An Electrochemical Cholesteric Liquid Crystalline Device for Quick and Low-Voltage Color Modulation
Summary
A protocol for the fabrication of a reflective cholesteric liquid crystalline display device containing a redox-responsive chiral dopant allowing quick and low-voltage operation is presented.
Abstract
We demonstrate a method for fabricating a prototype reflective display device that contains cholesteric liquid crystal (LC) as an active component. The cholesteric LC is composed of a nematic LC 4'-pentyloxy-4-cyanobiphenyl (5OCB), redox-responsive chiral dopant (FcD), and a supporting electrolyte 1-ethyl-3-methylimidazolium trifluoromethanesulfonate (EMIm-OTf). The most important component is FcD. This molecule changes its helical twisting power (HTP) value in response to redox reactions. Therefore, in situ electrochemical redox reactions in the LC mixture allow for the device to change its reflection color in response to electrical stimuli. The LC mixture was introduced, by a capillary action, into a sandwich-type ITO glass cell comprising two glass slides with patterned indium tin oxide (ITO) electrodes, one of which was coated with poly(3,4-ethylenedioxythiophene)-co-poly(ethylene glycol) doped with perchlorate (PEDOT+). Upon application of +1.5 V, the reflection color of the device changed from blue (467 nm) to green (485 nm) in 0.4 s. Subsequent application of 0 V made the device recover the original blue color in 2.7 s. This device is characterized by its fastest electrical response and lowest operating voltage among any previously reported cholesteric LC device. This device could pave the way for the development of next generation reflective displays with low energy consumption rates.
Introduction
Cholesteric liquid crystals (LCs) are known to exhibit bright reflection colors due to their internal helical molecular arrangements1,2,3,4. The reflection wavelength λ is determined by the helical pitch P and the average refractive index n of the LC (λ = nP). Such LCs can be generated by doping chiral compounds (chiral dopants) to nematic LCs and its helical pitch is defined by the equation P = 1/βMC, where βM is the helical twisting power (HTP) and C is the molar fraction of the chiral dopant. Based on this notion, various chiral dopants that can respond to a variety of stimuli such as light5,6,7,8, heat9, magnetic field10, and gas11 has been developed. Such properties are potentially useful for various applications such as sensors12 and lasers13,14,15 among others16,17,18.
Recently, we developed the first redox-responsive chiral dopant FcD (Figure 1A)19 that can change its HTP value in response to redox reactions. FcD is composed of a ferrocene unit, which can undergo reversible redox reactions20,21,22, and a binaphthyl unit, which is known to exhibit high HTP value23. The cholesteric LC doped with FcD, in the presence of a supporting electrolyte, can change its reflection color within 0.4 s and recover its original color in 2.7 s upon voltage application of +1.5 and 0 V, respectively. The high response speed and low operating voltage observed for the device is unprecedented among any other cholesteric LC device so far reported.
One of the important applications of the cholesteric LCs is in reflective displays, whose energy consumption rate is much lower than the conventional LC displays. For this purpose, cholesteric LCs should change its reflection color with electrical stimuli. However, most of the previous methodologies utilize an electrical coupling between the applied electrical stimuli and the host LC molecules, which requires high voltage over 40 V24,25,26,27,28. For the use of the electrically responsive chiral dopant, there are only few examples29,30 including our previous work31, which also requires high voltage with low response speed. Considering these previous works, the performance of our FcD-doped cholesteric LC device, especially for the fast color modulation speed (0.4 s) and low operating voltage (1.5 V), is a groundbreaking achievement that can greatly contribute to the development of next generation reflective displays. In this detailed protocol, we demonstrate the fabrication processes and the operating procedures of the prototype cholesteric LC display devices.
Protocol
1. Preparation of the cholesteric LC mixture
- Add 84.6 mg of 5OCB and 5.922 mg of FcD19 (3.1 mol% to 5OCB) into a clean 10 mL glass vial.
- Add 12.9 mg of EMIm-OTf and 10 mL of dichloromethane (CH2Cl2) into a new clean 10 mL glass vial and mix well. Transfer 2.1 mL of the EMIm-OTf solution into the 5OCB- and FcD-containing glass vial. Gently shake the vial to let all the components mix well.
- Cover the glass vial with an aluminum foil and make several holes at the top.
- Heat the above CH2Cl2 solution containing 5OCB, FcD (3.1 mol% to 5OCB) and EMIm-OTf (3.0 mol% to 5OCB) at 80 °C in a well-ventilated hood. After 60 min, most of the CH2Cl2 is evaporated. This procedure is important to ensure homogeneous mixing of the components.
- Evaporate the remaining CH2Cl2 under reduced pressure (~5.0 Pa) by oil rotary vacuum pump at 80 °C for 60 min in the well-ventilated hood to obtain a clear orange LC mixture.
2. Preparation of the sandwich-type ITO glass cell
- Cleaning procedure of the ITO coated glass
- Cut an ITO patterned glass (10 cm x 10 cm, resistance: ~30 Ω), which contains 100 pieces of a designated electrode to a smaller size (10 mm x 10 mm) by a diamond tipped glass cutter so that one piece includes one pattern of the electrode. Always check the resistance of the surface of the glass to know which side is patterned with ITO using, for example, digital multi-meter (ITO patterned side has low resistance).
- Cut a fully ITO coated glass (10 cm × 10 cm, resistance: ~30 Ω) to a smaller size (10 mm x 12 mm) by a diamond tipped glass cutter. Again, check the resistance of the surface of the glass to know which side is coated with ITO.
- Prepare a washing solution by mixing 60 mL of Extran MA01 and 240 mL of ultrapure water in a glass vessel (~500 mL). Soak the above prepared ITO glass plates into the solution thoroughly in such a way that the surface of each glass plate does not touch with one another. In case of washing many ITO glass plates, it is recommended to use some support (e.g., shampoo brush).
- Put the vessel containing ITO glass plates in an ultrasonic bath and sonicate it for 30 min. After decanting off the washing solution, rinse the vessel containing ITO glass plates by 200 mL of ultrapure water for three times.
- Add 300 mL of ultrapure water and sonicate the vessel for 20 min. Then, remove the water by decantation. Repeat this washing cycle using ultrapure water for three times. For each washing cycle, check the arrangement of the ITO glass plates in the vessel so that the surfaces of the plates are not attached to one another.
- After finishing the washing cycles, dry the ITO glass plates one by one through the nitrogen gas flow. When putting the ITO glass plates on the clean place, keep the ITO surface upward in order to avoid any damage or contamination of the surface.
- Fabrication of the PEDOT+ coated ITO glass plate
- Put the glass vial containing a nitromethane solution of poly(3,4-ethylenedioxythiophene)-co-poly(ethylene glycol) doped with perchlorate (PEDOT+, 0.7 wt%) into an ultrasonic bath and sonicate it for 60 min to obtain a well dispersed solution.
- Place the fully ITO coated glass plate on the rotator of the spin coater with the ITO surface facing upright. Blow off dust from the ITO surface by using a nitrogen blow gun. Carefully transfer 50 µL of freshly sonicated PEDOT+ solution by pipette.
- Fabricate the PEDOT+ film by spinning the plate at a rate of 1000 rpm for 60 s at ambient conditions (~25 °C, humidity: ~45%). Keep the PEDOT+ coated ITO glass plates under the ambient conditions for 1 h without baking.
- Fabrication of the ITO glass cell
- Blow off dust from ITO patterned glass plates by using a nitrogen blow gun.
- Rub the ITO face of the glass plates (10 mm x 10 mm) with rayon cloth thoroughly using a rubbing machine. During the whole process, use a nitrogen blow gun to avoid the contamination of dusts.
- Carry out the following procedures in a place that can avoid the contamination of dusts, ideally in a clean room.
- Mix a drop of an optical adhesive and a rice-sized amount of glass beads thoroughly.
- Lay down the PEDOT+ coated ITO glass plate on the table with the PEDOT+ surface facing upright. Put a very small amount of the adhesive mixture onto the PEDOT+ coated ITO glass plate where the four corners of the ITO patterned glass plate come.
- Put the ITO patterned glass plate onto the PEDOT+ coated ITO glass plate in such a way that the ITO surfaces of the two glass plates are facing to each other to fabricate a cell. Gently push the four corners of the cell. Confirm a uniform cell gap by the disappearance of a fringe pattern observed at the surface of the cell.
- Irradiate the above ITO glass cell with a 365 nm UV lamp for 20 s to strengthen the adhesion.
- Heat the above cell on a hot stage at 100 °C for 3 h to continue strengthening the adhesion.
- Connect two conducting wires to each of the ITO area of the glass plates in the cell by ultrasonic soldering.
3. Color modulation experiments
- Introduction of the cholesteric LC mixture into the ITO glass cell for the fabrication of the LC device
- For easily handling, fix the wires of the above prepared glass cell to a microscope slide with an insulating tape.
- Heat the glass vial containing the cholesteric LC mixture at 80 °C for 10 to 15 min on a hot stage. Also heat the ITO glass cell and a spatula, which is used for transferring the sample, at the same temperature.
- Transfer a small amount of the hot cholesteric LC mixture by using the heated spatula quickly to the gap of two ITO glass plates of the cell. Fill up the gap between the two glass plates by capillary action, which takes ~60 s.
- Lower the temperature of the hot stage so that the temperature of the cell reaches 37 °C.
- Push the center of the device to exhibit bright reflection color.
- Color modulation experiments by using a digital optical microscope.
- Apply +1.5 and 0 V alternately to the LC device for 4 s and 8 s, respectively, by using a potentiostat at 37 °C. The voltage values are defined for non-PEDOT+-coated ITO electrode in reference to that for PEDOT+-coated ITO electrode in the device. Observe and record the color change of the LC device by digital optical microscope.
- Spectrometric color modulation experiments
- Use the following UV-vis spectrophotometer setup parameters: photometric mode: %T, response: fast, bandwidth: 1.0 nm, scan speed: 2,000 nm/min, scan range: 800 to 300 nm
- For the baseline measurement, place the hot stage into the spectrophotometer without the LC device. Ensure that the observation hole is properly placed in the optical path of the spectrophotometer and the angle of incidence is 0°. Monitor the transmittance value in real-time at a certain wavelength whose value is maximized by adjusting the placement of the hot stage. Then start for the baseline measurement.
- Place the LC device into this hot stage, and then, place the hot stage to the appropriate position in a same way as described in section 3.3.2. Start the measurement and record the spectrum.
- Apply +1.5 V for 4 s and start the measurement. After the measurement, apply 0 V for 8 s and, again, start the measurement.
- Apply +1.5 and 0 V alternately for 100 times to the LC device for 4 s and 8 s, respectively, by using a potentiostat. Record transmittance at a designated wavelength (510 nm) during the voltage application cycles.
Representative Results
Photographs, transmittance spectra, and time dependent transmittance change profiles at 510 nm are collected for the LC device containing FcD-doped (3.1 mol%) cholesteric LC in the presence of EMIm-OTf (3.0 mol%) during the voltage application cycles between 0 and +1.5 V at 37 °C.
The LC mixture containing FcD (3.1 mol%), EMIm-OTf (3.0 mol%) and 5OCB exhibited a cholesteric mesophase from 46.8 °C to 3.2 °C on cooling and from 4.8 °C to 49.7 °C on heating confirmed by differential scanning calorimetry (DSC) measurement (scan rate: 5 °C/min). The LC device containing this mixture exhibited a bright reflection color (Figure 2A-I) whose reflection band centered at 467 nm was clearly observed in its transmittance spectrum (Figure 2B-I) at 37 °C. The shape of the transmittance spectrum of this LC material in the cell was typical of cholesteric LCs1,2, where the band width Δλ (= 45 nm) is in agreement with the estimated value (53 nm) calculated based on the ordinary (no = 1.53)32 and extraordinary (ne = 1.71)32 refractive indices of 5OCB. This indicates that the LC molecules are homogeneously aligned in the cell which was achieved simply by rubbing the surface of the glass substrate without orientation film, allowing for clear observation of the bright color and transmittance spectrum.
When a voltage of +1.5 V was applied to the LC device, the reflection color changed immediately from blue to green (485 nm, Figure 2A-II and Figure 2B-II). Subsequent application of 0 V resulted in the recovery of the initial blue color (467 nm, Figure 2A-III and Figure 2B-III). This cycle can be repeated many times with minimum degradation of transmittance (Figure 2C) due to an orientational disorder of the LC molecules which can be repaired simply by applying a shear. Quantitative analysis revealed that the forward and backward color changes were completed in only 0.4 s and 2.7 s, respectively, based on the 90% change in transmittance at 510 nm (Figure 2D). It is noted that this cholesteric reflective LC device is by far the fastest in response and lowest in operation voltage among those designed to be electrically driven24,25,26,27,28,29,30,31,33,34.
We also fabricated a cell with an ITO electrode patterned with a figure of "UT" using FcD-doped (3.1 mol%) cholesteric LC containing EMIm-OTf (3.0 mol%). Alternating application of +1.5 V and 0 V made the figure blink (Figure 3).
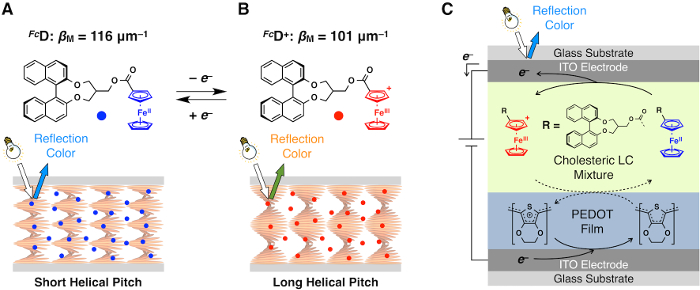
Figure 1: Chemical structure of the redox-responsive chiral dopant FcD and the mechanism for the reflection color change. (A,B) Chemical structures of FcD and its oxidized form FcD+. Helical pitch P of the cholesteric LC comprising 5OCB and FcD becomes longer upon oxidation of FcD which induces lowering of its helical twisting power βM. (C) Illustration of the mechanism of electrochemical modulation of the reflection color. Adapted with permission from J. Am. Chem. Soc.140, 10946-10949 (2018). Copyright 2018 American Chemical Society. Please click here to view a larger version of this figure.
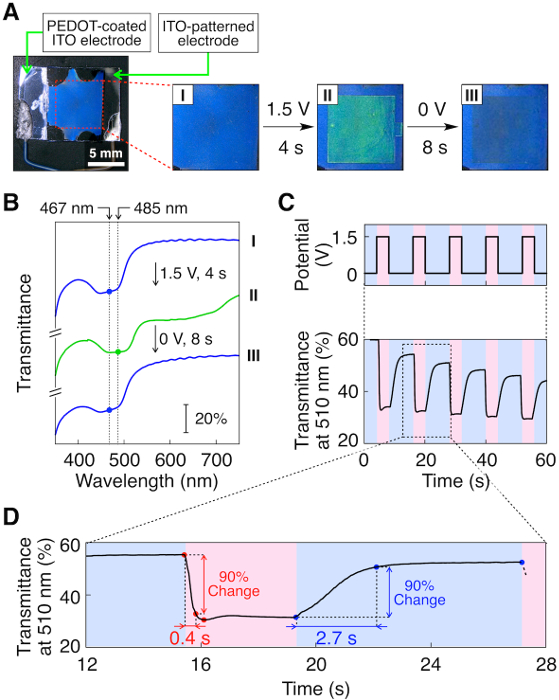
Figure 2: Electrical response of the reflection color of the cholesteric LC device. Photographs (A) and transmittance spectra (B) of the FcD-doped (3.1 mol%) LC device containing 3.0 mol% of EMIm-OTf in 5OCB in its initial state (I), after the application of +1.5 V for 4 s (II), and subsequent application of 0 V for 8 s (II) at 37 °C. (C) Changes in the transmittance of the LC device at 510 nm upon switching the applied voltage between +1.5 and 0 V. (D) Details of the transmittance change of the LC device at 510 nm. Adapted with permission from J. Am. Chem. Soc.140, 10946-10949 (2018). Copyright 2018 American Chemical Society. Please click here to view a larger version of this figure.

Figure 3: Prototype display device. An ITO-patterned cell containing FcD– and EMIm-OTf-doped cholesteric LC with a doping ratio of 3.1 and 3.0 mol%, respectively. The cell can blink the figure of "UT" by switching the applied voltage between +1.5 and 0 V alternately. Please click here to view a larger version of this figure.
Discussion
Upon application of +1.5 V to the top ITO electrode (Figure 1C), FcD undergoes an oxidation reaction to generate FcD+. As the helical twisting power of FcD+ (101 µm-1, Figure 1B) is lower than that of FcD (116 µm-1, Figure 1A)19, the helical pitch of the cholesteric LC becomes longer and thus shifts the reflection wavelength to a longer wavelength region from 467 nm to 485 nm. Based on the helical twisting power, the ratio between FcD and FcD+ in the LC mixture at the stationary state can be calculated to be 71:29. If all FcD in the LC mixture is oxidized to form FcD+, the reflection wavelength should be 536 nm, which is much longer than that observed for the LC device. The reason for the low conversion rate is likely due to the occurrence of a reverse reaction (reduction of FcD+) taking place at the interface of the LC mixture and the PEDOT+ film (Figure 1C). The application of a higher voltage induced a much wider range of the color shift19. For example, when we applied +2.5 V, the color shift was more significant (623 nm, orange). However, this color change was not reversible. When we took a cyclic voltammogram (CV) of FcD, its half-wave potential appears at +0.61 V and irreversible peak appears at +2.2 V19. Therefore, the appropriate driving voltage should be between +0.61 and +2.2 V.
The role of the film composed of poly(3,4-ethylenedioxythiophene)-co-poly(ethylene glycol) doped with perchlorate35 (PEDOT+) is a redox couple that can accept the electron from FcD to compensate for the charge. In fact, we can observe the decrease in the transmittance at around 600 nm (Figure 2B-II), which is characteristic for reduced PEDOT+36. If not using the PEDOT+ film, no reflection color change took place under the same voltage conditions19. Note that a film of PEDOT/PSS37, one of the most popular PEDOT derivatives, is not appropriate for this device as the reflection color gradually changes without voltage application. This is likely due to some irreversible reaction between FcD and highly acidic PSS.
The color modulation time for this device is 0.4 s and 2.7 s and thus the response speed is 45 nm/s and 7 nm/s for forward and backward color changes, respectively. The average speed is 26 nm/s. This is unprecedentedly fast among any other electrically color modulable cholesteric LCs. In 2010, Bunning and co-authors reported27 an electromechanically color tunable cholesteric LC device that can change its reflection colors in 3-5 s. In the visible range, the color modulation speed could be calculated as ~17 nm/s. No other example26,29,30,31,33,34 was reported to exceed this speed before our study19. It is also noted that the voltage of 1.5 V required for the color modulation in the device is significantly lower compared to the previously reported ones24,25,26,27,28 as they typically required over 40 V.
We have demonstrated the protocol for the fabrication of a reflective cholesteric LC display device containing FcD-doped LC as an active component. This is the first example for a cholesteric LC that can change its reflection color upon application of a voltage as low as 1.5 V. Under this voltage conditions, the reflection color change takes place within 0.4 s, which is also an unprecedented speed. Previously, the reflection color modulation of cholesteric LCs can be achievable only by applying high voltage (typically over 40 V). This methodology, on the other hand, can modulate the reflection color even by using ordinary 1.5 V dry-cell battery. This cholesteric-LC-based display device would pave the way to the development of next generation reflective displays.
Divulgaciones
The authors have nothing to disclose.
Acknowledgements
We thank Dr. Keisuke Tajima from RIKEN Center for Emergent Matter Science for valuable discussions. A part of this work was conducted at the Advanced Characterization Nanotechnology Platform of the University of Tokyo, supported by the Ministry of Education, Culture, Sports, Science and Technology (MEXT), Japan. This work was financially supported by a JSPS Grant-in-Aid for Scientific Research (S) (18H05260) on "Innovative Functional Materials based on Multi-Scale Interfacial Molecular Science" for T.A. Y.I. is grateful for a JSPS Grant-in-Aid for Challenging Exploratory Research (16K14062). S.T. thanks the JSPS Young Scientist Fellowship.
Materials
| 1-Ethyl-3-methylimidazolium Trifluoromethanesulfonate, 98% | TCI | E0494 | |
| 4-Cyano-4'-pentyloxybiphenyl, 98% | TCI | C1551 | |
| Diamond tipped glass cutter | AS ONE | 6-539-05 | |
| Dichloromethane, 99.5% | KANTO CHEMICAL | 10158-2B | HPLC grade |
| Differential Scanning Calorimeter | METTLER TOLEDO | DSC 1 | |
| Digital microscope | KEYENCE | VHX-5000 | |
| Extran MA01 | Merck | 107555 | |
| Fully ITO-coated glass plate | Costum order, Resistance: ~30Ω | ||
| Glass beads | Thermo Fisher Scientific | 9005 | 5 ± 0.3 μm in diameter |
| Hot stage | INSTEC | mK1000 | |
| ITO-patterned glass plate | Costum order, Resistance: ~30Ω | ||
| Oil rotary vacuum pump | SATO VAC | TSW-150 | Pressure: ~5 Pa |
| Optical adhesive | Noland | NOA81 | |
| Poly(3,4-ethylenedioxythiophene), bis-poly(ethyleneglycol), lauryl terminated | Sigma Aldrich | 687316 | 0.7 wt% (dispersion in nitromethane) |
| Potentiostat | TOHO TECHNICAL RESEARCH | PS-08 | |
| Rubbing machine | EHC | MRJ-100S | |
| Spectrophotometer | JASCO | V-670 UV/VIS/NIR | |
| Spin coater | MIKASA | 1H-D7 | |
| Ultrapure water | Merck | Milli-Q Integral 3 | |
| Ultrasonic bath | AS ONE | ASU-2 | Power: 40 W |
| Ultrasonic soldering | KURODA TECHNO | SUNBONDER USM-IV | |
| UV lamp | AS ONE | SLUV-4 | Power: 4 W |
Referencias
- Chandrasekhar, S. . Liquid Crystals. , (1992).
- Blinov, L. M., Chigrinov, V. G. . Electrooptic Effects in Liquid Crystal Materials. , (1994).
- Pieraccini, S., Masiero, S., Ferrarini, A., Spada, G. P. Chirality transfer across length-scales in nematic liquid crystals: fundamentals and applications. Chemical Society Reviews. 40 (1), 258-271 (2011).
- Eelkama, R., Feringa, B. L. Amplification of chirality in liquid crystals. Organic & Biomolecular Chemistry. 4 (20), 3729-3745 (2006).
- Wang, L., Li, Q. Stimuli-Directing self-organized 3D liquid-crystalline nanostructures: from materials design to photonic applications. Advanced Functional Materials. 26 (1), 10-28 (2016).
- Bisoyi, H. K., Li, Q. Light-directing chiral liquid crystal nanostructures: from 1D to 3D. Accounts of Chemical Research. 47 (10), 3184-3195 (2014).
- van Delden, R. A., Koumura, N., Harada, N., Feringa, B. L. Unidirectional rotary motion in a liquid crystalline environment: color tuning by a molecular motor. Proceedings of the National Academy of Sciences of the United States of America. 99 (8), 4945-4949 (2002).
- Mathews, M., Tamaoki, N. Planar chiral azobenzenophanes as chiroptic switches for photon mode reversible reflection color control in induced chiral nematic liquid crystals. Journal of the American Chemical Society. 130 (34), 11409-11416 (2008).
- Huang, Y., Zhou, Y., Doyle, C., Wu, S. -. T. Tuning the photonic band gap in cholesteric liquid crystals by temperature-dependent dopant solubility. Optics Express. 14 (3), 1236-1242 (2006).
- Hu, W., et al. Magnetite nanoparticles/chiral nematic liquid crystal composites with magnetically addressable and magnetically erasable characteristics. Liquid Crystals. 37 (5), 563-569 (2010).
- Han, Y., Pacheco, K., Bastiaansen, C. W. M., Broer, D. J., Sijbesma, R. P. Optical monitoring of gases with cholesteric liquid crystals. Journal of the American Chemical Society. 132 (9), 2961-2967 (2010).
- Kelly, J. A., et al. Responsive photonic hydrogels based on nanocrystalline cellulose. Angewandte Chemie International Edition. 52 (34), 8912-8916 (2013).
- Coles, H., Morris, S. Liquid-crystal lasers. Nature Photonics. 4 (10), 676-685 (2010).
- Xiang, J., et al. Electrically tunable laser based on oblique heliconical cholesteric liquid crystal. Proceedings of the National Academy of Sciences of the United States of America. 113 (46), 12925-12928 (2016).
- Song, M. H., et al. Effect of phase retardation on defect-mode lasing in polymeric cholesteric liquid crystals. Advanced Materials. 16 (9-10), 779-783 (2004).
- White, T. J., McConney, M. E., Bunning, T. J. Dynamic color in stimuli-responsive cholesteric liquid crystals. Journal of Materials Chemistry. 20 (44), 9832-9847 (2010).
- Bisoyi, H. K., Bunning, T. J., Li, Q. Stimuli-driven control of the helical axis of self-organized soft helical superstructures. Advanced Materials. 30 (25), 1706512 (2018).
- Bisoyi, H. K., Li, Q. Light-driven liquid crystalline materials: from photo-induced phase transitions and property modulations to applications. Chemical Reviews. 116 (26), 15089-15166 (2016).
- Tokunaga, S., Itoh, Y., Tanaka, H., Araoka, F., Aida, T. Redox-responsive chiral dopant for quick electrochemical color modulation of cholesteric liquid crystal. Journal of the American Chemical Society. 140 (35), 10946-10949 (2018).
- Step̌nicǩa, P. . Ferrocenes: Ligands, Materials and Biomolecules. , (2008).
- Togni, A., Hayashi, T. . Ferrocenes: Homogeneous Catalysis, Organic Synthesis, Materials Science. , (1995).
- Fukino, T., Yamagishi, H., Aida, T. Redox-responsive molecular systems and materials. Advanced Materials. 29 (25), 1603888 (2017).
- Goh, M., Akagi, K. Powerful helicity inducers: axially chiral binaphthyl derivatives. Liquid Crystals. 35 (8), 953-965 (2008).
- Xianyu, H., Faris, S., Crawford, G. P. In-plane switching of cholesteric liquid crystals for visible and near-infrared applications. Applied Optics. 43 (26), 5006-5015 (2004).
- Lin, T. H., et al. Electrically controllable laser based on cholesteric liquid crystal with negative dielectric anisotropy. Applied Physics Letters. 88 (6), 061122 (2006).
- Bailey, C. A., et al. Surface limitations to the electro-mechanical tuning range of negative dielectric anisotropy cholesteric liquid crystals. Journal of Applied Physics. 111 (6), 063111 (2012).
- Bailey, C. A., et al. Electromechanical tuning of cholesteric liquid crystals. Journal of Applied Physics. 107 (1), 013105 (2010).
- Xiang, J., et al. Electrically tunable selective reflection of light from ultraviolet to visible and infrared by heliconical cholesterics. Advanced Materials. 27 (19), 3014-3018 (2015).
- Hu, W., et al. Electrically controllable selective reflection of chiral nematic liquid crystal/chiral ionic liquid composites. Advanced Materials. 22 (4), 468-472 (2010).
- Choi, S. S., Morris, S. M. M., Huck, W. T. S., Coles, H. J. Electrically tuneable liquid crystal photonic bandgaps. Advanced Materials. 21 (38-39), 3915-3918 (2009).
- Tokunaga, S., et al. Electrophoretic deposition for cholesteric liquid-crystalline devices with memory and modulation of reflection colors. Advanced Materials. 28 (21), 4077-4083 (2016).
- Sen, M. S., Brahma, P., Roy, S. K., Mukherjee, D. K., Roy, S. B. Birefringence and order parameter of some alkyl and alkoxycyanobiphenyl liquid crystals. Molecular Crystrals and Liquid Crystals. 100 (3-4), 327-340 (1983).
- McConney, M. E., et al. Electrically induced color changes in polymer-stabilized cholesteric liquid crystals. Advanced Optical Materials. 1 (6), 417-421 (2013).
- Choi, S. S., Morris, S. M., Huck, W. T. S., Coles, H. J. The switching properties of chiral nematic liquid crystals using electrically commanded surfaces. Soft Matter. 5 (2), 354-362 (2009).
- Sapp, S., Luebben, S., Losovyj, Y. B., Jeppson, P., Schulz, D. L., Caruso, A. N. Work function and implications of doped poly(3,4-ethylenedioxythiophene)-co-poly(ethylene glycol). Applied Physics Letters. 88 (15), 152107 (2006).
- Groenendaal, L., Jonas, F., Freitag, D., Pielartzik, H., Reynolds, J. R. Poly(3,4-ethylenedioxythiophene) and its derivatives: past, present, and future. Advanced Materials. 12 (7), 481-494 (2000).
- Kirchmeyer, S., Reuter, K. Scientific importance, properties and growing applications of poly(3,4-ethylenedioxythiophene). Journal of Materials Chemistry. 15 (21), 2077-2088 (2005).

