Gold Nanoparticle Modified Carbon Fiber Microelectrodes for Enhanced Neurochemical Detection
Summary
In this study, we modify carbon-fiber microelectrodes with gold nanoparticles to enhance the sensitivity of neurotransmitter detection.
Abstract
For over 30 years, carbon-fiber microelectrodes (CFMEs) have been the standard for neurotransmitter detection. Generally, carbon fibers are aspirated into glass capillaries, pulled to a fine taper, and then sealed using an epoxy to create electrode materials that are used for fast scan cyclic voltammetry testing. The use of bare CFMEs has several limitations, though. First and foremost, the carbon fiber contains mostly basal plane carbon, which has a relatively low surface area and yields lower sensitivities than other nanomaterials. Furthermore, the graphitic carbon is limited by its temporal resolution, and its relatively low conductivity. Lastly, neurochemicals and macromolecules have been known to foul at the surface of carbon electrodes where they form non-conductive polymers that block further neurotransmitter adsorption. For this study, we modify CFMEs with gold nanoparticles to enhance neurochemical testing with fast scan cyclic voltammetry. Au3+ was electrodeposited or dipcoated from a colloidal solution onto the surface of CFMEs. Since gold is a stable and relatively inert metal, it is an ideal electrode material for analytical measurements of neurochemicals. Gold nanoparticle modified (AuNP-CFMEs) had a stability to dopamine response for over 4 h. Moreover, AuNP-CFMEs exhibit an increased sensitivity (higher peak oxidative current of the cyclic voltammograms) and faster electron transfer kinetics (lower ΔEP or peak separation) than bare unmodified CFMEs. The development of AuNP-CFMEs provides the creation of novel electrochemical sensors for detecting fast changes in dopamine concentration and other neurochemicals at lower limits of detection. This work has vast applications for the enhancement of neurochemical measurements. The generation of gold nanoparticle modified CFMEs will be vitally important for the development of novel electrode sensors to detect neurotransmitters in vivo in rodent and other models to study neurochemical effects of drug abuse, depression, stroke, ischemia, and other behavioral and disease states.
Introduction
Carbon-fiber microelectrodes (CFMEs)1 are best used as biosensors to detect the oxidation of several crucial neurotransmitters2, including dopamine3, norepinephrine4, serotonin5, adenosine6, histamine7, and others8. The biocompatibility and size of carbon fibers make them optimal for implantation as there is mitigated tissue damage compared to larger standard electrodes.9 CFMEs are known to possess useful electrochemical properties and are capable of making quick measurements when used with fast electrochemical techniques, most commonly fast-scan cyclic voltammetry (FSCV)10,11. FSCV is a technique that scans the applied potential rapidly and provides a specific cyclic voltammogram for specific analytes12,13. The large charging current produced by fast scanning is stable on carbon fibers and can be background-subtracted to produce specific cyclic voltammograms.
Due to its optimal electrochemistry and neurobiological importance, dopamine has been widely studied. The catecholamine dopamine is an essential chemical messenger that plays a pivotal role in the control of movement, memory, cognition, and emotion within the nervous system. A surplus or deficiency of dopamine can cause numerous neurological and psychological interference; among these are Parkinson’s disease, schizophrenia, and addictive behavior. Today, Parkinson’s disease continues to be a prevalent disorder due to the degeneration of midbrain neurons involved in dopamine synthesis14. Parkinson’s disease symptoms include tremor, slowness of movement, stiffness, and problems in maintaining balance. On the other hand, stimulants such as cocaine15 and amphetamine16,17 promote the overflow of dopamine. Drug abuse eventually substitutes the regular flow of dopamine and conditions the brain to require a surplus of dopamine, which eventually leads to addictive behaviors.
In recent years, there has been an emphasis on improving electrode functionality in neurotransmitter detection18. The most widespread method of enhancing electrode sensitivity is by coating the fiber surface. Surprisingly, there has been limited research done on metal nanoparticle electrodeposition onto carbon-fibers19. Noble metal-nanoparticles such as gold, may be electrodeposited onto the fiber surface with other functional materials20. For example, increasing the electroactive surface area for neurotransmitter adsorption to occur. Electrodeposited metal nanoparticles form rapidly, can be purified, and adhere to the carbon-fiber. Electrochemistry continues to be significant for both the deposition of noble metal nanoparticles and surface enhancement of carbon-fibers, as it allows for the control of nucleation and growth of these nanoparticles. Finally, the increased catalytic and conductive characteristics, and improved mass transport are among other advantages of utilizing metal nanoparticles for electroanalysis.
The Advanced Laboratory sequence course of American University (Experimental Biological Chemistry I and II CHEM 471/671-472/672) is a combination of Analytical, Physical, and Biochemistry laboratories. The first semester is an overview of laboratory techniques. The second semester is a student-driven and led research project21. For these projects, students have previously examined the mechanism of biomolecule, protein, peptide, and amino acid-facilitated synthesis of gold nanoparticles22,23. More recent work has focused on the formation of gold nanoparticle (AuNP) production on electrode surfaces and the evaluation of AuNPs effects on the ability of CFMEs to detect neurotransmitters. In the present work, the laboratory has applied this technique to demonstrate that the sensitivity of CFMEs in detecting the dopamine-oxidation is enhanced through the electrodeposition of AuNP onto the fiber surface. Each bare-CFME is characterized by varying scan-rate, stability and dopamine-concentration when detecting dopamine-oxidative currents to measure dopamine oxidation on the surface of the CFME. Au3+ was then electroreduced to Au0 and concurrently electrodeposited onto the fiber surface as nanoparticles, followed by a series of characterization experiments. After a direct comparison, the AuNP-CFMEs were found to possess higher sensitivity of dopamine detection. The uniform coating of AuNP onto the fiber surface via electrodeposition renders a higher electroactive surface area; thus, increasing the adsorption of dopamine onto the modified electrode surface. This led to higher dopamine oxidative currents. The potential separation of the dopamine oxidation and reduction peaks (∆Ep) of AuNP-CFMEs was also smaller, suggesting faster electron transfer kinetics. Future works of this study includes the in vivo testing of both the bare- and AuNP-CFMEs for the detection of dopamine.
Protocol
1. Construction of carbon-fiber microelectrodes
- Preparation of carbon fibers
- To create carbon-fiber microelectrodes, first separate the carbon fibers (carbon fiber,7 μm in diameter) one by one using hands, gloves, and spatula.
- Pull or yank one fiber from the twisted yarn.
- Aspirate an isolated carbon fiber into a glass capillary (single-barrel borosilicate capillary glass without microfilament, 1.2 mm outer diameter, 0.68 mm inner diameter).
- Create an electrode holder for the electrodes by cutting a piece of cardboard that is approximately 10 cm in length by 25 cm in width.
- Pull the electrodes using a vertical capillary puller.
- Open the sliding door of the vertical capillary puller.
- Loosen and remove the metallic holder rod, by rotating the drill-chuck counterclockwise with enough space to insert the glass capillary.
- Insert the glass capillary into the electrode holder. Raise the glass capillary to the top of the vertical capillary manually by hand.
- Tighten the glass capillary with the drill-chucks clockwise without breaking or shattering the glass capillaries.
- Adjust the Heater 1, Heater 2, and Magnet settings to the manufacturer suggested levels to pull glass capillaries to a fine taper for electrode materials.
- Press the red start button to heat the coiled coil to pull the electrodes via pressure, gravity, and heating.
- Let the coiled coil cool from its red hot state. Cut the carbon fiber with scissors connecting the two pulled electrodes from top to bottom. Use the drill-chuck method to remove the glass capillary from the vertical capillary puller by twisting in the counter-clockwise direction.
2. Carbon-Fiber Microelectrode Preparation
- Under a stereoscope or microscope, cut the carbon fiber protruding from the surface of the glass capillary with surgical scissors or a sharp razor blade to approximately 100 –150 μm in length.
- Prepare a solution of epoxy by mixing 10 g of epoxy with 0.2 mL of hardener in a 25 mL vial using a cotton swab.
- Dip only the tip of each electrode into the epoxy and hardener solution for approximately 15 s.
- Dip the aforementioned top of the carbon fiber microelectrode in acetone for approximately 3 s to wash away any excess epoxy from the barrel of the carbon fiber microelectrode.
3. Electrodeposition
- Place the working electrode (carbon-fiber microelectrode) in the solution of 0.5 mM HAuCl4 in addition to the reference electrode, silver-silver chloride (Ag/AgCl) using the micromanipulator.
- Connect the working electrode and the reference electrode to the potentiostat and headstage.
- Open the UNC HDCV Software. Change the settings on the software to apply the waveform. Enter the following waveform into the computer settings: scan from 0.2 V to −1.0 V in 0.1 M KCl solution containing 0.5 mM HAuCl4 at a scan rate of 50 mV/s for 10 cycles. Press the green arrow to apply the waveform. Then, press the start button to begin recording of the measurements.
4. Scanning electron microscopy
NOTE: Image bare and gold nanoparticle modified carbon fiber microelectrodes using scanning electron microscopy instrument (SEM). Load the sample onto black conductive tape and following the manufacturer described instructions.
- Turning on the instrument
- Turn the key to START and release.
- Open the InTouchScope software by double clicking it.
- Release the key. It should land on the I symbol on its own.
- Wait for the EVAC button to stop blinking.
- Once the EVAC button stops blinking, press the VENT button.
- Wait for the VENT button to stop blinking.
- Ensure that the working distance (WD) is at 20 mm – 30 mm.
- While waiting, prepare the sample(s).
- Scanning
- Once the VENT button stops blinking, load the sample(s) into the instrument.
- Ensure that the curved part of the sample-holder is pointed toward the instrument when loading sample(s).
- Press the EVAC button once the sample(s) is loaded.
- Adjust the working distance to 10 mm.
- Once the EVAC button stops blinking, turn on the computer.
- Click on the In Touch Scope software, located on the desktop. There are two In Touch Scope software, click on the one without the green and yellow circle.
- Once the software opens up, click on OBSERVE (top right of the screen), to turn on the beam. Ensure the EVAC button has stopped blinking before clicking on OBSERVE.
- Start analyzing the sample(s).
- Ensure that the voltage, working distance (WD) and probe current (PC) settings are to acceptable.
- Zoom out (~50X) for a higher setting and zoom in for a lower setting.
- Set the working distance at 10 mm.
- Before taking a picture of sample(s), ensure that the picture will be saved to the desired destination folder.
- To choose desired folder, click on the settings (top left of the screen).
- Export the pictures from the computer via a flash drive.
- Turning off
- Click on OBSERVE to turn off the beam.
- Press the VENT button and wait for it to stop blinking.
- While waiting for the VENT button to stop blinking, adjust the working distance back to 20 mm – 30 mm.
- Once the VENT button stops blinking, unload the sample(s) from the instrument.
- Press the EVAC button, and wait for it to stop blinking.
- Once the EVAC button stops blinking, exit out of the software and shut down the computer.
- Turn the key to the O symbol to completely turn off the instrument.
5. Fast scan cyclic voltammetry testing
- Connect the carbon-fiber microelectrode to potentiostat and headstage along with the Ag/AgCl reference electrode.
- Using the micromanipulator, lower the carbon fiber microelectrode into the flow cell well by manually adjusting the X, Y, and Z measurement knobs.
- Prepare buffer solution in DI water (131.5 mM NaCl, 3.25 mM KCl, 1.2 mM CaCl2, 1.25 mM NaH2PO4, 1.2 mM MgCl2, and 2.0 mM Na2SO4 with the pH adjusted to 7.4).
- Fill the flow cell with phosphate buffered saline (PBS) buffer (pH = 7.4).
- Using a filled 60 mL buffer syringe, inject PBS buffer into the flow cell at approximately 1 mL/min.
- Place the electrode into the flow cell and apply the waveform by pressing the green button. Observe the oscilloscope and either cut the electrode or adjust the gain to prevent overloading. Allow for approximately 10 min of equilibration between each electrode run.
- Set the default waveform to the dopamine waveform. Scan from – 0.4 V to 1.3 V at 10 Hz and 400 V/s.
- Prepare stock solution of 10 mM dopamine, serotonin, norepinephrine, and others in perchloric acid. Dilute neurochemicals to final concentration of 1 μM in buffer by pipetting 1 μM of the dopamine stock solution in 10 mL of PBS buffer using a pipette.
- To begin measurements, press the record button. After 10 s, inject 0.2 mL of 1 μM dopamine into the flow cell or any other concentration of neurotransmitter. Adjust the concentration, scan rate, waveform (holding potential or switching potential) accordingly. Set the total run time for 30 s.
- Analyze the run using the HDCV analysis software. Change the parameters as necessary.
- After the experiment is complete, clean the flow cell by injecting 3 mL of water and then air into the buffer and injection ports of the flow cell three times each.
- Turn off the waveform and the instrument.
Representative Results
For Figure 1, we show a schematic where FSCV testing is utilized to measure the concentration of neurotransmitters in vitro. Figure 1 displays the dopamine waveform applied. The triangle waveform scans from -0.4 V to 1.3 V at 400 V/s. In the second part of the figure to the left, it displays the oxidation of dopamine to dopamine-ortho-quinone (DOQ), a two electron transfer process occurs from the surface of the analyte to the surface of the electrode. Lastly, a current vs. time plot is overlaid with a color plot. The current vs. time plot is a representation of dopamine oxidation. It is flat when there is no dopamine oxidation, and it rises vertically when dopamine is oxidized to dopamine-orthoquinone and reduced back down to dopamine as the analyte adsorbs, and subsequently, desorbs from the surface of the electrode. The color plot is a 3-dimensional plot of current. The yellow current is the background current (close to zero), while the green plot is the positive oxidation current (dopamine oxidation to dopamine orthoquinone), and the blue plot is the negative reduction current (dopamine orthoquinone reduction to dopamine).
SEM was utilized to image surface features of the bare and modified carbon electrodes. In Figure 2, we see unique difference in surface features amongst three different types of electrode materials. In Figure 2a, a bare carbon fiber microelectrode is shown. The fiber is approximately 7 μm diameter with cylindrical ridges along the exterior. Figure 2b shows gold nanoparticles electrodeposited onto the surface of the carbon fiber for approximately 20 min with large sharp ridge of gold protruding from the surface of the carbon fiber. The presence of gold was further verified with EDS/EDX measurements. We then reduced the electrodeposition time to 5 min where we observed a thin uniform coating gold as shown in Figure 2c.
Comparison of Sensitivity and Electron Transfer
Figure 3a shows a comparison of sensitivity and electron transfer. As shown with the overlapping cyclic voltammograms, gold-modified carbon fiber microelectrodes have significantly higher peak oxidative currents (Figure 3b) and faster electron transfer kinetics (ΔEP). Significance was measured with an unpaired t-test (P = .004 and .0016, respectively). Error bars are standard error of the mean.
Stability
The bare (Figure 4a) and gold nanoparticle modified (Figure 4b) CFMEs were placed in the flow cell for 4 h. Measurements were taken for the detection of 1 μM dopamine every hour over 4 h. Both electrodes had a stable response with respect to dopamine. A stable response to dopamine (without water oxidation) is critically important for performing measurements in biological tissue. Error bars are standard error of the mean.
Scan Rate
The scan rate was varied from 100 V/s to 1,000 V/s. Both Bare (Figure 5a) and gold nanoparticle (Figure 5b) modified electrodes showed a linear response with respect to dopamine detection, therefore, indicating adsorption control to the surface of the bare and gold nanoparticle modified microelectrode. Error bars are standard error of the mean.
Concentration
The concentration was varied from 100 nM to 100 μM dopamine for bare (Figure 6a) and gold nanoparticle modified (Figure 6b) carbon fiber microelectrodes. The linear range was from 100 nM to 10 μM. After 10 μM, we observe an asymptotic curve denoting that dopamine is supersaturated at the surface of the carbon fiber microelectrode. The linear response for the peak oxidation current of dopamine with respect to dopamine concentration denotes adsorption control to the surface of the electrode. The physiologically relevant concentrations of dopamine in the brain are within this range and vary between brain regions.
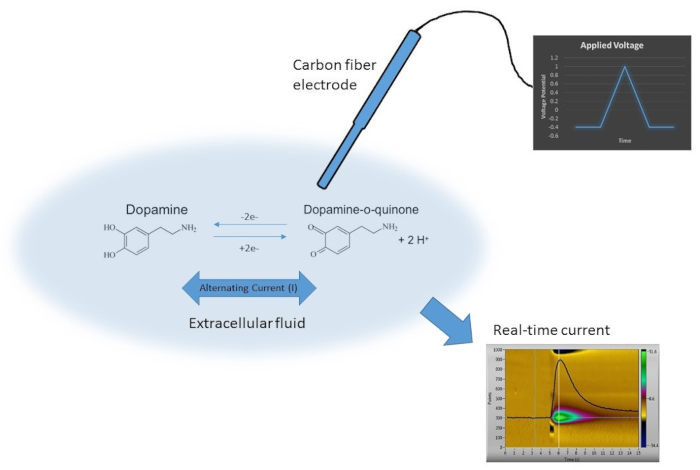
Figure 1. A schematic of dopamine oxidation. Overlay of carbon-fiber microelectrode oxidizing dopamine. Charge transfer is shown from the surface as dopamine is oxidized to dopamine-orthoquinone and back to dopamine as the triangle dopamine waveform is applied (-0.4 V to 1.3 V at 400 V/s). The current vs. time and color plots are shown denoting dopamine oxidation (green) and dopamine reduction (blue). Please click here to view a larger version of this figure.
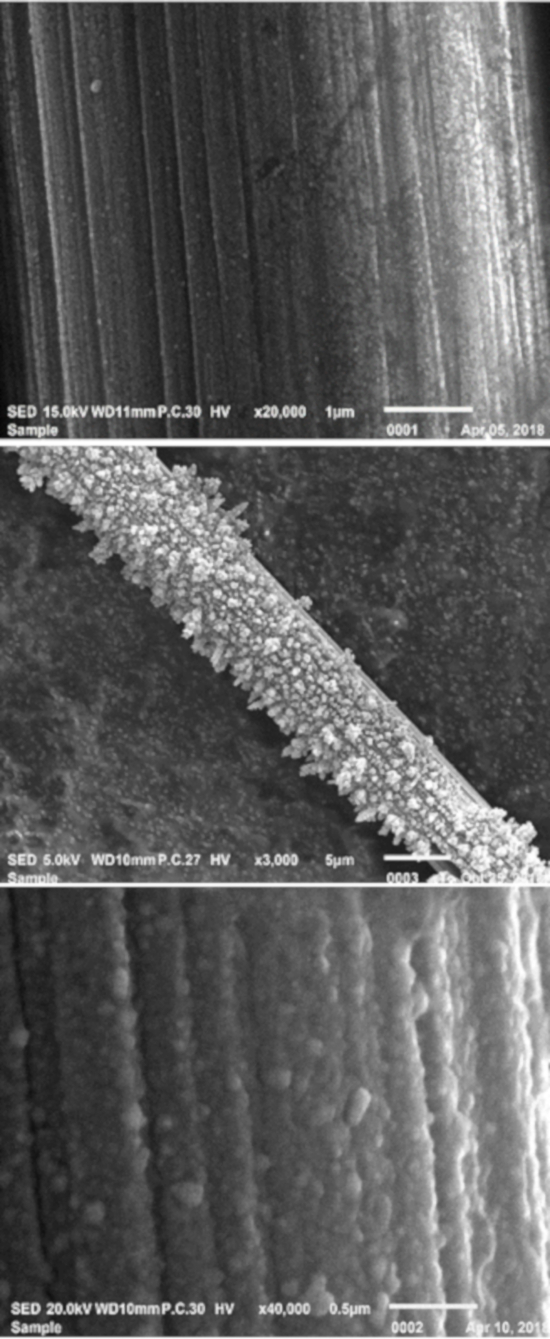
Figure 2. SEM images of (a) bare carbon fiber microelectrode, (b) gold-nanoparticle modified carbon fiber microelectrodes with a 20 min electrode deposition time, and (c) gold-nanoparticle modified microelectrodes with a 5-min electrode deposition time. This provides proof of principle results that the size and thickness of gold nanoparticle coatings can be controlled by the electrodeposition time. Please click here to view a larger version of this figure.
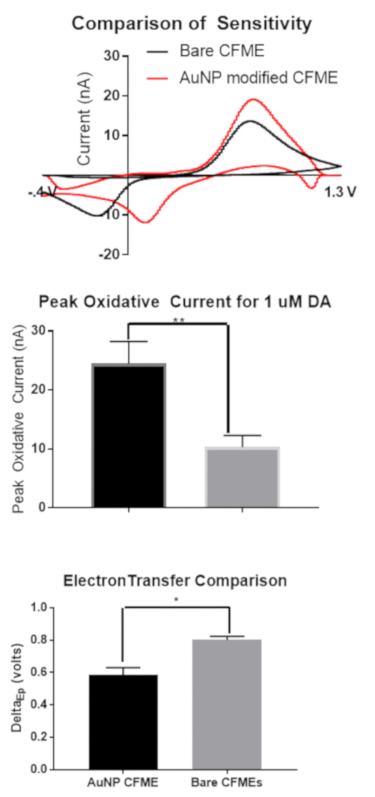
Figure 3. Sensitivity comparison of bare and gold-nanoparticle modified electrodes. (A) Overlay of cyclic voltammograms of bare and gold nanoparticle modified microelectrodes. (B). Bar graph denoting differences in peak oxidative current of bare and gold nanoparticle modified microelectrodes. (C). Bar graph showing difference in ΔEP between bare and gold nanoparticle modified microelectrodes. Error bars are standard error of the mean. Please click here to view a larger version of this figure.
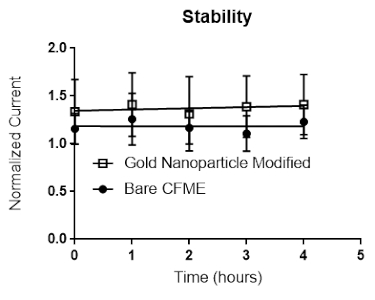
Figure 4. Stability experiment. (A) Bare and (B) gold nanoparticle-modified microelectrodes were placed in a flow cell for a total of at least 4 h. Their sensitivity towards 1 μM dopamine was measured over 4 h. Both had a uniform response to dopamine over 4 h. Error bars are standard error of the mean. Please click here to view a larger version of this figure.
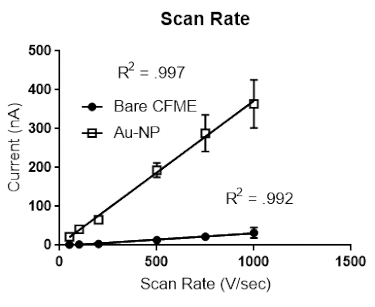
Figure 5. Scan rate experiment. (A) Bare and (B) gold nanoparticle-modified microelectrodes were placed in a flow cell, and the scan rate was varied from 100 V/s to 1,000 V/s. Both bare and gold nanoparticle modified microelectrodes had a linear response with respect to scan rate, thus denoting adsorption control of dopamine to the surface of the bare and gold nanoparticle modified carbon fiber microelectrode. Error bars are standard error of the mean. Please click here to view a larger version of this figure.
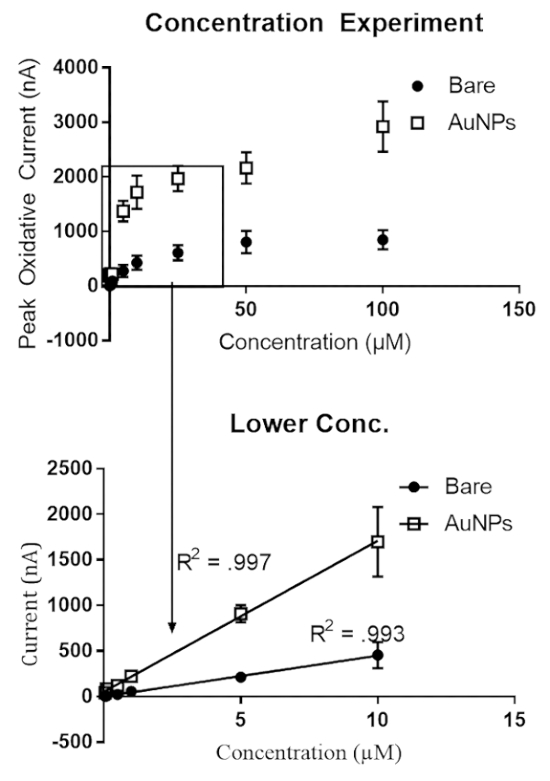
Figure 6. Concentration experiment. (A) Bare and (B) gold nanoparticle-modified microelectrodes were exposed to various concentrations of dopamine 100 nM – 100 μM. Both bare and gold nanoparticle modified microelectrodes had a linear response with respect to dopamine up to 10 μM, thus denoting adsorption control to the surface of the electrode. At concentrations higher than 10 μM, we observe an asymptotic curve, which is indicative of dopamine saturation at the surface of the electrode by occupying all adsorption sites and resulting in more diffusion control. Please click here to view a larger version of this figure.
Discussion
In this study, we demonstrate a novel method to construct gold-nanoparticle modified carbon fiber microelectrodes for the detection of neurotransmitters such as dopamine using fast scan cyclic voltammetry. The method is an efficient, green, and relatively inexpensive approach to enhancing the sensitivity of biomolecule detection. The thickness of gold deposited onto the surface of the carbon fiber can be controlled by the time of electrodeposition and the concentration of gold present in the electrodeposition solution. Gold modified carbon-fiber microelectrodes were shown to have significantly higher electroactive surface areas than bare electrodes in addition to faster electron transfer kinetics. They also had higher sensitivities and lower limits of detection than bare unmodified electrode materials. Furthermore, the electrodes showed a stability towards dopamine detection when tested in the flow cell for at least 4 h. There was a linear response with respect to peak oxidative current for dopamine detection with respect to both scan rate and concentration for the gold modified carbon fiber electrodes denoting adsorption control to the surface of the electrode.
Critical steps in the protocol include the pulling of the carbon-fiber microelectrodes with the vertical capillary puller and achieving interfacial adhesion between the glass capillary and carbon fiber using epoxy. Furthermore, the electrodeposition of gold onto the surface of the carbon fiber is quite challenging as to maintain a balance between having a thin uniform coating of gold on the surface of the electrode and over-depositing excess gold onto the surface of the electrode, which would hinder neurotransmitter detection through noise and signal overload. Modifications and troubleshooting the method include optimizing the method of electrodeposition with respect to both time and concentration. Different sources of gold (AuCl3, HAuCl4, and other gold hydrates) should be utilized to perform these experiments. Limitations of the method include the possibility of the electrodeposited gold overloading the signal of the potentiostat due to over-deposition. Furthermore, as a metal electrode material, gold modified electrodes could potentially oxidize water when scanning to higher potentials (over 1.45 V), which could interfere with analyte signal.
The method is a marked advancement in the field as gold nanoparticle modified microelectrodes significantly enhance neurotransmitter detection and have not been thoroughly examined for neurotransmitter detection using FSCV. Another method of enhancing electrochemical signals for CMFEs is through modification with carbon nanotubes24,25,26. Dipcoating electrodes into carbon nanotube suspensions often increases signal. However, the noise is also increased as the layer of deposited carbon nanotubes is heterogeneous. Gold nanoparticle deposition is a quick, reproducible, and effective method to create enhanced biomolecule sensors. Future method development will include the optimization of gold nanoparticle modification of carbon-fiber microelectrodes create thin, uniform layers of gold over the surface over the carbon fiber microelectrodes. Moreover, the study and optimization of the detection of other neurochemicals (norepinephrine, serotonin, histamine, adenosine, and others) will also be carried out. Lastly, these enhanced gold-modified microelectrodes will be used to perform in vivo measurements of neurotransmitters in rodent or fruit fly models. The enhancement of dopamine detection through gold nanoparticle modification allows for many possible applications and studies in the neurosciences such as studying Parkinson’s disease, drug abuse, and other disorders.
Divulgaciones
The authors have nothing to disclose.
Acknowledgements
We would like to thank American University, the Faculty Research Support Grant, NASA DC Space Grant, and NSF-MRI#1625977.
Materials
| Dopamine hydrochloride | Sigma Aldrich | H8502-5G | |
| Phosphate Buffered Saline | Sigma Aldrich | P5493-1L | |
| Pine WaveNeuro Potentiostat | Pine Instruments | NEC-WN-BASIC | This orders comes in bulk with all other accessories such as headstages, adapters, cords, and other electronics |
| Pine Flow Cell and Micromanipulator | Pine Instruments | NEC-FLOW-1 | This is also another bulk order including the micromanipulator, flow cell, knobs, tubing, connectors, etc. |
| Glass-Capillary | A-M Systems | 602500 | |
| T-650 Carbon Fiber | Goodfellow | C 005711 | |
| Epon 828 Epoxy | Miller-Stephenson | EPON 828 TDS | |
| Diethelynetriamine | Sigma Aldrich | D93856-5ML | |
| Gold (III) chloride | Sigma Aldrich | 254169 | Comes as either HAuCl4 or AuCl3 |
| pH meter | Fisher | S90528 | |
| Farraday Cage | AMETEK TMC | 81-334-03 | |
| Syringe Pump | NEW ERA PUMP | NE-1000 | |
| Eppendorf Pipettes and Tips | Eppendorf | 2231000222 | This is also a bulk order containing multiple pipettes and tips |
| 10 -1,000 mL beakers | VWR | 10536-390 | |
| Carbon fiber | Goodfellow | C 005711 | |
| SEM | JEOL | JSM-IT100 |
Referencias
- Zestos, A. G., Nguyen, M. D., Poe, B. L., Jacobs, C. B., Venton, B. J. Epoxy insulated carbon fiber and carbon nanotube fiber microelectrodes. Sensors and Actuators B: Chemical. 182, 652-658 (2013).
- Bucher, E. S., Wightman, R. M. Electrochemical analysis of neurotransmitters. Annual review of analytical chemistry. 8, 239-261 (2015).
- Zestos, A. G., Venton, B. J. Communication—Carbon Nanotube Fiber Microelectrodes for High Temporal Measurements of Dopamine. Journal of The Electrochemical Society. 165, G3071-G3073 (2018).
- Park, J., Takmakov, P., Wightman, R. M. In vivo comparison of norepinephrine and dopamine release in rat brain by simultaneous measurements with fast-scan cyclic voltammetry. Journal of neurochemistry. 119, 932-944 (2011).
- Abdalla, A., et al. In Vivo Ambient Serotonin Measurements at Carbon-Fiber Microelectrodes. Analytical chemistry. 89, 9703-9711 (2017).
- Ganesana, M., Venton, B. J. Early changes in transient adenosine during cerebral ischemia and reperfusion injury. PloS one. 13, e0196932 (2018).
- Denno, M. E., Privman, E., Borman, R. P., Wolin, D. C., Venton, B. J. Quantification of histamine and carcinine in Drosophila melanogaster tissues. ACS chemical neuroscience. 7, 407-414 (2016).
- Sanford, A. L., et al. Voltammetric detection of hydrogen peroxide at carbon fiber microelectrodes. Analytical chemistry. 82, 5205-5210 (2010).
- Heien, M. L., Johnson, M. A., Wightman, R. M. Resolving neurotransmitters detected by fast-scan cyclic voltammetry. Analytical chemistry. 76, 5697-5704 (2004).
- Raju, D., et al. Polymer modified carbon fiber-microelectrodes and waveform modifications enhance neurotransmitter metabolite detection. Analytical Methods. 11, 1620-1630 (2019).
- Jacobs, C. B., Ivanov, I. N., Nguyen, M. D., Zestos, A. G., Venton, B. J. High temporal resolution measurements of dopamine with carbon nanotube yarn microelectrodes. Analytical chemistry. 86, 5721-5727 (2014).
- Zestos, A. G., Yang, C., Jacobs, C. B., Hensley, D., Venton, B. J. Carbon nanospikes grown on metal wires as microelectrode sensors for dopamine. Analyst. 140, 7283-7292 (2015).
- Zestos, A. G. Carbon Nanoelectrodes for the Electrochemical Detection of Neurotransmitters. International Journal of Electrochemistry. , (2018).
- Kim, J. H., et al. Dopamine neurons derived from embryonic stem cells function in an animal model of Parkinson’s disease. Nature. 418, 50 (2002).
- Zestos, A. G., et al. Ruboxistaurin Reduces Cocaine-Stimulated Increases in Extracellular Dopamine by Modifying Dopamine-Autoreceptor Activity. ACS Chemical Neuroscience. 10, 1960-1969 (2019).
- Zestos, A. G., Kennedy, R. T. Microdialysis Coupled with LC-MS/MS for In Vivo Neurochemical Monitoring. The AAPS Journal. 19, 1284-1293 (2017).
- Carpenter, C., et al. Direct and systemic administration of a CNS-permeant tamoxifen analog reduces amphetamine-induced dopamine release and reinforcing effects. Neuropsychopharmacology. 42, 1940 (2017).
- Zestos, A. G., Venton, B. J. Carbon Nanotube-Based Microelectrodes for Enhanced Neurochemical Detection. ECS Transactions. 80, 1497-1509 (2017).
- Zachek, M. K., Hermans, A., Wightman, R. M., McCarty, G. S. Electrochemical Dopamine Detection: Comparing Gold and Carbon Fiber Microelectrodes using Background Subtracted Fast Scan Cyclic Voltammetry. J Electroanal Chem (Lausanne Switz). 614, 113-120 (2008).
- Li, J., Xie, H., Chen, L. A sensitive hydrazine electrochemical sensor based on electrodeposition of gold nanoparticles on choline film modified glassy carbon electrode. Sensors and Actuators B: Chemical. 153, 239-245 (2011).
- Hartings, M. R., Fox, D. M., Miller, A. E., Muratore, K. E. A hybrid integrated laboratory and inquiry-based research experience: replacing traditional laboratory instruction with a sustainable student-led research project. Journal of Chemical Education. 92, 1016-1023 (2015).
- Hart, C., et al. Protein-templated gold nanoparticle synthesis: protein organization, controlled gold sequestration, and unexpected reaction products. Dalton Transactions. 46, 16465-16473 (2017).
- Hartings, M. R., et al. Concurrent zero-dimensional and one-dimensional biomineralization of gold from a solution of Au3+ and bovine serum albumin. Science and technology of advanced materials. 14, 065004 (2013).
- Xiao, N., Venton, B. J. Rapid, sensitive detection of neurotransmitters at microelectrodes modified with self-assembled SWCNT forests. Analytical chemistry. 84, 7816-7822 (2012).
- Zestos, A. G., Jacobs, C. B., Trikantzopoulos, E., Ross, A. E., Venton, B. J. Polyethylenimine Carbon Nanotube Fiber Electrodes for Enhanced Detection of Neurotransmitters. Analytical chemistry. 86, 8568-8575 (2014).
- Yang, C., et al. Carbon nanotubes grown on metal microelectrodes for the detection of dopamine. Analytical chemistry. 88, 645-652 (2015).

