Preparation of Drosophila Larval and Pupal Testes for Analysis of Cell Division in Live, Intact Tissue
Summary
The goal of this protocol is to analyze cell division in intact tissue by live and fixed cell microscopy using Drosophila meiotic spermatocytes. The protocol demonstrates how to isolate whole, intact testes from Drosophila larvae and early pupae, and how to process and mount them for microscopy.
Abstract
Experimental analysis of cells dividing in living, intact tissues and organs is essential to our understanding of how cell division integrates with development, tissue homeostasis, and disease processes. Drosophila spermatocytes undergoing meiosis are ideal for this analysis because (1) whole Drosophila testes containing spermatocytes are relatively easy to prepare for microscopy, (2) the spermatocytes’ large size makes them well suited for high resolution imaging, and (3) powerful Drosophila genetic tools can be integrated with in vivo analysis. Here, we present a readily accessible protocol for the preparation of whole testes from Drosophila third instar larvae and early pupae. We describe how to identify meiotic spermatocytes in prepared whole testes and how to image them live by time-lapse microscopy. Protocols for fixation and immunostaining whole testes are also provided. The use of larval testes has several advantages over available protocols that use adult testes for spermatocyte analysis. Most importantly, larval testes are smaller and less crowded with cells than adult testes, and this greatly facilitates high resolution imaging of spermatocytes. To demonstrate these advantages and the applications of the protocols, we present results showing the redistribution of the endoplasmic reticulum with respect to spindle microtubules during cell division in a single spermatocyte imaged by time-lapse confocal microscopy. The protocols can be combined with expression of any number of fluorescently tagged proteins or organelle markers, as well as gene mutations and other genetic tools, making this approach especially powerful for analysis of cell division mechanisms in the physiological context of whole tissues and organs.
Introduction
Cell division is often studied using cell lines grown in culture1. While we have gained a wealth of invaluable insight and understanding of fundamental mechanisms from these studies2, cells grown in culture cannot fully recapitulate the physiology of cell division as it occurs in intact, living tissue. For example, in intact tissues and organs, cells must divide at the right place and at the right time so that progeny cells are properly situated within the tissue, so that they can undergo appropriate differentiation or functional programs, and so that cell proliferation is properly coordinated with tissue growth or homeostasis3. For cells grown in culture on the other hand, cell division is generally regulated by growth factors in the culture medium4, and thus we cannot learn from these cells how in vivo environmental factors such as tissue architecture or developmental signaling influence the division process. It is also important to note that many of the cell lines used to study cell division, such as HeLa and U2OS cells, were derived from metastatic tumors5. Therefore, many aspects of the basic physiology of these cancer cells, such as cell cycle regulatory mechanisms and chromosomal stability, have likely been altered compared to healthy cells. Complete understanding of cell division physiology, therefore, depends on our ability to study dividing cells in their native, in vivo environments that preserve physiological regulatory mechanisms and tissue architecture.
Advances in understanding how cell division operates within intact tissues and organs are hampered by difficulties inherent to in vivo or ex vivo analysis. First, it can be difficult or impossible to access dividing cells for microscopic analysis within large organs or thick tissues. Second, it is often difficult to predict when individual cells will divide in vivo. Third, tissue physiology may rapidly deteriorate during ex vivo culture. In this protocol, we describe readily accessible methods for live and fixed analysis of Drosophila melanogaster spermatocytes as they undergo meiotic cell divisions within fully intact testes. These cells are ideal for live, ex vivo analysis because they are readily accessible with standard optical methods such as confocal microscopy, they divide at predictable times and locations, and intact testes can be maintained in ex vivo culture for up to about 24 h. In addition, Drosophila spermatocytes are large round cells (approximately 20–30 µm in diameter) that do not change shape when they divide, making them ideal for high resolution, time-lapse imaging of cellular components such as the spindle apparatus and cytoplasmic organelles. Although these cells undergo meiosis as opposed to mitosis, many of the essential cell division processes are very similar between these two cell division mechanisms6. These advantages, combined with powerful Drosophila genetic tools, have made Drosophila spermatocytes an extensively used model for ex vivo analysis of essential cell division processes including spindle formation and regulation, cytokinesis, and organelle remodeling and partitioning7,8,9,10,11.
Spermatocytes are cells that are at the meiotic stage of spermatogenesis. In Drosophila, spermatogenesis begins with a group of germline stem cells that are located in a small region or “hub” at one pole of the testis12,13. These cells divide by an asymmetric mitosis, giving rise to a single differentiated spermatogonium. Spermatogonia then undergo four synchronous mitoses to produce a group of 16 cells that remain closely associated within a single cyst. At this stage, the cells transition from a mitotic to a meiotic cell cycle and are referred to as spermatocytes. Spermatocytes spend about two to three days in an extended G2 stage of the cell cycle, during which they grow dramatically and undergo cytological changes in preparation for the two meiotic divisions and subsequent spermiogenesis13,14. The entire group of 16 spermatocytes within a single cyst then enter the first meiotic division at the same time. Thus, several meiotic spermatocytes can be imaged simultaneously as they proceed through cell division. The first meiotic division proceeds for approximately 1.5 h and is followed almost immediately by the second meiotic division, yielding 64 total spermatids that go on to differentiate into mature spermatozoa.
A unique advantage of using spermatocytes to study cell division in live, intact tissue is that groups or cysts of cells constantly progress through the different stages of spermatogenesis, and cells at all stages of spermatogenesis can usually be identified in any given testis (see Figure 3A). Therefore, it is relatively easy to find meiotic cells in whole testes. We generally focus our analyses on the first as opposed to second meiosis because these cells are much larger and more amenable to high resolution imaging, but the entire process encompassing both meiotic divisions can be successfully imaged. It should also be noted that the general protocol for testis preparation and culture can be used to analyze other processes of spermatogenesis as well, such as the earlier mitotic stem cell or spermatogonial divisions or the cytological changes that occur as spermatids mature into spermatozoa15. Many of these aspects of spermatogenesis are highly conserved between Drosophila and humans16.
Drosophila spermatocytes begin to reach the meiotic phase of spermatogenesis during the third instar larval stage of Drosophila development13. Therefore, testes isolated from lifecycle stages beginning with third instar larvae and including pupae and adults can be used for analysis of dividing spermatocytes. Several excellent protocols are available for extraction of testes from adult male flies for live and fixed analysis of spermatogenesis17,18,19. These protocols may be preferable for studying late stages of spermatogenesis or if genetic markers that are only visible in adults must be used. The protocol focuses instead on testis preparation from larvae and early pupae, because testes at these stages have several advantages that are specifically pertinent to cell division analysis by high resolution, time-lapse microscopy. First, testes from larvae and pupae are smaller than those from adults, and the cells within the organ are less crowded. Because of this, dividing spermatocytes can often be imaged near the outer surface of larval testes, without having to penetrate through multiple layers of light scattering tissue. Second, adult testes move rhythmically due to contractions of the attached accessory organs, and these movements make time-lapse imaging of individual cells challenging. And third, larval testes are advantageous when studying gene mutations that cause pupal or adult lethality. Our methods are optimized for long-term culture of testes on the microscope stage, allowing for imaging of multiple rounds of cell division or the progression of individual cells through multiple stages of spermatogenesis in the same preparation. We also describe a protocol for fixation and immunostaining of whole testes. Overall, our protocols are particularly useful for those interested in studying cell division in intact tissue, and the ability to combine spermatocyte analysis with highly tractable Drosophila genetic tools makes this an especially powerful approach.
Protocol
1. Prepare Animals, Tools, and Media for Dissection
- Cross male and female flies to obtain progeny of the desired genotype. Useful transgenic markers for identification and staging of meiotic spermatocytes include GFP-tubulin to label the meiotic spindle and RFP-histone 2A to label chromsomes8. Use five to ten females per cross and maintain crosses on standard fly food in vials in a 25 °C incubator until third instar larvae begin crawling up the sides of the vial (4–5 days). Early white pupae will begin forming one day later.
- Obtain two pairs of straight forceps with fine tips. Ensure that the tips are sharp, even in length, and straight (Figure 1A). Use these forceps only for dissections, and cap with 10 μL pipette tips when not in use.
- Prepare a scalpel tool.
- Insert the blunt end of a black anodized steel insect pin into a pin holder, and twist the screw of the holder to secure the pin in place. Using a pair of forceps and under a dissecting microscope, grasp the insect pin in the middle and bend it to form an internal angle of 135° (Figure 1B). The sharp end is for cutting tissue, while the edge is used for transferring testes between drops of media.
- Cap with a 1,000 μL pipette tip when not in use.
- Working in a tissue culture hood, prepare 5 mL aliquots of Schneider’s media and store at 4 °C until the time of use. When ready to begin dissections, warm one 5 mL media aliquot to room temperature. Then transfer the warmed dissection media to a 10 mL syringe and attach a 0.22 µm syringe filter.
2. Dissection of Testes from Larvae and Early Pupae
- Use the media filled filter syringe to expel three drops of media (50 µL each) at the top, middle and bottom of a glass slide.
- Use a dissecting probe to transfer five to ten third instar larvae or early pupae (pupae that are still white or yellow) to the top drop. Gently agitate the larvae and pupae in the media with the dissecting probe to remove any food or debris.
- Under a dissecting microscope, turn a single larva or pupa on its side with a dissecting probe to identify the two bilateral testes if present, which appear as oval shaped translucent structures in the posterior third of the body (Figure 2A, B). Animals that lack testes are female and should be discarded.
- When a male larva or pupa is found and is clean, move it to the second drop of media. Repeat until 3 or 4 male larvae and/or pupae have been transferred to the second drop of media.
- Working in the second drop of media, grasp a single male larva or pupa with a pair of forceps at its mid-region, just anterior to the testes. Use the second pair of forceps to gently tear the animal in half.
- Hold the posterior end of the larva or pupa down on the glass slide with a pair of forceps. Starting just next to the forceps, push down on the cuticle using the edge of the scalpel tool and move the scalpel toward the cut end of the animal. This will expel the animal’s internal organs, which include the guts, fat bodies, and testes.
- Gently tease apart the testes from the rest of the organs. The testes are clear, oval shaped organs embedded in ribbons of fat body (Figure 2C).
- Transfer the testes one at a time to the third drop of media. To accomplish this, insert the edge of the scalpel under the fat body, lift the tissue out of the media, and quickly move it to the third drop.
- Alternatively, if there is not enough fat body still attached to the testes to successfully lift the tissue, gently transfer the tissue using a glass Pasteur pipette that has been pre-wetted with dissection media.
- Use the sharp end of the scalpel tool to gently slice away excess fat body from the testes, leaving just a small rim of fat body around the edges (Figure 2C). It is not necessary to remove all of the fat body, and attempting to do so will likely result in damaging or rupturing of the testes.
- Repeat the above steps for all of the male larvae on the glass slide.
- Proceed either to step 3 or step 5.
3. Mounting Testes for Live Microscopic Imaging
NOTE: This mounting procedure was adapted from a recently published protocol for imaging of Drosophila larval brain neuroblasts20. Additional details can be found in this reference.
- Use the filter syringe to deposit a single drop of Schneider’s media (about 30 µL) onto the center of a 50 mm gas-permeable culture dish.
- Use a pre-wetted Pasteur pipette to transfer the prepared testes to the drop on the gas-permeable dish. The testes will generally float to the surface of the drop.
- Use the scalpel tool to gently push the testes down onto the gas-permeable membrane. Take care not to rupture the gas-permeable membrane with the sharp point of the scalpel. Push all of the testes to the center of the drop.
- Use a 1 mL syringe filled with halocarbon oil to make four drops of oil, about 30 μL each, on the gas permeable membrane. Space the drops of oil around the drop of media containing the testes to correspond to the four corners of a 22 mm glass coverslip.
- Line up the corners of a 22 mm glass coverslip with the four drops of halocarbon oil and gently lower the coverslip onto the media and oil. Allow the coverslip to settle and for the media containing the testes to spread between the drops of oil.
- Remove excess media to allow the coverslip to settle onto the surface of the testes. While observing the testes under a dissecting microscope, insert the corner of a delicate task wipe into the media under the coverslip to wick away a small amount of liquid. Wick away enough media until the glass coverslip just makes contact with the surface of the largest testis. It is critical not to remove too much media and lower the coverslip too far, as this will exert pressure on the testes and cause them to rupture (see Figure 5).
4. Live Imaging of Meiotic Spermatocytes
NOTE: Spermatocytes can be imaged using a laser scanning or spinning disk confocal microscope. The system must have adequate speed and sensitivity to avoid photobleaching of fluorescent proteins or photodamage of the tissue.
- Place a drop of immersion oil on the glass coverslip just above the testes. Flip over the dish and place it on the microscope stage and move the microscope objective (40x or 60x) into the oil until it is just below the testes. Use transmitted light to find a single testis and bring it into focus.
- While capturing fast images with the confocal, move the testis around to examine the organization of the cells. One end of the testis will have many small, densely packed cells. This end contains the germline stem cells and spermatogonia. Moving toward the other end of the organ, identify progressively larger cells organized into discrete clusters or cysts. These large cells are the spermatocytes (Figure 3A).
- If imaging GFP-tubulin, identify spermatocytes that will commence meiosis within 30–60 min by the presence of two bright microtubule asters (i.e., centrosomes) located at the cortex of the cell (Figure 3B, C).
- Once a cyst of meiotic spermatocytes has been identified, set up acquisition parameters to capture z-stacks of images over time. Typically, acquisition of 15–20 images spaced 1.5 μm apart in the z-dimension is sufficient to capture the full volume of several spermatocytes within the same cyst.
- Acquire full z-stacks every 2 min if imaging the entire first meiotic division, which takes about 1.5 hours. It is possible to use faster acquisition rates, particularly if only a specific event of meiosis is to be analyzed. However, take care not to cause photobleaching or photodamage due to repeated exposure of the sample to laser excitation.
- Use a continuous, automatic focus control system to prevent focal drift over the long time-course of acquisition. Temperature control of the sample on the microscope stage generally is not necessary, assuming room temperature of approximately 22–23 °C. If temperature control is available, maintain the sample at 25 °C on the microscope stage.
- If meiotic spermatocytes are not found, store the sample for several hours or overnight in a 25 °C incubator and image again.
5. Fixation and Immunostaining
- From step 2.10 above, use a glass Pasteur pipette to transfer testes to a well in a 9-well glass dissecting dish containing 0.5 mL of 8% paraformaldehyde in PBSTx (phosphate buffered saline with 0.3% Triton X-100). Ensure that the testes are laying on the bottom of the dish.
NOTE: Paraformaldehyde is toxic and should be handled with gloves in a fume hood. - Fix the testes for 20 min at room temperature.
- Transfer the testes to a well containing 0.5 mL PBSTx. Wash for 5 min with gentle agitation. Repeat the wash step in new wells with fresh PBSTx two more times.
- Dilute primary antibody to the desired concentration in 0.5 mL PBSTx containing 5% bovine serum albumin (BSA) in a 1.5 mL microcentrifuge tube. Transfer testes from the 9-well glass dissecting dish to the tube of diluted primary antibody and incubate overnight at 4 °C with gentle rocking or nutation.
- Wash the testes in 0.5 mL of PBSTx for 5 min in a well of a 9-well glass dissecting dish. Repeat the wash step with fresh PBSTx two more times.
- Dilute secondary antibody in 250 μL of PBSTx containing 5% BSA and dispense into a clean well of a 9-well glass dissecting dish. If desired, add DAPI to stain DNA. Transfer testes to the well containing secondary antibody, cover with plastic wrap, and incubate in the dark with gentle agitation for 4 hours at room temperature.
- Transfer the testes to a clean well filled with 0.5 mL of PBSTx. Wash with fresh PBSTx twice for 5 min and a third time for 30 min.
6. Mounting Fixed Testes
- Transfer the testes from the last wash onto a glass microscopy slide using a Pasteur pipette. If necessary, use the edge of the scalpel tool to push the testes down onto the surface of the slide.
- Use the corner of a delicate task wipe to wick away excess liquid from the testes. Remove as much liquid as possible.
- Apply a 30–50 µL drop of microscopy mounting medium to the testes.
- Place a 22 mm coverslip onto the drop of mounting medium and allow the coverslip to settle and the mounting medium to spread under the coverslip. Apply gentle pressure to the coverslip if necessary to squeeze out excess mounting medium and allow the coverslip to rest on the surface of the testes.
- Use nail polish to seal the edges of the coverslip.
Representative Results
When this protocol is successfully executed, testes will remain fully intact for imaging by confocal microscopy or other fluorescence microscopy methods. As seen in Figure 3A, the cellular organization of the testes is preserved, and the progression of cell differentiation from one end of the testis to the other including spermatogonia, spermatocytes, and haploid spermatids is visible. GFP-tubulin is a useful marker for identifying dividing spermatocytes and for live imaging of the progression of the cells through meiosis. As shown in Figure 3B, spermatocytes about the begin meiosis can be identified by their two prominent microtubule asters. By metaphase the large meiotic spindles are beautifully labeled by GFP-tubulin (Figure 3C).
Drosophila spermatocytes’ large size and their relative slow progression through meiosis make them ideal for analysis of the dynamics and reorganization of cellular components such as cytoplasmic organelles during cell division8,11,21. Figure 4 and Video 1 show live imaging analysis of the dynamic reorganization of the endoplasmic reticulum (ER) with respect to microtubules through the course of meiosis. As described above, during late interphase just prior to the onset of meiosis, two centrosomes located at the cortex of the cell are clearly visible by GFP-tubulin fluorescence. At this stage, ER-targeted RFP (RFP-KDEL) fluorescence reveals that the ER is evenly distributed throughout the cytoplasm. The centrosomes then begin migrating to opposite sides of the nuclear envelope to establish the two spindle poles. During this time, the ER rapidly accumulates around the centrosomal microtubules and is progressively cleared from the rest of the cytoplasm. Nuclear envelope breakdown (NEB) is revealed by the appearance of GFP-tubulin fluorescence within the nuclear region. It should be noted that Drosophila cells divide by semi-open mitosis or meiosis, meaning that nuclear envelope components break down and disperse and large cytoplasmic molecules enter the nuclear region, but an envelope of ER-derived membranes surrounds the spindle and chromatids throughout cell division22,23,24. By the time of NEB and progressing through metaphase, the ER is nearly completely associated with the astral microtubules that surround the two spindle pole centrosomes. These two astral microtubule accumulations of the ER then partition to the two daughter cells during anaphase, ensuring proper ER inheritance by the newly formed cells8,11.
Figure 5 shows the effects of testis rupture on live imaging of spermatocytes. As described in the protocol, great care must be taken not to lower the coverslip too far onto the surface of the testes during the mounting steps, as this will apply pressure to the testes and cause them to burst. As seen in Figure 5 and Video 2, cysts of spermatocytes rapidly flow out of the ruptured testis, and this movement of the cells makes live, time-lapse imaging extremely difficult.
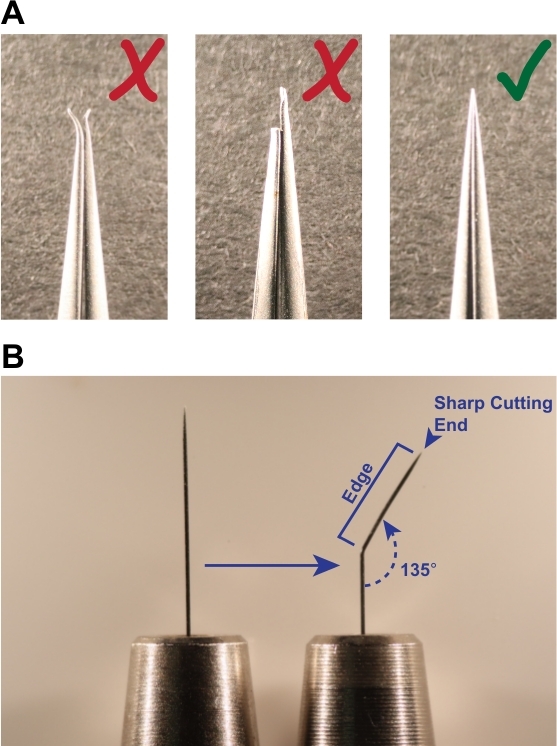
Figure 1: Tools required for successful dissection. (A) Forceps used for dissection must be straight and have sharp, unbroken tips (indicated by green check mark). Do not use forceps with bent or broken tips (red X marks), as they will be ineffective at grasping larvae and teasing apart tissues. (B) To prepare the scalpel tool, bend a black anodized steel insect pin to an internal angle of approximately 135°. The sharp point is used as a knife to cut through tissue, and the straight edge is used for moving tissues. Please click here to view a larger version of this figure.
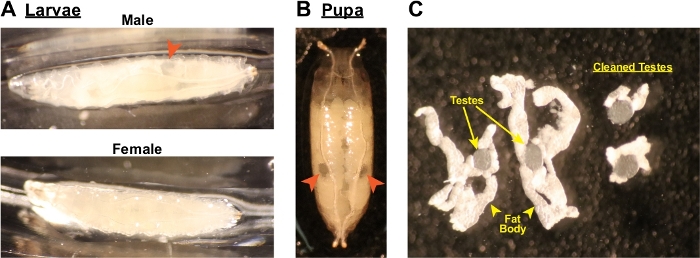
Figure 2: Identification of larval and pupal testes. (A) Images of male and female larvae. The testes are identifiable in the male as the circular or oval translucent structures in the posterior third of the animal (arrowhead). Note the lack of a similar structure in the female. (B) Image of an early male pupa, with the two translucent testes indicated by arrowheads. (C) Testes with attached fat bodies after dissection. The two testes on the left have excessive fat bodies still attached that need to be trimmed away. The two testes on the right have been properly trimmed of their fat bodies and are ready for imaging. Please click here to view a larger version of this figure.
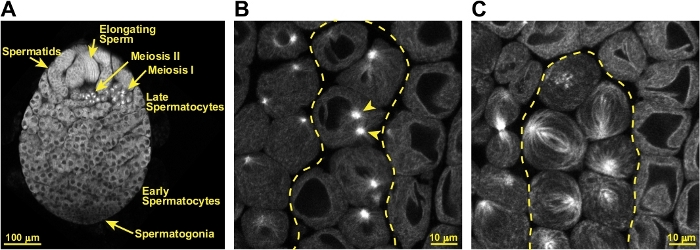
Figure 3: Identification of meiotic spermatocytes in a successfully prepared testis. (A) Confocal image of a successfully prepared live, intact testis expressing GFP-tubulin. The hub of stem cells is located toward the lower tip of the testis, though it is not identifiable in this preparation. Several layers of small spermatogonia are indicated, and these are followed by numerous cysts of spermatocytes. A cyst of spermatocytes in the first meiotic division is identifiable based on the large size of the cells (approximately 20 – 30 µm in diameter) and presence of GFP-tubulin labeled meiotic spindles. Spermatocytes in the second meiotic division similarly have spindles, but these cells are approximately half the size meiosis I cells. Post-meiotic spermatids are also visible, as are elongating spermatozoa. (B) Spermatocytes that will begin meiosis within 30 – 60 min can be identified by their two large, very bright microtubule asters (indicated by arrow heads) labeled by GFP-tubulin. The cyst of pre-meiotic cells is delineated by the dashed yellow line. (C) Cells in meiosis are identifiable by their large meiotic spindles that are easily visualized with GFP-tubulin. The cyst of meiotic cells is delineated by the dashed yellow line. Please click here to view a larger version of this figure.
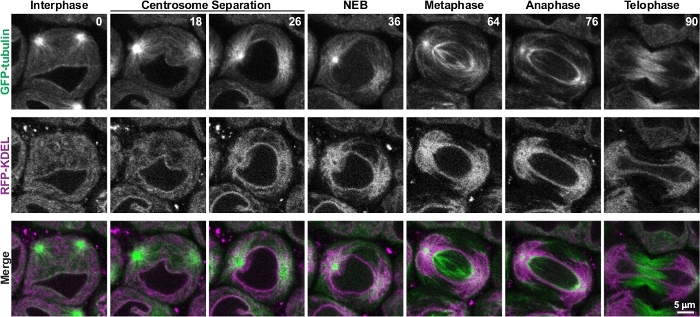
Figure 4: Time-lapse imaging of meiosis in a single spermatocyte. A single spermatocyte co-expressing GFP-tubulin and RFP targeted to the ER (RFP-KDEL) was imaged every two minutes through the course of meiosis. Shown are representative images of the spermatocyte at specific stages of meiosis, beginning in late interphase and progressing through telophase. Times are in minutes. Each image is a single optical plane from a stack of fifteen optical slices acquired at each time point. This figure was modified from Karabasheva and Smyth, 20198. Please click here to view a larger version of this figure.
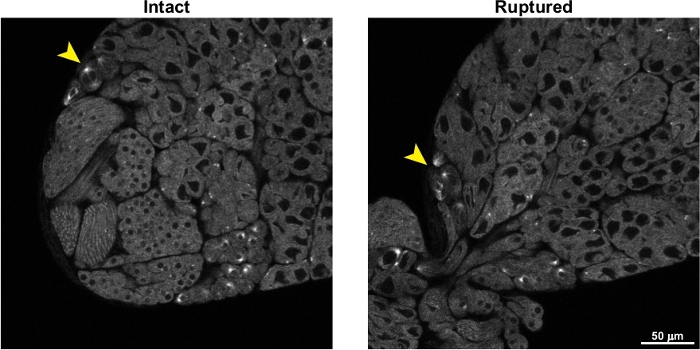
Figure 5: Representative images of a testis before and after rupturing. The image on the left, labeled “Intact”, shows a GFP-tubulin expressing testis just before rupturing. The image on the right shows the same testis after rupturing. Cysts of spermatocytes can be seen streaming out of the ruptured testis. The arrowheads indicate a cyst of meiotic spermatocytes; note the significant movement of these spermatocytes after the testis ruptures, making imaging these spermatocytes over time extremely challenging. Please click here to view a larger version of this figure.
Video 1: Full meiotic division of a single spermatocyte. Shown is the full time-lapse of the GFP-tubulin and RFP-KDEL expressing spermatocyte in Figure 4. Images were captured every two minutes, and the playback rate is three frames per second. This video was modified from Karabasheva and Smyth, 20198. Please click here to download this video.
Video 2: Ruptured testis. Time-lapse of a testis before and after rupturing. The rupture is evident due to the sudden streaming of cysts of cells through the lower left corner of the testis. Images were captured every two minutes, and the playback rate is three frames per second. Please click here to download this video.
Discussion
We have described a protocol for the preparation of larval or early pupal Drosophila testes, optimized for long-term, live imaging of spermatocyte cell division. This is a powerful method for analysis of cell division in the physiological context of intact tissue. The power of this method is further expanded when combined with Drosophila genetic tools, such as specific gene mutations, tissue-specific RNAi-mediated suppression, and fluorescently labeled protein and organelle markers. In addition, the small size of larval and pupal testes, and the ability to image dividing cells very close to the glass coverslip, make the method ideal for high resolution imaging including super-resolution approaches. The versatility of this experimental system is demonstrated by the representative results describing the dynamic reorganization of the ER during cell division. Drosophila spermatocytes are particularly useful for such analysis of organelle dynamics during cell division due to these cells’ large size and relatively slow transit through meiosis. Further, the same cells can be imaged post-meiotically to understand how changes in organelle dynamics or cell division mechanisms affect subsequent events of spermatogenesis. Thus, there is great potential with this experimental approach to understand the physiology of cell division within the larger context of tissue development and cell differentiation, outcomes that are unattainable when studying cells grown in culture.
The protocol includes several elements that facilitate long-term ex vivo culture of testes for up to about 24 hours. First, Schneider’s media, which was developed for maintenance of Drosophila tissue culture cells, contains readily available energy sources and is optimized to maintain physiological pH in atmospheric air. Second, the gas-permeable membrane used for mounting testes allows for rapid gas exchange. An important advantage of long-term culture is that if cells at the desired stage of development or meiosis are not found immediately after sample preparation, the sample can be stored and imaged again at a later time. We have verified that testes maintained in a 25 °C incubator overnight, for a total of 16-24 hours, are viable and appear healthy based on the routine presence of cells actively undergoing meiosis and progressing through later stages of sperm differentiation. However, precautions should be taken when culturing testes for longer than 2-3 hours to ensure that tissue physiology is not adversely affected. Most importantly, testes continue to grow in culture as cells proliferate and maturing sperm elongate. This may cause the testes to enlarge to the point that they burst due to the restrictive space between the membrane and coverslip, rendering the testes unsuitable for live imaging for reasons described below. In addition, if long-term cultures are to be routinely employed in experiments, it is advisable to test whether lab-specific extended culture conditions result in meiotic abnormalities such as prolonged meiotic durations or increased frequencies of lagging chromosomes or cytokinesis failure.
In order to image individual cells over long time-courses, it is important that cells do not move significantly once the prepared testes are on the microscope stage. Thus, it is essential when executing the protocol that the testes remain intact and undamaged, because cells spill out of damaged testes and all the cells within the organ move as a result (see Figure 5 and Video 2). It may still be possible to image spermatocytes in damaged testes if the cells eventually settle and stop moving, though by the time this occurs the stages of meiosis that one intends to analyze may have already occurred. In addition, effects of tissue damage on the cell division process itself cannot be discounted. Damage to the testes can occur when clearing away fat bodies following removal of the testes from larvae. Great care must therefore be taken to avoid piercing or tugging on the organs during this step of the protocol. In fact, it is often not necessary to remove very much of the fat bodies, so long as they do not obscure visualization of the testis. More commonly, testis damage is the result of removing too much culture medium following placement of the coverslip during the mounting procedure for live imaging (step 3.6). As medium is removed, the coverslip falls lower and exerts pressure on the testes, and excess pressure will cause the testes to rupture. Thus, while it is important for the coverslip to be as close as possible to the testes to facilitate optimal imaging of the spermatocytes, some practice is necessary to avoid removing too much medium and lowering the coverslip too far. It is also helpful to point out that for some applications, such as acute exposure of spermatocytes to drugs or other small molecules, it can be advantageous to disrupt the testes and disperse the cysts of spermatocytes for analysis. Several excellent protocols are available for the preparation and culture of dispersed spermatocyte cysts18,25.
An important consideration when using spermatocytes for cell division analysis is that these cells execute meiotic as opposed to mitotic divisions. Importantly, many of the essential aspects of cell division mechanics, such as spindle architecture and cytokinesis, are similar between male meiosis and mitosis6. Thus, mechanistic insights gathered from studying spermatocyte meiosis can be broadly applicable to general mechanisms of animal cell division7. However, depending on the mechanistic questions being addressed, important differences between spermatocytes and mitotic cells in processes such as chromosomal dynamics and cell cycle regulation may need to be considered26. At the same time, Drosophila spermatocytes present the unique opportunity to compare mitotic and meiotic cells within the same tissue and germline lineage. For example, the same testis preparation can be used to analyze germline stem cells or spermatogonia undergoing mitosis and spermatocytes undergoing meiosis. We typically do not attempt to image germline stem cell or spermatogonial mitoses in live testis preparations because the timing of these divisions is difficult to predict. However, we have fortuitously captured these divisions in our experiments, demonstrating that the general protocol can be applied to analysis of mitotic cells within testes as well.
The methodology presented focuses on testis preparation for live imaging of spermatocyte meiosis, as this allows for real-time analysis of cellular and molecular dynamics. We also provide a method for fixation and immunostaining of intact testes, as this approach may be preferable if required fluorescently labeled expression constructs are not available or to avoid confounding effects of protein overexpression. An important consideration though is that fixation does not always faithfully preserve native tissue and cellular architecture. For example, we and others have found that fixation often results in fragmentation or disruption of the ER11,27. Some of these problems can be alleviated by using different fixatives or tissue preparation methods, and some troubleshooting may therefore be necessary to identify the optimal fixation protocol for preservation of a particular cell component or protein organization. Fortunately, a number of additional protocols for Drosophila spermatocyte fixation are also available18,28,29.
In conclusion, we have described versatile methods for the preparation of Drosophila testes for live and fixed cell analysis of spermatocyte meiosis. Specific strengths of this experimental approach include analysis of cell division in intact tissue, high resolution imaging of large cells, and integration with Drosophila genetic tools. Experiments employing the methodology have the potential to make important contributions to our understanding of how cell division integrates with complex mechanisms of tissue development and homeostasis.
Divulgaciones
The authors have nothing to disclose.
Acknowledgements
This work was supported by Department of Defense start-up funds to J.T.S.
Materials
| 5" Dissecting Probe | Fisher | 08-965-A | |
| 5.75" Glass Pasteur Pipet | Fisher | 13-678-20A | |
| Bovine Serum Albumin (BSA) | Fisher | BP9703-100 | |
| Dumont #5 Forceps | Fine Science Tools | 11252-20 | Straight forcepts with fine tips |
| Frosted Microscope Slides | Fisher | 12-544-2 | Slides for mounting fixed tissue, with frosted writing surface |
| Halocarbon oil 700 | Sigma | H8898-100 mL | |
| Lumox Dish 50 | Sarstedt | SAR946077410 | Gas-permeable tissue culture dish |
| Microscope Cover Glass | Fisher | 12-541-B | 22×22 mm, #1.5 glass coverslip |
| Minutien Pins | Fine Science Tools | 26002-15 | Insect pins used to make scalpel tool |
| Nickel Plated Pin Holder | Fine Science Tools | 26018-17 | |
| Paraformaldehyde 32% Solution, EM Grade | Electron Microsocopy Sciences | 15714 | |
| Plan Beveled Edge Microscope Slides | Fisher | 12-549-5 | Slides used for dissections |
| PYREX Spot Plates | Fisher | 13-748B | 9-well glass dissecting dish |
| Schneider's Drosophila medium | Fisher | 21720-024 | |
| Syringe Filter, 0.22 µm | EMD Millipore | SLGS033SB | |
| Triton X-100 | Fisher | BP151-500 | |
| Vectashield | Vector Labs | H-1000 | Microscopy mounting medium |
Referencias
- Khodjakov, A., Rieder, C. L. Imaging the division process in living tissue culture cells. Methods. 38 (1), 2-16 (2006).
- Ong, J. Y., Torres, J. Z. Dissecting the mechanisms of cell division. The Journal of Biological Chemistry. 294 (30), 11382-11390 (2019).
- Levine, E. M. Cell cycling through development. Development. 131 (10), 2241-2246 (2004).
- Gross, S. M., Rotwein, P. Unraveling Growth Factor Signaling and Cell Cycle Progression in Individual Fibroblasts. The Journal of Biological Chemistry. 291 (28), 14628-14638 (2016).
- Mittelman, D., Wilson, J. H. The fractured genome of HeLa cells. Genome Biology. 14 (4), 111 (2013).
- Bury, L., Coelho, P. A., Glover, D. M. From Meiosis to Mitosis: The Astonishing Flexibility of Cell Division Mechanisms in Early Mammalian Development. Current Topics in Developmental Biology. 120, 125-171 (2016).
- Giansanti, M. G., Fuller, M. T. What Drosophila spermatocytes tell us about the mechanisms underlying cytokinesis. Cytoskeleton. 69 (11), 869-881 (2012).
- Karabasheva, D., Smyth, J. T. A novel, dynein-independent mechanism focuses the endoplasmic reticulum around spindle poles in dividing Drosophila spermatocytes. Scientific Reports. 9 (1), 12456 (2019).
- Polevoy, G., et al. Dual roles for the Drosophila PI 4-kinase four wheel drive in localizing Rab11 during cytokinesis. Journal of Cell Biology. 187 (6), 847-858 (2009).
- Rebollo, E., Llamazares, S., Reina, J., Gonzalez, C. Contribution of noncentrosomal microtubules to spindle assembly in Drosophila spermatocytes. PLoS Biology. 2 (1), 8 (2004).
- Smyth, J. T., Schoborg, T. A., Bergman, Z. J., Riggs, B., Rusan, N. M. Proper symmetric and asymmetric endoplasmic reticulum partitioning requires astral microtubules. Open Biology. 5 (8), (2015).
- Demarco, R. S., Eikenes, &. #. 1. 9. 7. ;. H., Haglund, K., Jones, D. L. Investigating spermatogenesis in Drosophila melanogaster. Methods. 68 (1), 218-227 (2014).
- Fuller, M. T., Bate, M., Martinez-Arias, A. . The Development of Drosophila melanogaster. , 71-147 (1993).
- Dunleavy, E. M., et al. The Cell Cycle Timing of Centromeric Chromatin Assembly in Drosophila Meiosis Is Distinct from Mitosis Yet Requires CAL1 and CENP-C. PLOS Biology. 10 (12), 1001460 (2012).
- Fabian, L., Brill, J. A. Drosophila spermiogenesis: Big things come from little packages. Spermatogenesis. 2 (3), 197-212 (2012).
- Ramm, S. A., Scharer, L., Ehmcke, J., Wistuba, J. Sperm competition and the evolution of spermatogenesis. Molecular Human Reproduction. 20 (12), 1169-1179 (2014).
- Savoian, M. S. Microscopy Methods for Analysis of Spindle Dynamics in Meiotic Drosophila Spermatocytes. Methods in Molecular Biology. 1471, 265-276 (2017).
- Sitaram, P., Hainline, S. G., Lee, L. A. Cytological analysis of spermatogenesis: live and fixed preparations of Drosophila testes. Journal of Visualized Experiments. (83), e51058 (2014).
- Zamore, P. D., Ma, S. Isolation of Drosophila melanogaster testes. Journal of Visualized Experiments. (51), (2011).
- Lerit, D. A., Plevock, K. M., Rusan, N. M. Live imaging of Drosophila larval neuroblasts. Journal of Visualized Experiments. (89), (2014).
- Belloni, G., et al. Mutations in Cog7 affect Golgi structure, meiotic cytokinesis and sperm development during Drosophila spermatogenesis. Journal of Cell Science. 125 (22), 5441-5452 (2012).
- Harel, A., et al. Persistence of major nuclear envelope antigens in an envelope-like structure during mitosis in Drosophila melanogaster embryos. Journal of Cell Science. 94 (3), 463-470 (1989).
- Katsani, K. R., Karess, R. E., Dostatni, N., Doye, V. In vivo dynamics of Drosophila nuclear envelope components. Mol Biol Cell. 19 (9), 3652-3666 (2008).
- Tates, A. D. . Cytodifferentiation during spermatogenesis in Drosophila melanogaster: An electron microscope study. , (1971).
- Gartner, S. M., Rathke, C., Renkawitz-Pohl, R., Awe, S. Ex vivo culture of Drosophila pupal testis and single male germ-line cysts: dissection, imaging, and pharmacological treatment. Journal of Visualized Experiments. (91), 51868 (2014).
- Ohkura, H. Meiosis: an overview of key differences from mitosis. Cold Spring Harbor perspectives in biology. 7 (5), (2015).
- Friedman, J. R., Webster, B. M., Mastronarde, D. N., Verhey, K. J., Voeltz, G. K. ER sliding dynamics and ER-mitochondrial contacts occur on acetylated microtubules. Journal of Cell Biology. 190 (3), 363-375 (2010).
- Bonaccorsi, S., Giansanti, M. G., Cenci, G., Gatti, M. Methanol-acetone fixation of Drosophila testes. Cold Spring Harbor Protocols. 2011 (10), 1270-1272 (2011).
- Bonaccorsi, S., Giansanti, M. G., Cenci, G., Gatti, M. Paraformaldehyde fixation of Drosophila testes. Cold Spring Harbor Protocols. 2012 (1), 102-104 (2012).

