Stereotaxic Surgery for Implantation of Microelectrode Arrays in the Common Marmoset (Callithrix jacchus)
Summary
This work presents a protocol to perform a stereotaxic, neurosurgical implantation of microelectrode arrays in the common marmoset. This method specifically enables electrophysiological recordings in freely behaving animals but can be easily adapted to any other similar neurosurgical intervention in this species (e.g., cannula for drug administration or electrodes for brain stimulation).
Abstract
Marmosets (Callithrix jacchus) are small non-human primates that are gaining popularity in biomedical and preclinical research, including the neurosciences. Phylogenetically, these animals are much closer to humans than rodents. They also display complex behaviors, including a wide range of vocalizations and social interactions. Here, an effective stereotaxic neurosurgical procedure for implantation of recording electrode arrays in the common marmoset is described. This protocol also details the pre- and postoperative steps of animal care that are required to successfully perform such a surgery. Finally, this protocol shows an example of local field potential and spike activity recordings in a freely behaving marmoset 1 week after the surgical procedure. Overall, this method provides an opportunity to study the brain function in awake and freely behaving marmosets. The same protocol can be readily used by researchers working with other small primates. In addition, it can be easily modified to allow other studies requiring implants, such as stimulating electrodes, microinjections, implantation of optrodes or guide cannulas, or ablation of discrete tissue regions.
Introduction
Common marmosets (Callithrix jacchus) are gaining recognition as an important model organism in many fields of research, including neuroscience. These new-world primates represent an important complementary animal model to both rodents and other non-human primates (NHPs), such as the rhesus macaque. Like rodents, these animals are small, easy to manipulate, and relatively economical to care for and breed1,2,3,4, as compared with larger NHPs. Furthermore, these animals have a propensity for twinning and high fecundity relative to other NHPs1,2,3. Another advantage the marmoset has over many other primates is that modern molecular biology tools3,4,5,6,7 and a sequenced genome2,3,4,5,8 have been used to genetically modify them. Both knock-in animals using lentivirus5, and knock-out animals using zinc-finger nucleases (ZFNs) and transcription activator-like effector nucleases (TALENS)7, have yielded viable founder animals.
An advantage in relation to rodents is that marmosets, as primates, are phylogenetically closer to humans3,5,6,9,10,11. Like humans, marmosets are diurnal animals that depend on a highly developed visual system to guide much of their behavior10. Further, marmosets exhibit behavioral complexity, including a wide range of social behaviors such as the use of different vocalizations3, allowing researchers to address questions not possible in other species. From a neuroscientific perspective, marmosets have lissencephaly brains, unlike the more commonly used rhesus macaque9. Furthermore, marmosets have a central nervous system similar to humans, including a more highly developed prefrontal cortex9. Together, all these characteristics position marmosets as a valuable model to study brain function in health and disease.
A common method for studying brain function involves implanting electrodes in anatomically specific locations by means of stereotaxic neurosurgery. This allows for chronical recording of the neural activity in different target areas in awake and freely behaving animals12,13. Stereotaxic neurosurgery is an indispensable technique used in many lines of research, as it allows precise targeting of neuroanatomical regions. Compared to macaque and rodent literature, there are fewer published studies describing the stereotaxic neurosurgery specific to the marmoset, and they tend to provide sparse detail of the steps involved in the surgery. Moreover, those with greater detail mainly focus on procedures for electrophysiology recording in head-restrained animals14,15,16,17.
In order to facilitate wider adoption of marmosets as a model organism in neuroscience research, the present method defines specific steps necessary for a successful stereotaxic neurosurgery in this species. In addition to implantation of recording arrays, as detailed in the present method, the same technique can be adapted for many other experimental ends, including implantation of stimulating electrodes for the treatment of diseases18 or causally driving circuit behavior19; implantation of guide cannulas for extraction and quantification of neurotransmitters20, injections of reagents, including those for inducing disease models12 or for circuit tracing studies15; ablation of discrete tissue regions21; implantation of optrodes for optogenetic studies22; implantation of optical windows for cortical microscopic analysis23; and implantation of electrocorticographic (ECoG) arrays24. Thus, the overall goal of this procedure is to outline the surgical steps involved in the implantation of microelectrode arrays for chronic electrophysiological recordings in freely behaving marmosets.
Protocol
Animal experiments were performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and approved by the Santos Dumont Institute Ethics Committee (protocol 02/2015AAS).
1. Surgery preparation
- Attach each electrode array to an electrode holder compatible with the stereotaxic frame to be used.
- Connect one electrode holder to the stereotaxic micromanipulator and set one microwire to the interaural coordinates. Repeat this for the additional electrode arrays and holders, if necessary.
NOTE: The interaural coordinate of any microwire can be used to calculate the implantation coordinates for the entire array, because the relative distance between the microwires is constant. When the array has bundles with different lengths, the interaural coordinate of the longest wire is the most convenient to use for setting the interaural zero. - Detach the electrode holders from the stereotaxic micromanipulator and sterilize the assemblies (electrode attached to the electrode holder) in an ultraviolet light (UV) cabinet for at least 2 h.
- Attach a 24 G needle to a stereotaxic probe holder, connect it to the micromanipulator and set the interaural coordinates for the tip of the needle.
NOTE: Prior to the surgery, the coordinates of all craniotomies must be predefined as a perimeter 200 µm2 larger than the anteroposterior (AP) and mediolateral (ML) position of the array’s target implantation site. Use the 24 G needle-probe holder assembly to determine the position of the craniotomies in the skull based on the zero interaural coordinates. - Detach the probe holder from the micromanipulator and sterilize the assembly in a UV cabinet for at least 2 h.
- Gather 6−8 titanium or stainless-steel screws. Solder a ground wire to half of them.
- Organize and sterilize all remaining instruments, equipment, and disposables required for the surgery.
2. Preoperative procedures
NOTE: Two adult male marmosets (Callithrix jacchus) weighing 320–370 g were used in this study. Ensure that the animal has not eaten for 6 h prior to the induction of anesthesia.
- Anesthetize the animal with an intramuscular injection of atropine (0.05 mg/kg) to reduce salivation and bronchial secretions. Check for the lack of pedal responses.
- After 5 min apply ketamine (10−20 mg/kg) intramuscularly.
- Shave the animal’s head using an electric barber clipper.
- Administer tramadol (2 mg/kg) intramuscularly as a general analgesic.
- Intubate the animal.
- Using a mask, expose the marmoset to isoflurane in 1−2% oxygen with a flow rate of 1−5 L/min to induce deep anesthesia. When the animal is deeply anesthetized, reduce and maintain isoflurane to 1−3 L/min.
- Attach an elastic band to the surgical table with tape.
- Position the marmoset in a supine position with the head toward the technician and place the elastic band in the marmoset’s mouth behind its canines.
NOTE: It is best to position the head such that the dorsal surface is pointed toward the floor and its face is toward the technician. - Using a cotton-tipped probe, swab dry the marmoset’s tongue, and grasp it in one hand to keep the mouth open.
- Spray 10% lidocaine on the tip of the endotracheal tube.
- Insert the uncuffed, 2.0 mm diameter endotracheal tube into the trachea until the 4.0 cm mark is at the entrance of the trachea.
- Attach the tube to the anesthesia assembly with the artificial ventilator set to 40 breaths/min and confirm proper expansion and contraction of the chest.
NOTE: At this time the isoflurane and oxygen should be delivered via the endotracheal tube, not the mask. - Remove the elastic band from the marmoset’s mouth so the endotracheal tube can be taped to the jaw.
- Position the marmoset in a prone position in line with the stereotaxic frame and fix the animal’s head into the stereotaxic frame.
- First, insert the tip of the right ear bar into the animal’s right auditory canal.
- Next, insert the tip of the left ear bar into the left auditory canal.
- Center the animal’s head at the center of the stereotaxic frame and fix the ear bars in place.
- Insert the mouthpiece into the animal’s mouth and adjust its height so that it touches the animal’s palate. At the same time, position the orbital bars at the lower surface of the orbital bone.
- Make sure that the lower surface of the orbital bone is horizontally aligned with the center of the ear bars.
- Connect a portable pulse oximeter to the marmoset’s hand. Ensure that the heart rate is within 154−180 beats/min (bpm) for the duration of the surgery; often a heart rate above 200 bpm implies the animal is waking up. Ensure that the oxygen saturation is above 95%. It may occasionally drop to 90% without harm.
NOTE: Should the heart rate drop below 154 bpm, decrease the isoflurane. - Position the rectal temperature probe connected to a homeothermic heating pad into the anus, with the desired temperature set for 37 °C. Tape this sensor to the tail to keep it fixed in place.
- Apply sterile ophthalmic lubricant to the eyes.
- Clean and disinfect the animal’s head with chlorhexidine and povidone iodine before covering the animal with a surgical field.
NOTE: Carry out all the following surgical procedures under aseptic conditions.
3. Surgery procedures
- Apply local analgesic subcutaneously (e.g., lidocaine 20 mg/mL, 0.1 mL) at the site of the intended incision. Make an incision in the midline of the scalp.
- Expose and prepare the surface of the skull.
- Carefully detach the temporal muscle from the cranium. First, use a scalpel to cut the fascia at its insertion into the skull. Then, gently separate the temporal muscle from the cranium using a periosteal raspatory.
- Remove the periosteum from all exposed cranium using a periosteal raspatory.
- Control the bleeding with a sterile cotton swab, if necessary.
- Clean the bone surface with hydrogen peroxide.
- Delineate the location of the craniotomy by marking its corners with shallow burr holes into the bone surface. Then, drill out the perimeter of the craniotomy using a dental drill at maximum speed (i.e., 350,000 rpm). Add a few drops of sterile saline over the skull while drilling to prevent overheating. Measure the position of the craniotomy and the coordinates of the electrode implant with respect to the interaural coordinates.
- Implant screws into the skull.
- Drill 6−8 screw holes into the cranium.
- Implant the screws such that each ground wire fused screw is adjacent to and in the proximity of an unaltered screw (i.e., without a ground wire attached to it).
- Wind each ground wire around the adjacent, unaltered screw.
- Add a drop of silver paint between the ground wire and each screw.
- Remove the bone at the center of the craniotomy using forceps with a curved tip (e.g., McPherson forceps). Keep the dura mater hydrated with sterile saline.
- Remove the dura mater. Use a sterile hypodermic needle (25 or 26 G) with the bevel bent at approximately 90˚ to puncture and lift the surface of the dura mater away from the brain surface. Then, cut the dura mater with microscissors. Keep the exposed brain hydrated with saline.
NOTE: If significant dural bleeding is observed, use cautery or sterile absorbent gelatin sponges soaked in thrombine25. - Implant microelectrode arrays.
- Attach the sterilized electrode holder and electrode array to the stereotaxic micromanipulator.
- Position the micromanipulator such that the electrode is at the desired anteroposterior and mediolateral coordinates.
- Lower the electrode array until the tip of the longest bundle touches the surface of the brain.
- Slowly insert the array into the brain tissue until it reaches the dorsoventral coordinates.
- Cover the exposed cortex with small pieces of sterile, absorbent gelatin sponges.
- Secure the electrode to the skull by applying dental acrylic to the exposed skull, one screw, and the electrode.
- Detach the electrode holder and remove it from the micromanipulator.
- Repeat the implantation procedure from step 3.7 with the additional arrays, if necessary.
- Wind together and weld the ground wires of the separate arrays and screws. Use silver paint to form a bridge around the weld to ensure an electrical connection has been achieved.
- Using dental acrylic, make a sturdy headcap around the lateral extent of the arrays, and completely encase the ground wires and any exposed skull and screws.
- If necessary, insert a support bar into the headcap. This can be a sturdy plastic cylinder like those from a cotton swab. Seal it into place with the dental acrylic.
NOTE: This can be helpful in securing the electrophysiology cable connectors in place but may be unnecessary depending on the equipment used. In the present method, a similar support rod is affixed to the head stage such that an elastic band can robustly hold the head stages in place on the connectors. - Suture the skin around the headcap.
4. Postoperative recovery
- Apply antiseptic solution (e.g., chlorhexidine) around the wound.
- Turn off the isoflurane supply but not the oxygen and remove the animal from the stereotaxic frame.
- Place the animal onto the heating pad with the oxygen maintained through the endotracheal tube.
- Remove the endotracheal tube when the first signs of neurogenic reflexes, such as laryngospasms, are observed.
- Keep supplying the oxygen with a mask until the animal presents clear signs of anesthetic recovery, such as protective reflexes, postural tone, and attempts to ambulate.
- Place the animal inside a clean cage in a recovery room for 24−48 h before moving the animal to its home cage. House each implanted animal individually.
NOTE: Because marmosets tend to climb the cage walls, use a cage with smooth walls or cover the cage walls with a smooth surface to prevent the animal from falling. - In the first hour following surgery, observe the animal to watch for signs of distress or uncoordinated head contact against the side of the cage.
- Administer antibiotics (e.g., enrofloxacin 5 mg/kg, subcutaneously, once a day for 5−7 days), analgesics (e.g., oral tramadol 1 mg/kg, every 8 h for 3−5 days) and anti-inflammatory drugs (e.g., dexamethasone 0.5−1.5 mg/kg, subcutaneously, once a day for 1−3 days).
NOTE: After a successful surgery, animals will be fully recovered within 3−5 days.
5. Chronic electrophysiological recordings in freely behaving marmosets
- Start the electrophysiological recording sessions at least 1 week after the surgery.
NOTE: Habituate the animals to the researcher and experimental environments before starting all experimental procedures for at least 1 month. - At the beginning of each session, lightly anesthetize the animal using isoflurane (1−5 L/min, 1% O2).
NOTE: Follow the relevant institution’s guidelines regarding the sedation of small primates. If recording sessions are very frequent, habituate the animals to be handled so that cables can be connected without anesthesia. - Connect the electrode arrays to a commercial neural recording system.
- Place the animal inside the experimental chamber.
NOTE: The experimental chamber used here is a cubic acrylic box (0.45 m x 0.45 m x 0.45 m) designed to evaluate the amount and pattern of spontaneous motor activity26,27. - Wait for 30 min before starting the recordings to ensure the animal is fully recovered from anesthesia.
NOTE: Isoflurane has a rapid onset and offset action which allows for rapid sedation and awakening28. Once the isoflurane supply is turned off, the animal will start to wake up. The animal is awake when it stays in the upright position and can ambulate freely in the experimental chamber without falling. This takes less than 15 min. To ensure the absence of any sedative effects, begin the recordings 30 min after the isoflurane is discontinued. - Confirm the position of the microelectrode array implants postmortem by NISSL staining after fixing and sectioning the tissue29.
Representative Results
The purpose of this study was to describe a stereotaxic neurosurgical procedure for implantation of microelectrode arrays for electrophysiological recordings in the common marmoset. A typical surgery (from anesthesia induction to anesthesia recovery) will last for approximately 5−7 h, depending on the number of arrays implanted. Here, two arrays were symmetrically implanted, one in each brain hemisphere. Each array contained 32 stainless steel microwires arranged in seven bundles targeting several structures of the basal ganglia-corticothalamic circuit (Figure 1), but the electrode design and targeted brain regions may vary depending on the experiment. After successful surgery and postoperative procedures, the animal should be fully recovered within 3−5 days. If the array has been grounded and implanted properly it will be possible to record spikes (Figure 2A) and local field potentials (Figure 2B) in freely behaving animals over several weeks or months, before a mature gliotic scar is established13,30. As an example, the electrophysiological data collected in the experimental paradigm described here has been effectively used to study the concurrent activity of different regions of the basal ganglia-corticothalamic circuit during spontaneous, ground-based locomotion in a model of Parkinson’s disease26.
Finally, a successful surgery also involves implanting the arrays into the targeted structures. Non-invasive imaging methodologies, such as MRI or tomography may be performed following the surgery and prior to beginning experimental recordings. Use of such methodology will be possible only if the specific implants used are manufactured to be compatible with such techniques, and if the researcher has access to appropriate small animal equipment. Ultimate confirmation can also be performed postmortem. Nissl stained sections containing electrode tracks can be used to precisely determine the position of each implanted microwire (Figure 3). Notice that electrode tracks in coronal sections appear as tears in the tissue. Thus extreme care must be used when sectioning is performed to reduce the chance of creating artifacts that will confound interpretation.
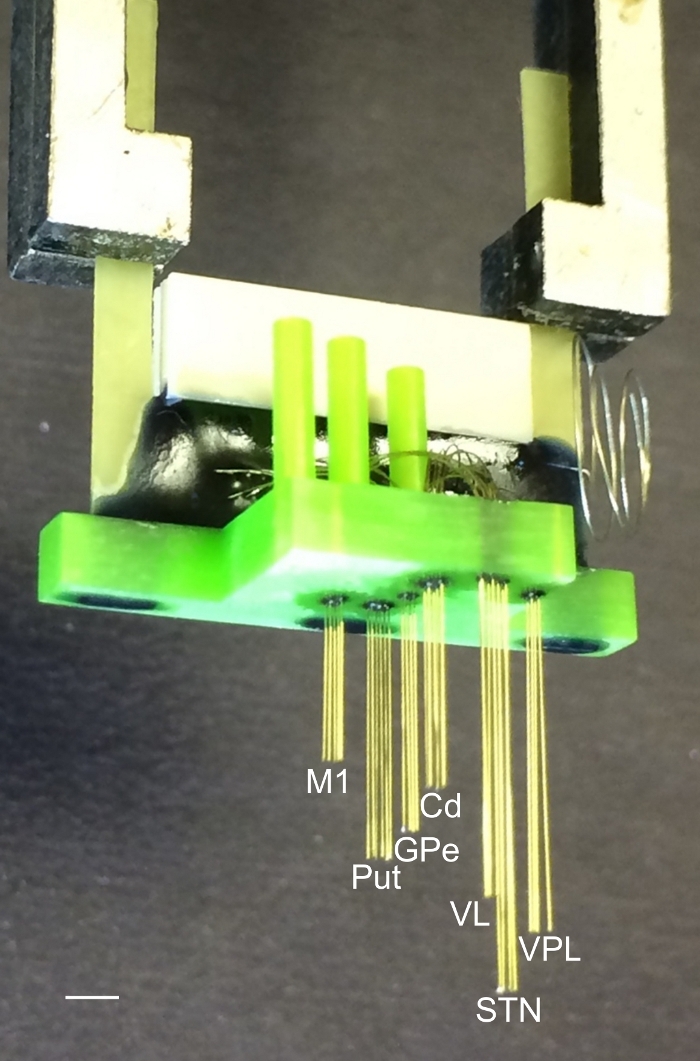
Figure 1: Microelectrode array for implantation in small primates. The array was composed of 32 stainless steel microwires. The wires were 50 µm in diameter and were organized in seven bundles aimed to reach the following areas: primary motor cortex (M1), putamen (Put), caudate (Cd), globus pallidus (GPe), ventrolateral and ventroposterior lateral thalamic nucleus (VPL), and subthalamic nucleus (STN). The interelectrode spacing in each bundle was 300 µm. The interbundle spacing depends on the target coordinates for each brain region. More detailed information about microelectrode array design and manufacturing can be found in Nicolelis31, Lehew and Nicolelis32, and Dizirasa et al.33. Scale bar = 5 mm. Please click here to view a larger version of this figure.
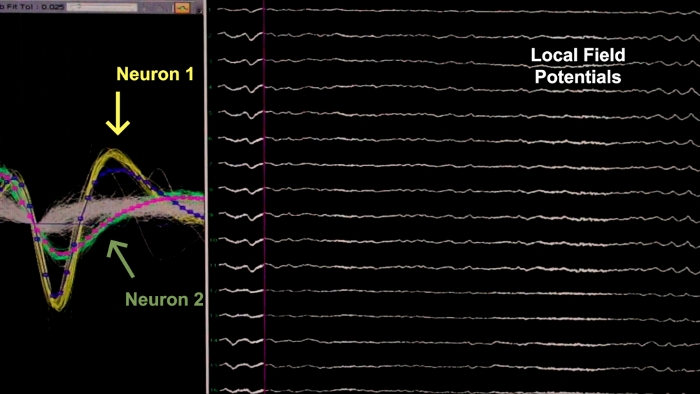
Figure 2: Representative electrophysiological result following a successful surgery. The left panel shows spike activity of two neurons (yellow and green waveforms) recorded from one electrode. The right panel shows local field potential oscillations recorded from 14 electrodes. Please click here to view a larger version of this figure.
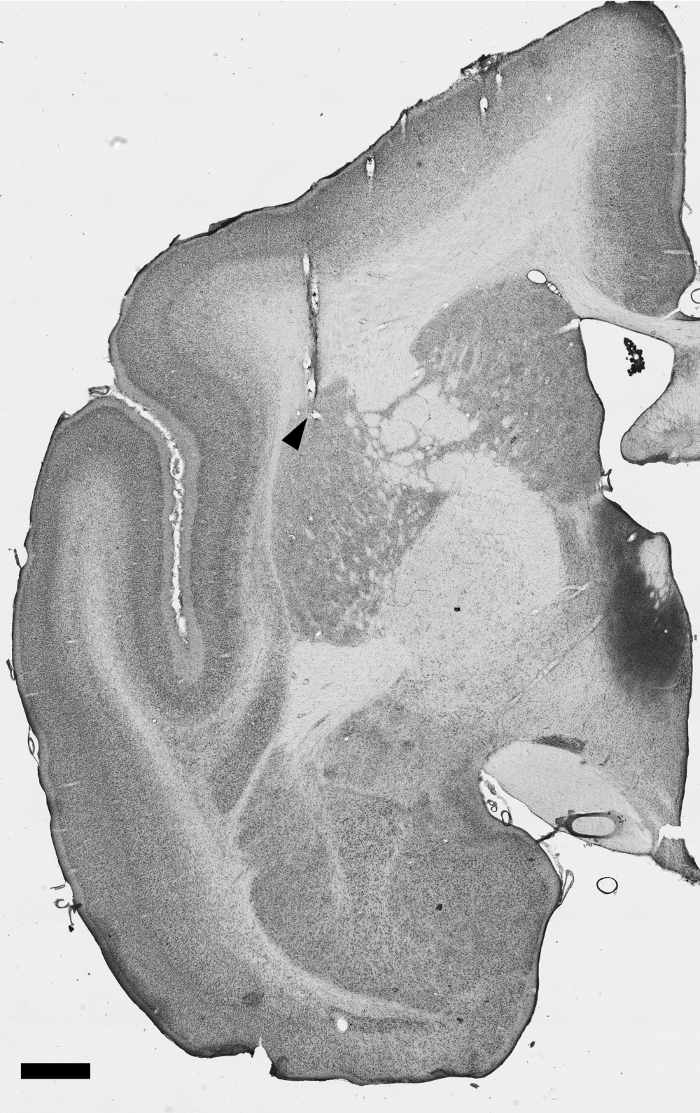
Figure 3: Nissl stained tissue section demonstrating an electrode track. This section (anteroposterior coordinate, relative to interaural line: +8.0, according to the atlas by Paxinos and Watson34) depicts an electrode track with the tip at the Putamen, as indicated by the black triangle. Scale bar = 1 mm. Please click here to view a larger version of this figure.
Discussion
This work provides a detailed description of the procedures involved in the implantation of microelectrode recording arrays in the marmoset brain. This same protocol can be readily used when implanting electrodes, whether homemade or commercially available, in other small primates. Additionally, it can be easily adapted for other experimental ends that require precise targeting of brain structures. Therefore, this protocol is purposefully vague regarding stereotaxic coordinates and cranial drilling techniques, because those are the aspects that may vary the most. For instance, to implant the arrays used in this surgery, craniotomies were performed to open two appropriately sized windows in each hemisphere. However, when implanting sturdy, individual structures, such as guide cannulas, neither this nor the durectomy is necessary. Rather, a simple burr hole to the level of the dura will suffice. Similarly, when nonelectrical implants are involved it is not necessary for the screws to be grounded. Thus, step 3.9 in the surgical protocol can be omitted. Instead, dental acrylic can be used to simply cover the exposed skull, implant, and screws.
Regardless of the specific experimental goal of the stereotaxic neurosurgery, successful implementation of the given procedure largely revolves around good surgical practices. This means that rigorous protocols must be followed to perform the surgery under aseptic conditions in order to prevent postoperative infections35. Some of the most critical moments are inducing and removing anesthesia. It is therefore essential that the vital signs of the animal (heart rate, blood oxygen saturation, and body temperature) be monitored throughout the entire surgical procedure36. If a decrease in the heart rate with a concurrent drop in oxygen saturation occurs, confirm that the chest is inflating and deflating normally, otherwise the connection to the breathing machine may be at fault. The first thing that can be done to attempt to recover the heart rate and oxygen saturation is to decrease the isoflurane concentration. If this does not resolve the issue, atropine may be administered intramuscularly to increase the heart rate and attempt to stabilize the animal. This must be done extremely cautiously, because previous experience shows that a heart rate above 200 bpm without sufficient isoflurane will awaken the animal.
Unlike rodents, in primates all coordinates are usually measured relative to the interaural coordinate, not the bregma and lambda34. Therefore, it is important to measure the interaural zero coordinates of the electrode arrays and other probes before fixing the animal’s head in the stereotaxic apparatus. Moreover, in marmosets the horizontal plane is defined as the plane passing through the lower margin of the orbital bone and the center of the external auditory meatus. Thus, it is important to align the lower surface of the orbital bone with the center of the ear bars before fixing the head in the stereotaxic frame. Furthermore, the temporal muscles of the marmoset cover a wide area of the cranium. Thus, many neural targets require craniotomies to be performed under or in very close proximity to this musculature. Because these muscles are important for marmoset communication38, the surgeon must slowly and carefully detach this musculature from the cranium to minimize damage.
Researchers familiar with behavioral work involving either rodents or marmosets should be aware of several limitations when performing electrophysiology in freely behaving NHPs. First, in the present arrangement and others involving high density arrays or multiple arrays, it is likely that inducing light anesthesia will be required to attach the cable connectors, even after appropriate habituation. This procedure, while within the scope of NIH’s and other countries’ regulatory guidelines, should be performed sparingly to reduce the mental and physical stress on the marmoset. Furthermore, it is critical that the researcher ensure the animal is fully recovered from anesthesia before beginning data acquisition, otherwise the anesthesia may confound the data39. Another related limitation is the physical presence of the cable itself. While wireless recording solutions are becoming available40, the more common wired options impose a physical restriction on the animal. Finally, the experimental chamber being used will also restrict the range of behaviors available to the marmoset. Unlike rodents, marmosets display unique behaviors (e.g., climbing) that will not be possible depending on the experimental chamber being used.
Advances in material science and engineering are leading to the novel neural interfaces41. Effective neurosurgical procedures, such as the one described in this manuscript, will allow researchers to implement these new and forthcoming tools in marmosets. Combined with the concurrent developments in molecular biology3,4,5, marmosets have the potential to allow investigations of important basic and clinical questions in neuroscience.
Divulgaciones
The authors have nothing to disclose.
Acknowledgements
The authors would like to thank Bernardo Luiz for technical assistance with filming and editing. This work was supported by Santos Dumont Institute (ISD), Brazilian Ministry of Education (MEC) and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES).
Materials
| Equipments | |||
| 683 Small Animal Ventilator | Harvard Apparatus, Inc. | 55-0000 | |
| Anesthesia Assembly | BRASMED | COLIBRI | |
| Barber Clippers | Mundial | HC-SERIES | |
| Dental Drill | Norgen | B07-201-M1KG | |
| Homeothermic Heating Pad and Monitor | Harvard Apparatus, Inc. | 50-7212 | |
| Marmoset Stereotaxic Frame | Narishige Scientific Instrument Lab | SR-6C-HT | |
| Patient Monitor and Pulse Oximeter | Bionet Co., Ltd | BM3 | |
| Stereotaxic Micromanipulator | Narishige Scientific Instrument Lab | SM-15R | |
| Surgical Microscope | Opto | SM PLUS IBZ | |
| Instruments | |||
| Allis tissue forceps | Sklar | 36-2275 | |
| Alm Retractor, rounded point, 4×4 teeth | Rhosse | RH11078 | |
| Angled McPherson Forceps | Oftalmologiabr | 11301A | |
| Curved Surgial Scissors | Harvard Apparatus, Inc. | 72-8422 | |
| Curved Tissue Forceps | Sklar | 47-1186 | |
| Delicate Dissection forceps | WPI | WP5015 | |
| Dental Drill Bit | Microdont | ISO.806.314.001.524.010 | |
| Essring Tissue Forceps | Sklar | 19-2460 | |
| FG 1/4 Dental Drill Bit | Microdont | ISO.700.314.001.006.005 | |
| Halsey Needle Holder | WPI | 15926-G | |
| Halstead Mosquito forceps | WPI | 503724-12 | |
| Hemostatic Forceps, Straight | Sklar | 17-1260 | |
| Jewler Forceps | Sklar | 66-7436 | |
| McPherson-Vannas Optathalmic microscissor, 3 mm point | Argos Instrumental | ARGOS-4004 | |
| Pereosteal Raspatory | Golgran | 38-1 | |
| Scalpal Handle | Harvard Apparatus, Inc. | 72-8354 | |
| Screwdrivers | Eurotool | SCR-830.00 | |
| Sodering Iron | Hikari | 21K006 | |
| Surgical Scissor | Harvard Apparatus, Inc. | 72-8400 | |
| Toothed forceps | WPI | 501266-G | |
| Disposables/Single Use | |||
| 1 ml sterile syringe with 26 G needle | Descarpack | 7898283812785 | |
| 130 cm x 140 cm surgical field, presterilized | ProtDesc | 7898467276344 | |
| 24G Needle, presterilized | Descarpack | 7898283812846 | |
| 50 cm x 50 cm surgical field, presterilized | Esterili-med | 110100236 | |
| Cotton Tipped Probes, Presterilized | Jiangsu Suyun Medical Materials Co. LTD | 23007 | |
| Cotton tipped Qutips | Higie Topp | 7898095296063 | |
| Electrode Array | Home made | ||
| Endotracheal tube without cuff, internal diameter 2.0 mm, outer diameter 2.9 mm | Solidor | 7898913077201 | |
| Tinned copper wire, 0.15 mm diameter | |||
| M1.4×3 Stainless steel screws | USMICROSCREW | M14-30M-SS-P | |
| Medical Tape | Missner | 7896544910102 | |
| Nylon surgical sutures | Shalon | N540CTI25 | |
| Scalpal Blade, presterilized | AdvantiVe | 1037 | |
| solder | Kester | SN63PB37 | |
| Sterile Saline 0.9% | Isofarma | 7898361700041 | |
| Sterile Surgical Gloves | Maxitex | 7898949349051 | |
| Sterile Surgical Gown | ProtDesc | 7898467281208 | |
| Surgical Gauze, 15 cm x 26 cm presterilized | Héika | 7898488470315 | |
| Gelfoam | Pfizer | ||
| Drugs/Chemicals | |||
| 0.25mg/ml Atropine | Isofarma | ||
| 10% Lidocaine Spray | Produtos Químicos Farmacêuticos Ltda. | 7896676405644 | |
| 2.5% Enrofloxacino veterinary antibiotic | Chemitec | 0137-02 | |
| Dexametasona Veterinary Anti inflammatory | MSD | R06177091A-00-15 | |
| Hydrogen Peroxide | Farmax | 7896902211537 | |
| Isoflourane | BioChimico | 7897406113068 | |
| Jet Acrylic polymerization solution | Artigos Odontológicos Clássico | ||
| Jet Auto Polymerizing Acrylic | Artigos Odontológicos Clássico | ||
| Ketamine 10% | Syntec | ||
| Lidocaine and Phenylephrine 1.8 ml local anesthetic | SS White | 7892525041049 | |
| Povidone-Iodine solutiom | Farmax | 7896902234093 | |
| Riohex 2% surgical Soap | Rioquímica | 7897780209418 | |
| Silver Paint | SPI Supplies | 05002-AB | |
| Tramadol chloride 50 mg/ml | União Química | 7896006245452 | |
| Refresh gel (polyacrylic acid) | Allergan |
Referencias
- Okano, H., Hikishima, K., Iriki, A., Sasaki, E. The common marmoset as a novel animal model system for biomedical and neuroscience research applications. Seminars in Fetal and Neonatal Medicine. 17 (6), 336-340 (2012).
- Harris, R. A., et al. Evolutionary genetics and implications of small size and twinning in callitrichine primates. Proceedings of the National Academy of Sciences. 111 (4), 1467-1472 (2014).
- Kishi, N., Sato, K., Sasaki, E., Okano, H. Common marmoset as a new model animal for neuroscience research and genome editing technology. Development, Growth & Differentiation. 56 (1), 53-62 (2014).
- Sasaki, E. Prospects for genetically modified non-human primate models, including the common marmoset. Neuroscience Research. 93, 110-115 (2015).
- Sasaki, E., et al. Generation of transgenic non-human primates with germline transmission. Nature. 459 (7246), 523-527 (2009).
- Sasaki, E. Creating Genetically Modified Marmosets. The Common Marmoset in Captivity and Biomedical Research. , 335-353 (2019).
- Sato, K., et al. Generation of a Nonhuman Primate Model of Severe Combined Immunodeficiency Using Highly Efficient Genome Editing. Cell Stem Cell. 19 (1), 127-138 (2016).
- Sato, K., et al. Resequencing of the common marmoset genome improves genome assemblies and gene-coding sequence analysis. Scientific Reports. 5, 16894 (2015).
- Chaplin, T. A., Yu, H. H., Soares, J. G. M., Gattass, R., Rosa, M. G. P. A Conserved Pattern of Differential Expansion of Cortical Areas in Simian Primates. Journal of Neuroscience. 33 (38), 15120-15125 (2013).
- Mitchell, J. F., Leopold, D. A. The marmoset monkey as a model for visual neuroscience. Neuroscience Research. 93, 20-46 (2015).
- Brok, H. P. M., et al. Non-human primate models of multiple sclerosis: Non-human primate models of MS. Immunological Reviews. 183 (1), 173-185 (2001).
- Santana, M. B., et al. Spinal Cord Stimulation Alleviates Motor Deficits in a Primate Model of Parkinson’s disease. Neuron. 84 (4), 716-722 (2014).
- MacDougall, M., et al. Optogenetic manipulation of neural circuits in awake marmosets. Journal of Neurophysiology. 116 (3), 1286-1294 (2016).
- Wakabayashi, M., et al. Development of stereotaxic recording system for awake marmosets (Callithrix jacchus). Neuroscience Research. 135, 37-45 (2018).
- Johnston, K. D., Barker, K., Schaeffer, L., Schaeffer, D., Everling, S. Methods for chair restraint and training of the common marmoset on oculomotor tasks. Journal of Neurophysiology. 119 (5), 1636-1646 (2018).
- Sedaghat-Nejad, E., et al. Behavioral training of marmosets and electrophysiological recording from the cerebellum. Journal of Neurophysiology. , (2019).
- Kringelbach, M. L., Owen, S. L., Aziz, T. Z. Deep-brain stimulation. Future Neurology. 2 (6), 633-646 (2007).
- Talakoub, O., Gomez Palacio Schjetnan, A., Valiante, T. A., Popovic, M. R., Hoffman, K. L. Closed-Loop Interruption of Hippocampal Ripples through Fornix Stimulation in the Non-Human Primate. Brain Stimulation. 9 (6), 911-918 (2016).
- Oddo, M., Hutchinson, P. J. Understanding and monitoring brain injury: the role of cerebral microdialysis. Intensive Care Medicine. 44 (11), 1945-1948 (2018).
- Metz, G. A., Whishaw, I. Q. Cortical and subcortical lesions impair skilled walking in the ladder rung walking test: a new task to evaluate fore- and hindlimb stepping, placing, and co-ordination. Journal of Neuroscience Methods. 115 (2), 169-179 (2002).
- Gradinaru, V., Mogri, M., Thompson, K. R., Henderson, J. M., Deisseroth, K. Optical Deconstruction of Parkinsonian Neural Circuitry. Science. 324, 354-359 (2009).
- Hammer, D. X., et al. Longitudinal vascular dynamics following cranial window and electrode implantation measured with speckle variance optical coherence angiography. Biomedical Optics Express. 5 (8), 2823-2836 (2014).
- Komatsu, M., Kaneko, T., Okano, H., Ichinohe, N. Chronic Implantation of Whole-cortical Electrocorticographic Array in the Common Marmoset. Journal of Visualized Experiments. (144), (2019).
- Oliveira, L. M. O., Dimitrov, D. . Surgical Techniques for Chronic Implantation of Microwire Arrays in Rodents and Primates. , (2008).
- Santana, M. B., et al. Spinal Cord Stimulation Alleviates Motor Deficits in a Primate Model of Parkinson’s disease. Neuron. 84 (4), 716-722 (2014).
- Santana, M., Palmér, T., Simplício, H., Fuentes, R., Petersson, P. Characterization of long-term motor deficits in the 6-OHDA model of Parkinson’s disease in the common marmoset. Behavioural Brain Research. 290, 90-101 (2015).
- Misra, S., Koshy, T. A review of the practice of sedation with inhalational anaesthetics in the intensive care unit with the AnaConDa device. Indian Journal of Anaesthesia. 56 (6), 518-523 (2012).
- Freire, M. A. M., et al. Distribution and Morphology of Calcium-Binding Proteins Immunoreactive Neurons following Chronic Tungsten Multielectrode Implants. PLOS ONE. 10 (6), 0130354 (2015).
- Budoff, S., et al. Astrocytic Response to Acutely- and Chronically Implanted Microelectrode Arrays in the Marmoset (Callithrix jacchus) Brain. Brain Sciences. 9 (2), 19 (2019).
- Dzirasa, K., Fuentes, R., Kumar, S., Potes, J. M., Nicolelis, M. A. L. Chronic in vivo multi-circuit neurophysiological recordings in mice. Journal of Neuroscience Methods. 195 (1), 36-46 (2011).
- Nicolelis, M. A. L., et al. Chronic, multisite, multielectrode recordings in macaque monkeys. Proceedings of the National Academy of Sciences. 100 (19), 11041-11046 (2003).
- Lehew, G., Nicolelis, M. A. L. . State-of-the-Art Microwire Array Design for Chronic Neural Recordings in Behaving Animals. , (2008).
- Paxinos, G., Watson, C., Petrides, M., Rosa, M., Tokuno, H. . The Marmoset Brain in Stereotaxic Coordinates. , (2012).
- Brown, M. J., Pearson, P. T., Tomson, F. N. Guidelines for animal surgery in research and teaching. American Journal of Veterinary Research. 54 (9), 1544-1559 (1993).
- Flecknell, P. A. Anaesthesia of Animals for Biomedical Research. British Journal of Anaesthesia. 71 (6), 885-894 (1993).
- Kurihara, S., et al. A Surgical Procedure for the Administration of Drugs to the Inner Ear in a Non-Human Primate Common Marmoset (Callithrix jacchus). Journal of Visualized Experiments. (132), (2018).
- Boer, R. A., de Vries, A. M. O., Louwerse, A. L., Sterck, E. H. M. The behavioral context of visual displays in common marmosets (Callithrix jacchus). American Journal of Primatology. 75 (11), 1084-1095 (2013).
- Kudo, C., Nozari, A., Moskowitz, M. A., Ayata, C. The impact of anesthetics and hyperoxia on cortical spreading depression. Experimental Neurology. 212 (1), 201-206 (2008).
- Ghomashchi, A., et al. A low-cost, open-source, wireless electrophysiology system. 2014 36th Annual International Conference of the IEEE Engineering in Medicine and Biology Society. , 3138-3141 (2014).
- Fu, T. M., Hong, G., Viveros, R. D., Zhou, T., Lieber, C. M. Highly scalable multichannel mesh electronics for stable chronic brain electrophysiology. Proceedings of the National Academy of Sciences. 114 (47), 10046-10055 (2017).

