure 4A-C). Also, to avoid unspecific background, the pH of the X-Gal staining solution must be maintained between 8 and 9 throughout the whole staining. In Figure 3E, β-gal-positive cells are visualized in whole mount HT embryos in the somites and the intersomitic vasculature at the E9.5 stage. However, to report the precise location of the stained cells, embryo sectioning is required to visualize under the microscope reported gene expression in tissue sections at cellular resolution. In this case, LacZ-positive cells are found in the somites, the dorsal aorta, the intersomitic vasculature as well as the perineural vascular plexus in tissue sections (arrows in Figure 3F-H). These results correlate with the expression pattern reported for Mt4-mmp in these mouse embryonic stages9, indicating that this technique is easily reproducible and reliable. As expected, levels of β-gal activity are higher in the KO embryos as compared with the HT due to the presence of two copies of the LacZ reporter gene (Figure 3I-L). However, only HT LacZ/+ embryos should be analyzed in studies of gene expression patterns and KO tissue is used as a proof of concept to demonstrate the feasibility of the method since due to the lack of the targeted gene of interest, β-gal positive cells can be abnormally located in the latter.
When performing this protocol, older embryos (from E10.5 to E12.5) are not translucent, and, therefore, the most visible X-gal stained regions are those close to the surface. Thus, an alternative step to this method is to clear embryos by washes in solutions with increasing glycerol concentrations. In Figure 4, the E12.5 LacZ-stained embryos became clear while maintaining the X-gal staining, allowing us to photograph deeper stained regions of the embryo in the stereomicroscope. As previously reported at this embryonic stage, labeling was localized in distinct regions of the brain, the limbs, the follicle of vibrissa, and the tip of the tail9. However, if one wishes to obtain a cellular resolution, embryos should be embedded in paraffin, sectioned and microscopically examined.
In Figure 5, immunohistochemistry with anti-β-gal antibodies was used to confirm the expression of Mt4-mmp observed by means of reporter LacZ staining. Thus, β-gal-positive cells were detected in the pool of motoneurons of the mouse spinal cord at E11.5 by using both approaches, which confirms the specificity of the protocol reported here (Figure 5A, B).
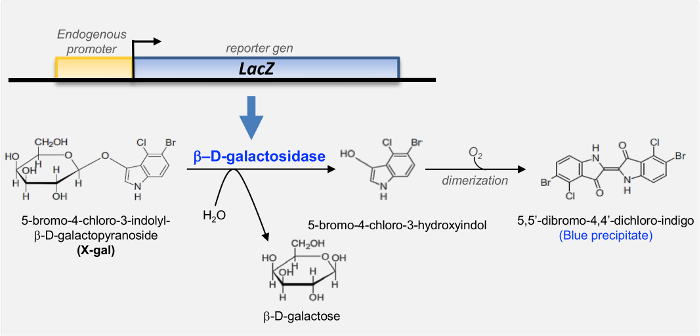
Figure 1. Schematic illustration of the E. coli LacZ reporter gene encoding for the β-galactosidase and the histochemical reaction catalyzed by this enzyme. In the traditional staining, β-galactosidase hydrolyzes the substrate 5-bromo-4-chloro-3-indolyl-β-D-galactopyranoside (X-gal) to 5-bromo-4-chloro-3-hydroxyindole. Subsequently, this intermediate is oxidized, which causes dimerization and formation of the final blue-colored product (5,5'-dibromo-4,4'-dichloro indigo). Please click here to view a larger version of this figure.
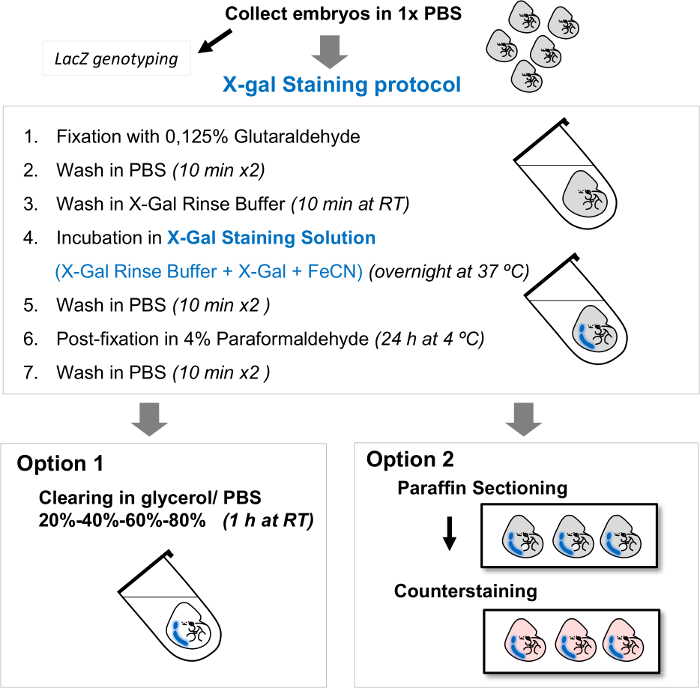
Figure 2. Diagram showing the main steps of the standard protocol for the detection of β-galactosidase activity. Mouse embryos are collected and processed for whole mount X-gal staining. After the histochemical staining performed according to the protocol, whole mount embryos can be (Option 1) cleared with washes in solutions with increasing glycerol concentrations and subsequently photographed in a stereomicroscope, or (Option 2) embedded in paraffin, sectioned and counterstained. Abbreviations: RT, room temperature. Please click here to view a larger version of this figure.
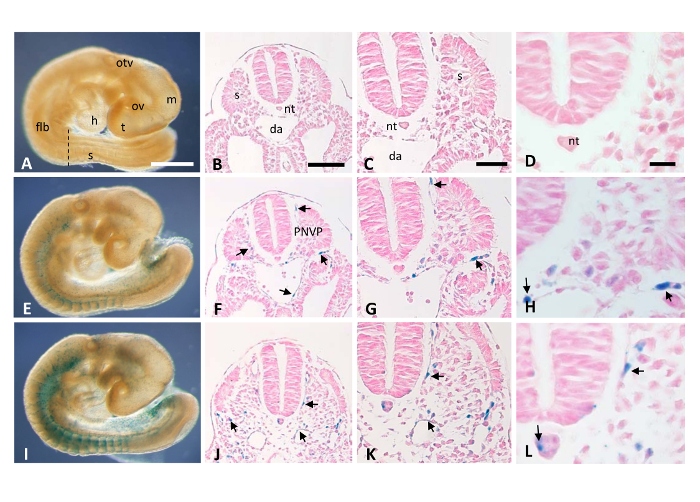
Figure 3. X-gal staining of E9.5 WT and Mt4-mmpLacZ/+ whole-mount embryos and tissue sections. By using the standard X-gal histochemical assay, transgenic embryos carrying the LacZ gene under the control of Mt4-mmp promoter were analyzed for β-gal activity. WT (A), HT (E) and KO (I) E9.5 whole mount embryos were processed for the detection of the β-galactosidase expression. Paraffin sections obtained from these embryos at the level of the spinal cord allow visualization of the staining at cellular resolution. As expected, β-gal positive cells were absent in WT sections (B-D), while the intensity of labeling was higher in the KO (J-L) as compared with the HT tissue sections (F-H). Cross sections through the neural tube reveal β-gal activity in the developing somites, dorsal aorta, and the perineural vascular plexus. Abbreviations: da, dorsal aorta; flb, forelimb bud; h, heart; m, mesencephalon; nt, notochord; otv, otic vesicle; ov, optic vesicle; PNVP, perineural vascular plexus; s, somite; t, telencephalon. Scale bars: 500 μm (A, E, I); 100 μm (B, F, J); 50 μm (C, G, K); 20 μm (D, H, L). Please click here to view a larger version of this figure.
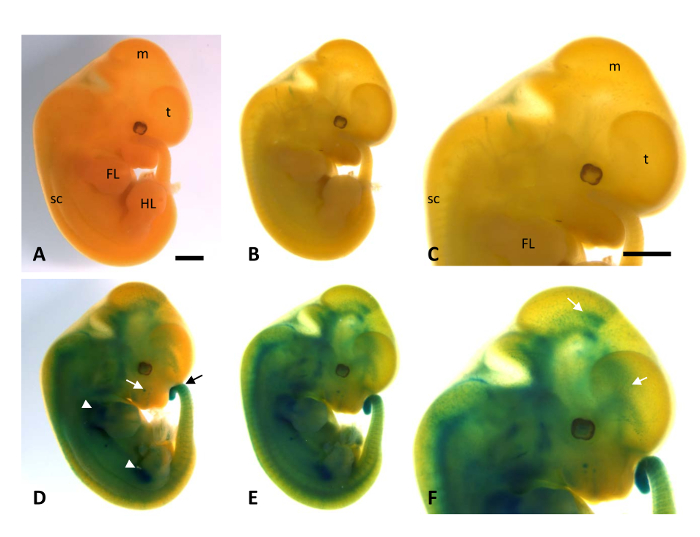
Figure 4. Photomicrographs of WT and HT X-gal stained mouse embryos at E12.5 before (A, D) and after (B, C, E, F) clearing with washes in increased glycerol. As expected, WT embryos show no reporter LacZ expression (A-C) while labeling was detected in the limbs (white arrowheads), the primordium of the follicle of vibrissa (white arrow), the tip of the tail (black arrow) and brain of Mt4-mmpLacZ/+ embryos at this stage (D-F)9. (E, F) After clearing in glycerol, embryos became translucent and labeling can be more easily visualized in deeper regions of the embryo, as shown in the mesencephalon and the telencephalon (white arrows in F). Scale bar: 1 mm. Please click here to view a larger version of this figure.
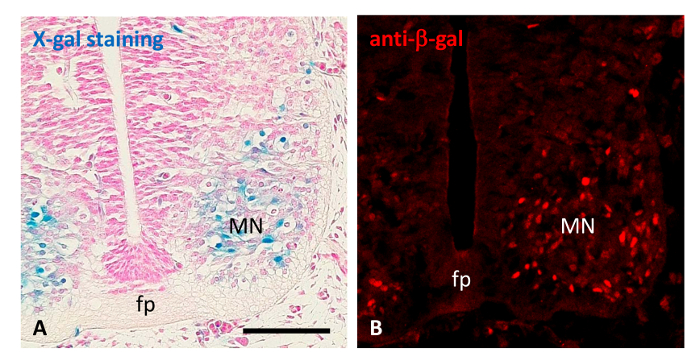
Figure 5. Histochemical and immunohistochemical detection of the β-galactosidase. (A) X-gal staining (in blue) reveals the expression of Mt4-mmp in the pool of motoneurons in a paraffin section of the spinal cord at the E11.5 embryonic stage. (B) Immunohistochemistry with anti-β-gal antibodies (in red) confirmed the expression of Mt4-mmp in the spinal cord motoneurons at the same embryonic stage in cryostat sections. Abbreviations: fp, floor plate; MN, motoneurons; Scale bar: 100 μm. Please click here to view a larger version of this figure.
Table 1. Recipes and Solutions required for this protocol. Please click here to download this file.
Discussion
The E. coli LacZ gene has been widely used as a reporter in studies of gene expression patterns because of its high sensitivity and ease of detection. The present protocol describes a classic method for detecting β-gal expression based on an enzymatic reaction that is easy and quick to perform as well as inexpensive. This method can be also applied without major modifications in whole mount embryos, intact organs, cryostat tissue sections or cultured cells.
Accurate application of this method results in a robust and interpretable staining. However, simultaneous controls and subsequent confirmatory steps are highly recommended. In this regard, the present protocol is carried out in parallel in wild type littermate embryos used as negative controls that serve to monitor non-specific X-gal staining. Moreover, expression data obtained when using β-gal staining is confirmed by western-blot and quantitative-PCR (Q-PCR) analysis or by means of β-gal immunohistochemistry (Figure 5), which combined with other antibodies also allows identification of specific cell types expressing LacZ9. Fixation steps need to be taken into consideration in order to preserve the enzymatic activity of the β-galactosidase and to minimize background during the staining. Thus, embryos must be fixed in glutaraldehyde, which gives the most consistent and reliable results compared with paraformaldehyde fixation17. Besides, fixation time is crucial and should be determined empirically for each embryonic stage, since over-fixation can reduce β-galactosidase activity. In this regard, whole mount embryos can be fixed by immersion or perfusion depending on the embryonic stage and size. Thus, transcardial perfusion is recommended for embryos older than E14.5 and for postnatal specimens to ensure that the fixative reaches all regions of the sample. Smaller embryos (from E8.5 up to E12.5) can be fixed by immersion and processed directly for in toto X-gal staining (Figure 2). It should be mentioned that X-gal staining of mouse embryos younger than E14.5 can be performed as the whole mount. In this case, stained cells close to the surface are easily detected by means of β-gal staining. Even so, it may occur that deeper labeling appears blurred or even dye does not penetrate the tissue, especially in older embryos. In this case, either organs or adult mice tissue can be dissected, X-gal stained, and visualized under a stereomicroscope. Nevertheless, sectioning and microscopic examination of the stained tissue of interest is more suitable (Figure 2).
Despite the protocol's efficiency, staining whole embryos and detection can be troublesome. For instance, the kidney, testis, secretory glands, epididymis and gastrointestinal tract contain high amounts of endogenous acidic β-galactosidase18 that can interfere with the interpretation of the results due to background activity. For that reason, the histochemical reaction should be performed under slightly alkaline pH conditions (pH 8-9) throughout the staining, since E. coli β-galactosidase has an optimum pH of 7.3. This will help to significantly reduce background and to favor bacterial β-galactosidase activity19,20,21,22. It should also be noted that long staining times may result in acidification of the X-gal staining solution, favoring an increased background. Moreover, the β-galactosidase activity is lost under incubation temperatures above 50 °C, but not below 42 °C. In our hands, incubation with X-gal substrate works better when left overnight at 37 °C. After staining and prior to the post-fixation step, it is important to wash all X-gal reaction material off as carefully and as quickly as possible to eliminate precipitates from the histochemical reaction that can be deposited in the tissue section.
As shown here, clearing embryos in glycerol is important for making the tissue translucent while preserving the X-gal staining and tissue histology. Thus, the embryos become clear and labeling can be visualized in deeper regions under the stereomicroscope. However, to analyze the exact location of the stained cells, sectioning of the sample is required and, therefore, clearing is not essential. In this case, by following recommendations indicated in the method, samples can be carefully paraffin embedded and sectioned. During the embedding process, ethanol dehydrated tissue needs to be immersed in a mixture of isopropanol-paraffin. Isopropanol is used to favor the replacement of ethanol in the sample and to facilitate the tissue embedding in paraffin23. In some protocols, xylene is used instead of isopropanol for the same purpose. Nevertheless, the use of xylene presents disadvantages versus isopropanol, such as the shrinkage and hardening of the sample due to the removal of tissue lipids24 and its toxicological profile as a flammable and irritant product25. Orange oil-based clearing agents are one of the safest alternatives to xylene since they allow rapid clearing without the toxicity of xylene as well as excellent tissue structure preservation26. Also, if using xylene, this part of the protocol should be reduced at maximum, since X-gal staining can diffuse and be lost19. After sectioning, counterstaining with dyes (e.g. Nuclear Fast Red) is recommended to facilitate histological identification of expressing cells. This staining procedure is rapid, requiring only one step, and its pink appearance provides good contrast with the blue-colored X-gal precipitate. However, this counterstaining is only suitable for the use with non-aqueous mounting media (i.e. DPX).
This classical method is suitable for the detection of β-galactosidase activity, since X-gal in combination with potassium ferro- and ferricyanide produces a characteristic blue precipitate that does not fade or bleach easily and, when well-built, can be detected even under the dissecting microscope. There are other modifications of the original protocol. Using available substrates such as Salmon-gal (6-Chloro-3-indolyl-β-D-galactopyranoside), Magenta-gal (5-Bromo-6-chloro-3-indolyl-β-D-galactopyranoside) or Bluo-gal (5-Bromo-3-indolyl β-D-galactopyranoside)13,19, or substituting the classic X-gal/FeCN for Tetrazolium salts can greatly enhance sensitivity of detection13,19. This is especially recommended to allow high sensitivity detection of low expression levels of protein and mRNA material. However, when used for too long, any of these procedures may lead to high background levels and so careful management of the conditions is always recommended, especially considering that prolonged incubation periods may facilitate unspecific staining due to endogenous β-galactosidase activity13,27. Regarding the latter, it is always advisable to use ferric potassium salts in order to optimize the reliability of the technique and avoid pitfalls.
An important limitation of this method is that the classical X-gal staining procedure produces an insoluble blue precipitate that can be easily detected by light microscopy but does not allow co-localization studies by fluorescent or confocal microscopy in tissue sections. One possible way to overcome this limitation would be to use antibodies against β-gal (see Figure 5) combined with immunodetection for a specific cell type. However, if the expression levels are low or the background high, immunolabeling can make it difficult to detect whether the reporter gene is expressed in a particular cell. Notably, a new technique has been reported to obtain high quality confocal images based on the fluorescence emission produced by the X-gal precipitate28. Moreover, this method is compatible with immunofluorescence and can clearly identify X-gal positive cells28. Enzymatic reporters such as the LacZ gene offer the advantage of being more sensitive than fluorescent ones, which is noteworthy when labeling, if expression levels are especially low. In this regard, the present technique has been proven ideal for the detection of microRNA29, therefore permitting both qualitative evaluation of the expression patterns and quantification of expression levels. Double staining is used to detect β-gal activity and mRNA presence via in situ hybridization techniques, which can provide very useful information to examine the expression pattern of endogenous genes while monitoring β-gal activity in the same sample30. Nevertheless, both β-gal stain and in situ hybridization detection reactions may display color incompatibility during double staining. Choosing a more suitable substrate, such as S-gal, is recommended in specifically optimized methods30.
This versatile method has been extensively used to study gene function in the context of developmental biology. Thus, the LacZ gene has been adopted for the analysis of gene expression patterns during embryonic development by knocking it into a targeted gene locus. LacZ is a common reporter gene for studying cis-acting regulatory elements (enhancers) involved in directing gene expression into restricted regions of the embryo, providing information on the transcription activity of the promoter of interest31,32,33. Also, it is frequently used in the detection of genetic recombination events by Cre-mediated recombination of loxP sites, which is especially useful for the detection of embryonic stem cell derivatives in cell fate mapping experiments or in cell transplants. Gene-trap mutagenesis in embryonic stem cells produces truncated proteins fused to β-gal that permits evaluation of promoter activity under physiological conditions34. For all these approaches, transgenic animals carrying LacZ and expressing bacterial β-galactosidase have been generated in rat35,36, chicken37,38, Xenopus39 or zebrafish40. Hitherto, this technique has been used in Drosophila to improve the visualization of gene expression spatially and temporally due to the availability of inducible gene expression systems, and tissue and cell-specific p-LacZ marker lines41,10. It is worth noting that apart from these many applications involving transgenic and knockout animals, LacZ reporter gene has been used in whole tissues as well as cultured cells to predict the role of genes in disease in biomedical research. One relevant example of the latter is the study of the aging process regarding responses to stress, tumor suppression, or development. Senescent cells are easy to detect both in situ and in vivo due to their characteristic overexpression and accumulation of endogenous lysosomal beta-galactosidase42,43. Hence, β-galactosidase is used as a senescent biomarker in quantitative assays in cancer cultured cells44.
In summary, we describe a feasible and optimized procedure to facilitate easy detection of β-galactoside activity in mouse embryonic tissue. The present method allows modifications based on the tissue selected and the substrate used. Hence, this versatile and cost-effective method employed with appropriate controls is especially suitable for the use with whole mouse embryos as well as on thin histological sections.
Divulgaciones
The authors have nothing to disclose.
Acknowledgements
We would like to thank the Histopathological Service for their technical assistance at the Centro Nacional de Investigaciones Cardiovasculares (CNIC). We also thank Dr. Motoharu Seiki for kindly providing Mt4-mmpLacZ mice, and Dr. Alicia G. Arroyo for supporting our project and for her critical reading of the manuscript. We wish to thank Peter Bonney for proofreading this article. This work was supported by Universidad Europea de Madrid by means of a grant (# 2017UEM01) awarded to C.S.C.
Materials
| REAGENTS | |||
| 2-Propanol | SIGMA-ALDRICH | 24137-1L-R | |
| Agarose | SCHARLAU | 50004/ LE3Q2014 | |
| Aqueous mounting medium | VECTOR LABS | H-5501 | |
| Synthetic mounting media | MERCK | 100579 | |
| 96% Ethanol | PROLABO | 20824365 | |
| 99.9% Ethanol absolute | SCHARLAU | ET00021000 | |
| 50% Glutaraldehyde solution | SIGMA-ALDRICH | G6403-100ml | |
| 85% Glycerol | MERCK | 104094 | |
| 99.9% Glycerol | SIGMA-ALDRICH | G5516 | |
| Magnesium chloride hexahydrate | SIGMA-ALDRICH | 63064 | |
| Nonionic surfactant (Nonidet P-40) | SIGMA-ALDRICH | 542334 | |
| Nuclear Fast Red counterstain | SIGMA-ALDRICH | N3020 | |
| Paraffin pastilles | MERCK | 111609 | |
| Paraformaldehyde | SIGMA-ALDRICH | 158127-500g | |
| Phosphate buffered saline (tablets) | SIGMA-ALDRICH | P4417-50TAB | |
| Potassium ferrocyanate | MERCK | 1049840500 | |
| Potassium ferrocyanide | MERCK | 1049731000 | |
| Sodium azide | SIGMA-ALDRICH | S8032 | |
| Sodium deoxycholate | SIGMA-ALDRICH | 30970 | |
| Sodium dihydrogen phosphate monohydrate | SIGMA-ALDRICH | 106346 | |
| Sodium phosphate dibasic dihydrate | SIGMA-ALDRICH | 71638 | |
| Thymol | SIGMA-ALDRICH | T0501 | |
| Tris hydrochloride (Tris HCl) | SIGMA-ALDRICH | 10812846001 (Roche) | |
| X-GAL | VENN NOVA | R-0004-1000 | |
| Xylene | VWR CHEMICALS | VWRC28973.363 | |
| EQUIPMENT | |||
| Disposable plastic cryomolds 15x15x5 mm | SAKURA | 4566 | |
| Rotatory Microtome | Leica | RM2235 | |
| Cassettes | Oxford Trade | OT-10-9046 | |
| Microscope Cover Glasses 24×60 mm | VWR | ECN631-1575 | |
| Microscope slides | Thermo Scientific, MENZEL-GLÄSER | AGAA000001#12E | |
| Adhesion microscope slides | Thermo Scientific, MENZEL-GLÄSER | J1820AMNZ | |
| Flotation Water bath | Leica | HI1210 | |
| Disposable Low Profile Microtome Blades | Feather | UDM-R35 | |
| Paraffin oven | J.R. SELECTA | 2000205 | |
| Wax Paraffin dispenser | J.R. SELECTA | 4000490 | |
| Stereomicroscope | Leica | DM500 | |
| Polypropylene microcentrifuge tubes 2.0 mL | SIGMA-ALDRICH | T2795 | |
| Polypropylene microcentrifuge tubes 1.5 mL | SIGMA-ALDRICH | T9661 | |
| Orbital shaker | IKA Labortechnik | HS250 BASIC | |
| Stirring Hot Plate | Bibby | HB502 | |
| Vortex Shaker | IKA Labortechnik | MS1 | |
| Laboratory scale | GRAM | FH-2000 | |
| Precision scale | Sartorius | ISO9001 | |
| pHmeter | Crison | Basic 20 | |
| Optic fiber | Optech | PL2000 |
Referencias
- Shuman, H. A., Silhavy, T. J., Beckwith, J. R. Labeling of proteins with beta-galactosidase by gene fusion. Identification of a cytoplasmic membrane component of the Escherichia coli maltose transport system. The Journal of Biological Chemistry. 255, 168-174 (1980).
- Cui, C., Wani, M. A., Wight, D., Kopchick, J., Stambrook, P. J. Reporter genes in transgenic mice. Transgenic Research. 3, 182 (1994).
- Takahashi, E., et al. Expression analysis of Escherichia coli lacZ reporter gene in transgenic mice. Brain Research. Brain Research Protocols. 5 (2), 159-166 (2000).
- Burn, S. F. Detection of β-Galactosidase Activity: X-gal Staining. Methods Mol Biol. 886, 241-250 (2012).
- Jacob, F., Monod, J. Genetic regulatory mechanisms in the synthesis of proteins. J Mol Biol. 3, 318-356 (1961).
- Pearson, B., Wolf, P. L., Vazquez, J. A comparative study of a series of new indolyl compounds to localize beta-galactosidase in tissues. Laboratory Investigation; a Journal of Technical Methods and Pathology. 12, 1249-1259 (1963).
- Kothary, R., et al. A transgene containing lacZ inserted into the dystonia locus is expressed in neural tube. Nature. 335 (6189), 435-437 (1988).
- Kothary, R., et al. Inducible expression of an hsp68-lacZ hybrid gene in transgenic mice. Development. 105 (4), 707-714 (1989).
- Blanco, M. J., et al. Developmental expression of membrane type 4-matrix metalloproteinase (Mt4-mmp/Mmp17) in the mouse embryo. PLOS ONE. 12 (9), e0184767 (2017).
- Hartenstein, V., Jan, Y. N. Studying Drosophila embryogenesis with P-lacZ enhancer trap lines. Roux’s Archives of Developmental Biology. 201 (4), 194-220 (1992).
- Gierut, J. J., Jacks, T. E., Haigis, K. M. Whole-mount X-Gal staining of mouse tissues. Cold Spring Harb Protoc. 1 (4), 417-419 (2014).
- Cooper, M. A., Zhou, R. β-Galactosidase Staining of LacZ Fusion Proteins in Whole Tissue Preparations. Methods Mol Biol. 1018, 189-197 (2013).
- Sundararajan, S., Wakamiya, M., Behringer, R. R., Rivera-Pérez, J. A. A fast and sensitive alternative for β-galactosidase detection in mouse embryos. Development. 139 (23), 4484-4490 (2012).
- Wei, Q., Manley, N. R., Condie, B. G. Whole mount in situ hybridization of E8.5 to E11.5 mouse embryos. Journal of Visualized Experiments. 56, 2797 (2011).
- Rikimaru, A., et al. Establishment of an MT4-MMP-deficient mouse strain representing an efficient tracking system for MT4-MMP/MMP-17 expression in vivo using beta-galactosidase. Genes Cells. 12 (9), 1091-1100 (2007).
- Leight, J. L., Alge, D. L., Maier, A. J., Anseth, K. S. Direct measurement of matrix metalloproteinase activity in 3D cellular microenvironments using a fluorogenic peptide substrate. Biomaterials. 34 (30), 7344-7352 (2013).
- Ma, W., Rogers, K., Zbar, B., Schmidt, L. Effects of different fixatives on β-galactosidase activity. Journal of Histochemistry & Cytochemistry. 50 (10), 1421-1424 (2002).
- Lojda, Z. Indigogenic methods for glycosidases. II. An improved method for beta-D-galactosidase and its application to localization studies of the enzymes in the intestine and in other tissues. Histochemie. 23 (3), 266-288 (1970).
- Trifonov, S., Yamashita, Y., Kase, M., Maruyama, M., Sugimoto, T. Overview and assessment of the histochemical methods and reagents for the detection of β-galactosidase activity in transgenic animals. Anat Sci Int. 91, 56-67 (2016).
- Sanchez-Ramos, J., et al. The X-gal Caution in Neural Transplantation Studies. Cell Transplantation. 9 (5), 657-667 (2000).
- Weiss, D. J., Liggitt, D., Clark, J. G. In situ histochemical detection of beta-galactosidase activity in lung: assessment of X-Gal reagent in distinguishing lacZ gene expression and endogenous beta-galactosidase activity. Hum Gene Ther. 8 (13), 1545-1554 (1997).
- Merkwitz, C., Blaschuk, O., Schulz, A., Ricken, A. M. Comments on Methods to Suppress Endogenous β-Galactosidase Activity in Mouse Tissues Expressing the LacZ Reporter Gene. The Journal of Histochemistry and Cytochemistry. 64 (10), 579-586 (2016).
- Buesa, R. J., Peshkov, M. V. Histology without xylene. Annals of Diagnostic Pathology. 13, 246-256 (2009).
- Richardson, D. S., Lichtman, J. W. Clarifying Tissue Clearing. Cell. 162 (2), 246-257 (2015).
- Kandyala, R., Raghavendra, S. P. C., Rajasekharan, S. T. Xylene: An overview of its health hazards and preventive measures. Journal of Oral and Maxillofacial Pathology. 14 (1), 1-5 (2010).
- Hinds, H. L. A Comparison of Three Xylene Substitutes. Laboratory Medicine. 17 (12), 752-755 (1986).
- Merkwitz, C., Blaschuk, O., Winkler, J., Schulz, A., Prömel, S., Ricken, A. M. Advantages and Limitations of Salmon-Gal/Tetrazolium Salt Histochemistry for the Detection of LacZ Reporter Gene Activity in Murine Epithelial Tissue. Journal of Histochemistry & Cytochemistry. 65 (4), 197-206 (2017).
- Levitsky, K. L., Toledo-Aral, J. J., López-Barneo, J., Villadiego, J. Direct confocal acquisition of fluorescence from X-gal staining on thick tissue sections. Scientific Reports. 3, 2937 (2013).
- Shen, X., Bao, W., Yu, W., Liang, R., Nguyen, B., Yu Liu, Y. An improved method with high sensitivity and low background in detecting low β-galactosidase expression in mouse embryos. PLoS One. 12 (5), (2017).
- Komatsu, Y., Kishigami, S., Mishina, Y. In situ Hybridization Methods for Mouse Whole Mounts and Tissue Sections with and Without Additional β-Galactosidase. Methods Mol Biol. 1092, 1-15 (2014).
- Jeong, Y., Epstein, D. J. Distinct regulators of Shh transcription in the floor plate and notochord indicate separate origins for these tissues in the mouse node. Development. 130 (16), 3891-3902 (2003).
- Eid, R., Koseki, H., Schughart, K. Analysis of LacZ reporter genes in transgenic embryos suggests the presence of several cis-acting regulatory elements in the murine Hoxb-6 gene. Developmental Dynamics. 196 (3), 205-216 (1993).
- Will, A. J., et al. Composition and dosage of a multipartite enhancer cluster control developmental expression of Ihh (Indian hedgehog). Nature Genetics. 49 (10), 1539-1545 (2017).
- Stanford, W. L., Cohn, J. B., Cordes, S. P. Gene-trap mutagenesis: past, present and beyond. Nature Reviews Genetics. 2 (10), 756-768 (2001).
- Ménoret, S., et al. lacZ transgenic rats tolerant for beta-galactosidase: recipients for gene transfer studies using lacZ as a reporter gene. Human Gene Therapy. 13 (11), 1383-1390 (2002).
- Takahashi, M., et al. Establishment of lacZ-transgenic rats: a tool for regenerative research in myocardium. Biochemical and Biophysical Research Communications. 305 (4), 904-908 (2003).
- Mozdziak, P. E., Borwornpinyo, S., McCoy, D. W., Petitte, J. N. Development of transgenic chickens expressing bacterial betagalactosidase. Developmental Dynamics. 226 (3), 439-445 (2003).
- Mozdziak, P. E., Wu, Q., Bradford, J. M., Pardue, S. L., Borwornpinyo, S., Giamario, C., Petitte, J. N. Identification of the lacZ insertion site and beta-galactosidase expression in transgenic chickens. Cell Tissue Research. 324 (1), 41-53 (2006).
- Hartley, K. O., Nutt, S. L., Amaya, E. Targeted gene expression in transgenic Xenopus using the binary Gal4-UAS system. Proceedings of the National Academy of Science U.S.A. 99, 1377-1382 (2002).
- Scheer, N., Campos-Ortega, J. A. Use of the Gal4-UAS technique for targeted gene expression in the zebrafish. Mechanisms of Development. 80 (2), 153-158 (1999).
- Brand, A. H., Perrimon, N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development. 118 (2), 401-415 (1993).
- Lee, B. Y., et al. Senescence-associated beta-galactosidase is lysosomal beta-galactosidase. Aging Cell. 5 (2), 187-195 (2006).
- Morais, K. S., Guimarães, A. F. R., Ramos, D. A. R., Silva, F. P., de Oliveira, D. M. Long-term exposure to MST-312 leads to telomerase reverse transcriptase overexpression in MCF-7 breast cancer cells. Anti-Cancer Drugs. 28 (7), 750-756 (2017).
- Dimri, G. P., et al. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proceedings of the National Academy of Science U.S.A. 92 (20), 9363-9367 (1995).

