Alignment of Visible-Light Optical Coherence Tomography Fibergrams with Confocal Images of the Same Mouse Retina
Summary
The present protocol outlines the steps for aligning in vivo visible-light optical coherence tomography fibergraphy (vis-OCTF) images with ex vivo confocal images of the same mouse retina for the purpose of verifying the observed retinal ganglion cell axon bundle morphology in the in vivo images.
Abstract
In recent years, in vivo retinal imaging, which provides non-invasive, real-time, and longitudinal information about biological systems and processes, has been increasingly applied to obtain an objective assessment of neural damage in eye diseases. Ex vivo confocal imaging of the same retina is often necessary to validate the in vivo findings especially in animal research. In this study, we demonstrated a method for aligning an ex vivo confocal image of the mouse retina with its in vivo images. A new clinical-ready imaging technology called visible light optical coherence tomography fibergraphy (vis-OCTF) was applied to acquire in vivo images of the mouse retina. We then performed the confocal imaging of the same retina as the "gold standard" to validate the in vivo vis-OCTF images. This study not only enables further investigation of the molecular and cellular mechanisms but also establishes a foundation for a sensitive and objective evaluation of neural damage in vivo.
Introduction
Retinal ganglion cells (RGCs) play a critical role in visual information processing, receiving synaptic inputs through their dendritic trees in the inner plexiform layer (IPL) and transmitting the information via their axons in the retinal nerve fiber layer (RNFL) to the brain1,2,3,4. In diseased conditions such as glaucoma, early RGC degeneration may result in subtle changes in the RNFL, the ganglion cell layer (GCL), the IPL, and the optic nerve in both patients and rodent models5,6,7,8,9. Early detection of these morphological changes in RGCs is thus essential for timely intervention to prevent RGC and vision loss.
We have recently developed a new clinical-ready imaging technology called visible-light optical coherence tomography (vis-OCT) to satisfy the need for in vivo monitoring of RGC damage. Vis-OCT improved the axial resolution, reaching 1.3 µm in the retina10, 11, allowing for the visualization of individual RGC axon bundles in the RNFL. Subsequently, vis-OCT fibergraphy (vis-OCTF) was established to track and quantify RGC damage at the single axon bundle level in mice11,12,13. However, ex vivo confocal imaging of the same retina as the gold standard is often necessary to validate the in vivo findings. Therefore, this study will demonstrate how to align in vivo images acquired by vis-OCTF with ex vivo confocal images of the same mouse retina. The protocol aims to validate the in vivo findings by ex vivo confocal imaging and establish a foundation for examining the molecular and cellular changes underlying RGC damage in diseased conditions.
Protocol
All animal procedures were approved by the Institutional Animal Care and Use Committee at the University of Virginia and conformed to the guideline on Use of Animals from the National Institute of Health (NIH). See the Table of Materials for details related to all materials, reagents, and instruments used in this protocol.
1. In vivo vis-OCT imaging
- The vis-OCT system
- Image the mice eyes using a small animal vis-OCT system that uses a supercontinuum light source, which delivers visible-light illumination between 480 nm and 650 nm. Ensure that the power incident on the cornea is 1 mW and use an A-line rate of 25 kHz and an integration time of 39.3 µs per A-line.
- Ensure that the spectral detection range of the spectrometer is between 508 nm and 613 nm, which provides an axial resolution of 1.3 µm in the retina. The total imaging volume is approximately 700 µm (x) x 700 µm (y) x 1,500 µm (z). The lateral resolution is between 4.5 µm at the center of the field of view and 8.7 µm at 350 µm from the center11,13.
- Mouse anesthesia
- Anesthetize mice of C57BL/6 background and either sex with an intraperitoneal injection of Ketamine (114 mg/kg) and Xylazine (17 mg/kg) cocktail and dilate their pupils using 1% tropicamide drops. Confirm adequate anesthetization by loss of pedal reflex after a firm toe pinch.
- During imaging, keep the mouse warm by using an infrared heat lamp. After each image acquisition, apply artificial tears to prevent corneal dehydration.
- Positioning of the mouse for imaging
- Place the anesthetized mouse on the animal holder and keep the mouse in position with the help of two Velcro straps.
NOTE: The animal holder allows movement in three dimensions (vertical adjustment, fine horizontal adjustment, as well as pitch and yaw adjustments) to place the laser into the mouse's eye.
- Place the anesthetized mouse on the animal holder and keep the mouse in position with the help of two Velcro straps.
- Adjusting imaging parameters
- Turn on the computer and open the referenced software, which will turn on the laser automatically.
- Adjust the animal holder until the laser is stable and centered into the mouse's eye. Visualize the posterior part of the eye through an En Face preview in the software interface, the field of view (FOV) of the superficial vascular plexus, and a B-scan, the cross-section of the retina within the FOV.
- Acquire a vis-OCT volume by clicking on the Acquire button on the software interface after performing minor adjustments of the optical focus, which consists of 512 A-lines/B-scan and 512 B-scans/volume.
NOTE: This process takes ~10.5 s. Image acquisition is guided by a built-in quality index (QI) estimator to ensure that images below a predetermined threshold (QI < 45) are not included.- For each mouse, acquire four vis-OCT volumes from the same eye. Align the optic nerve head (ONH) in each of the four corners in the FOV to cover different areas of the retina.
NOTE: Such placement minimizes the retinal curvature, which maximizes RNFL reflectance throughout the FOV. It requires ~1 min to reposition the eye between each acquisition (Figure 1A,B).
- For each mouse, acquire four vis-OCT volumes from the same eye. Align the optic nerve head (ONH) in each of the four corners in the FOV to cover different areas of the retina.
- Vis-OCTF analysis
NOTE: MATLAB is used to perform image analysis.- To generate vis-OCT fibergrams from the vis-OCT volume, use an intensity-based threshold method (MATLAB code line 808) to detect the surface of the retina.
NOTE: These lines use the imadjust function to adjust the intensity values of the bscan. The [0.0087 0.08] argument passed to imadjust specifies the range of intensities to map to the full dynamic range of the output image. - Crop the RNFL by selecting the first ~16 µm in depth. See Matlab code line 782.
NOTE: The typical in vivo RNFL thickness in an adult wild-type C57BL/6 mouse is ~14 µm and can vary among different segmentation methods13. - Change the paths of the RAW file (line 9), Files for reconstruction (line 11), and file name (line 15), click Run, and wait for the OCT image to be analyzed by the MATLAB code. Calculate the mean intensity projection along the axial (z) direction (MATLAB code lines 905-908) to generate the fibergram image composed of RGC axon bundles and surrounding vasculature. Montage the four images after fibergram processing for each FOV by aligning the blood vessels with a graphics editor of choice, covering ~1.2 x 1.2 mm in total. The RAW files are usually saved in the folder: Halo Data, under the date of OCT imaging (e.g., 0606 Opticent).
NOTE: The MATLAB codes are available in Supplementary File 1.
- To generate vis-OCT fibergrams from the vis-OCT volume, use an intensity-based threshold method (MATLAB code line 808) to detect the surface of the retina.
2. Ex vivo confocal imaging
- Mouse euthanasia
- After acquiring vis-OCT data, euthanize the mice with a mixture of pentobarbital sodium (390 mg/mL) and phenytoin sodium (50mg/mL). Phenytoin sodium functions by amplifying the effects of pentobarbital sodium, which has been widely applied in rodent euthanasia14,15. For each mouse, use 0.2 mL/20 g of the diluted mixture (156 mg/mL) and perfuse with 20 mL of phosphate-buffered saline (PBS) and then, 20 mL of 4% paraformaldehyde (PFA) in PBS16,17.
- Eye dissection and orientation
- Enucleate the eyes and make a mark on the temporal side to indicate the orientation.
- After carefully removing the anterior chamber, the ocular lens, vitreous, and fix the eyecups in PFA for 30 min.
- Wash the eyecups with PBS for 30 min, and replace the PBS solution 3x during the washing. Then, wash with 0.5% Triton X-100 in PBS (pH 7.5) for 30 min.
- Incubate the eyecups in blocking buffer (5% donkey serum with 2.5% bovine serum albumin and 0.5% Triton X-100 in Tris-buffered saline [pH 7.5]) for 2 h at room temperature.
- Immunostaining
NOTE: The eyecups are now ready to be stained with the primary antibodies of mouse anti-Tuj1 to detect RGC axon bundles and rat anti-ICAM-2 to detect blood vessels.- Incubate the eye cups overnight with the primary antibodies, mouse anti-Tuj1 (1:200 in blocking buffer) and rat anti-ICAM-2 (1:500 in blocking buffer), at 4 °C.
- Wash the eyecups 3-5x for 1 h each time, with a phosphate-buffered saline solution containing 0.5% Triton X-100 (PBST) to minimize background and remove any unbound antibody.
- After washing, incubate the eye cups overnight with the secondary antibodies, donkey anti-mouse Immunoglobulin G conjugated to Alexa Fluor 488 dye (green fluorescence) and donkey anti-rat IgG conjugated to Alexa Fluor 594 dye (red fluorescence), all diluted 1:1000 in blocking buffer, at 4 °C.
- The following day, wash the eyecups 3-5x for 1 h each with PBST to minimize background and remove unbound antibody.
- Transfer the eyecups to a Petri dish of PBS before the flat mount.
- Flat-mounted retina
- After the immunostaining process, isolate the retinas from the eyecups under the microscope.
- Cut the retinas into four leaves and flat mount them with the RGC layer facing up. Leave the mark on the temporal side attached to the retina to indicate orientation.
- Coverslip the retinas with mounting medium13,18. Seal the slides with nail polish13,18,19.
- Confocal imaging
NOTE: The image processing of confocal images was performed using ZEN Microscopy Software.- Turn on the confocal microscope, and under Locate mode, find the area of interest using the eyepiece of the microscope.
- Under acquisition mode, set up tiles to cover the whole retina and z-stack slices to cover all layers of information. Image a least 25 tiles across the whole retina to cover the total volume of 5.99 mm (x) x 5.88 mm (y) x 30 µm (z) at a pixel size of 1.24 µm/pixel.
- Project the Z-stack slices to create two-dimensional en face confocal microscopy images (Figure 1C,D)11,13,19,20,21.
3. Alignment of in vivo and ex vivo images
- After processing the fibergram, create a composite image that includes the four images acquired from each mouse by aligning all the blood vessels with a graphics editor of choice.
NOTE: On average, the final composite image is approximately 1.2 mm (x) x 1.2 mm (y), as shown in Figure 1B. - Use the optic nerve head (ONH) and the pattern of blood vessels as landmarks to align the composite fibergram. Obtain the in vivo and ex vivo alignment by overlapping the blood vessel pattern of the composite OCT images with the confocal images of the same retina stained with ICAM-2.
Representative Results
The composite vis-OCT fibergram is compared with the corresponding confocal image of flat-mounted retina immunostained with Tuj-1 for RGC axons (Figure 1D, top panel). Axon bundles imaged by vis-OCTF can be matched with the Tu-j1-labeled axon bundles on the confocal image. Blood vessels usually exhibit distinguishable branching structures compared with surrounding axon bundles in fibergram images, which can be matched with the ICAM-2-labeled blood vessels on the confocal image (Figure 1D, bottom panel).
Side-by-side comparison between ex vivo confocal microscopy and in vivo vis-OCT revealed identical RGC axon bundle networks and surrounding retinal vasculature. Note that the confocal image may not match perfectly with the in vivo images, especially in the peripheral region; this is because the retina has been flat-mounted on the slide. Taken together, these results validate the capability of vis-OCT fibergraphy to resolve two adjacent RGC axon bundles with varying sizes in vivo.
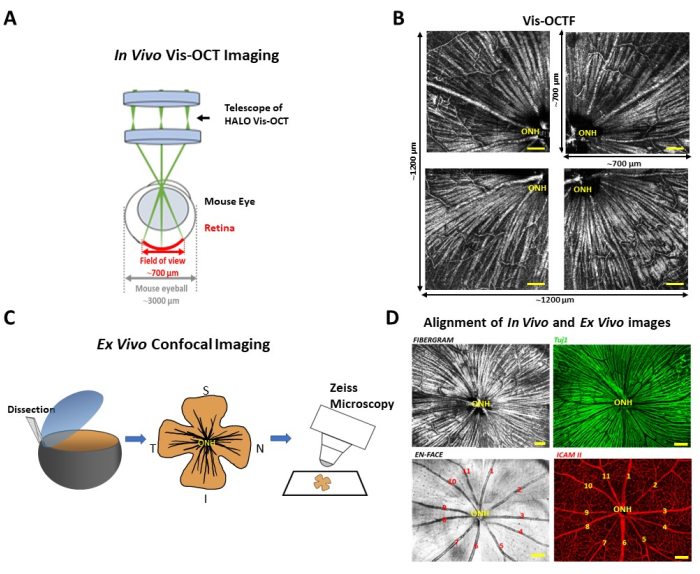
Figure 1: Schematic illustration of the alignment of in vivo image with the ex vivo image of the same mouse retina. (A) Schematic of the small-animal vis-OCT system imaging of a mouse eye; (B) Alignment of the four images acquired from the same eye with the optic nerve head placed in the four corners of the field of view; (C) Schematic representation of retina dissection (left), retina flat-mounted with tracked orientation (middle), and retina being imaged by confocal microscopy (right); (D) Comparing vis-OCT fibergraphy and confocal microscopy RGC axon bundle images (top panel), and in vivo En-Face with ex vivo confocal microscopy image of the immunostained for blood vessels (bottom panel). The four images are from the same eye. Yellow scale bars = 100 µm. Numbers (1-11) in panel D each represent blood vessels. Abbreviations: ONH = optic nerve head; vis-OCTF = visible-light optical coherence tomography fibergraphy; RGC = retinal ganglion cell; S = superior; I = inferior; T = temporal; N = nasal. Please click here to view a larger version of this figure.
Supplementary File 1: MATLAB codes for image analysis. Please click here to download this File.
Discussion
There are two steps in this protocol that require attention. First, it is necessary to ensure that the animal is under deep anesthesia and that their eyes are fully dilated before vis-OCT imaging. If the mice are not adequately anesthetized, their fast breathing may lead to unstable movements of the en face images, which can adversely affect the quality of the fibergram. Moreover, insufficient dilation can also have a negative impact on image quality since the iris may obstruct the light, preventing it from reaching the retina. Second, it is important to mark the left or right eye, as well as the temporal side of the eye, after perfusion but before removing the eyeball from the mouse's eye socket. Since the flat-mounted retina faces upwards with the RNFL on the superficial layer, marking the temporal side will enable proper orientation of the flat-mounted retina.
One of the advantages is that the protocol can be applied to different mouse models of eye diseases, such as retinal ischemia and diabetic retinopathy, as long as the anterior eyes are clear for optical imaging. However, one limitation of this method is that even if the retina is properly fixed for immunostaining and confocal imaging, the axon bundle morphology may change. This occurs due to mis-operations during retina dissection, which may cause ruptures in the axon bundles. Additionally, while retinas are curved bowl-shaped structures when imaged in vivo, they are flattened on slides for confocal imaging. As a result, there may be incomplete overlap between in vivo vis-OCT images and ex vivo confocal images of the peripheral retina.
For troubleshooting: this technique mainly includes two parts. First, for the vis-OCT part, the quality of the mouse eye can greatly impact the success of acquiring clear fibergrams. Therefore, artificial tears were constantly applied to the mouse's eye to keep it moist and bright. The body position of the mouse was also fine-tuned to make the laser shine as perpendicularly onto the retina as possible. These measures together ensured image quality. Second, for the retina dissection part, we found that cutting off the sclera surrounding the retina, rather than ripping it off, was crucial to maintaining the integrity of the ONH structure. When the sclera was ripped off with forceps, the ONH appeared as a fair-sized dark hole under confocal microscopy, with the retina tissue missing from the center. Maintaining a complete ONH structure is essential for in vivo and ex vivo alignments.
In summary, we have established vis-OCTF to directly quantify and track changes at the single axon bundle level in vivo11,12,13. This protocol provides instructions for aligning the in vivo vis-OCTF and ex vivo confocal imaging of the same retinas. These studies lay the foundation for establishing an objective evaluation of neural damage in humans, which can significantly improve glaucoma diagnosis and treatment in the future.
Divulgaciones
The authors have nothing to disclose.
Acknowledgements
This study is supported by the Glaucoma Research Foundation Shaffer Grant, 4-CA Cavalier Collaborative Award, R01EY029121, R01EY035088, and Knights Templar Eye Foundation.
Materials
| Equipment | |||
| Halo 100 | Opticent Health, Evanston, IL | ||
| Zeiss LSM800 microscope | Carl Zeiss | ||
| Drugs and antibodies | |||
| 4% paraformaldehyde (PFA) | Santz Cruz Biotechnology, SC-281692 | 1-2 drops | |
| Bovine serum albumin powder | Fisher Scientific, BP9706-100 | 1:10 | |
| Donkey anti Mouse Alexa Fluor 488 dye | Thermo Fisher Scientific, Cat# A-21202 | 1:1,000 | |
| Donkey anti rat Alexa Fluor 594 dye | Thermo Fisher Scientific, Cat# A-21209 | 1:1,000 | |
| Euthasol (a mixture of pentobarbital sodium (390 mg/mL) and phenytoin sodium (50 mg/mL)) | Covetrus, NDC 11695-4860-1 | 15.6 mg/mL | |
| Ketamine | Covetrus, NADA043304 | 114 mg/kg | |
| Mouse anti-Tuj1 | A gift from Anthony J. Spano, University of Virginia | 1:200 | |
| Normal donkey serum(NDS) | Millipore Sigma, S30-100 mL | 1:100 | |
| Phosphate-buffered saline (PBS, 10x), pH 7.4 (Contains 1370 mM NaCl, 27 mM KCl, 80 mM Na2HPO4, and 20 mM KH2PO4) |
Thermo Fisher Scientific, Cat# J62036.K3 | 1:10 | |
| Rat anti-ICAM-2 | BD Pharmingen, Cat#553325 | 1:500 | |
| Tropicamide drops | Covetrus, NDC17478-102-12 | ||
| Triton X-100 (Reagent Grade) |
VWR, CAS: 9002-93-1 | 1:20 | |
| Vectashield mounting medium | Vector Laboratories Inc. H2000-10 | ||
| Xylazine | Covetrus, NDC59399-110-20 | 17 mg/kg |
Referencias
- Sernagor, E., Eglen, S. J., Wong, R. O. Development of retinal ganglion cell structure and function. Progress in Retinal and Eye Research. 20 (2), 139-174 (2001).
- Sanes, J. R., Masland, R. H. The types of retinal ganglion cells: current status and implications for neuronal classification. Annual Review of Neuroscience. 38, 221-246 (2015).
- Seabrook, T. A., Burbridge, T. J., Crair, M. C., Huberman, A. D. Architecture, function, and assembly of the mouse visual system. Annual Review of Neuroscience. 40, 499-538 (2017).
- Cang, J., Savier, E., Barchini, J., Liu, X. Visual function, organization, and development of the mouse superior colliculus. Annual Review of Vision Science. 4, 239-262 (2018).
- Quigley, H. A. Understanding glaucomatous optic neuropathy: the synergy between clinical observation and investigation. Annual Review of Vision Science. 2, 235-254 (2016).
- Whitmore, A. V., Libby, R. T., John, S. W. Glaucoma: thinking in new ways-a role for autonomous axonal self-destruction and other compartmentalised processes. Progress in Retinal and Eye Research. 24 (6), 639-662 (2005).
- Syc-Mazurek, S. B., Libby, R. T. Axon injury signaling and compartmentalized injury response in glaucoma. Progress in Retinal and Eye Research. 73, 100769 (2019).
- Puyang, Z., Chen, H., Liu, X. Subtype-dependent morphological and functional degeneration of retinal ganglion cells in mouse models of experimental glaucoma. Journal of Nature and Science. 1 (5), (2015).
- Tatham, A. J., Medeiros, F. A. Detecting structural progression in glaucoma with optical coherence tomography. Ophthalmology. 124, S57-S65 (2017).
- Shu, X., Beckmann, L., Zhang, H. Visible-light optical coherence tomography: a review. Journal of Biomedical Optics. 22 (12), 1-14 (2017).
- Miller, D. A., et al. Visible-light optical coherence tomography fibergraphy for quantitative imaging of retinal ganglion cell axon bundles. Translational Vision Science and Technology. 9 (11), (2020).
- Beckmann, L., et al. In vivo imaging of the inner retinal layer structure in mice after eye-opening using visible-light optical coherence tomography. Experimental Eye Research. 211, 108756 (2021).
- Grannonico, M., et al. Global and regional damages in retinal ganglion cell axon bundles monitored non-invasively by visible-light optical coherence tomography fibergraphy. Journal of Neuroscience. 41 (49), 10179-10193 (2021).
- Allen-Worthington, K. H., Brice, A. K., Marx, J. O., Hankenson, F. C. Intraperitoneal Injection of Ethanol for the Euthanasia of Laboratory Mice (Mus musculus) and Rats (Rattus norvegicus). J Am Assoc Lab Anim Sci. 54 (6), 769-778 (2015).
- Boivin, G. P., Bottomley, M. A., Schiml, P. A., Goss, L., Grobe, N. Physiologic, Behavioral, and Histologic Responses to Various Euthanasia Methods in C57BL/6NTac Male Mice. J Am Assoc Lab Anim Sci. 56 (1), 69-78 (2017).
- Chen, H., et al. Progressive degeneration of retinal and superior collicular functions in mice with sustained ocular hypertension. Investigative Ophthalmology and Visual Science. 56 (3), 1971-1984 (2015).
- Feng, L., Chen, H., Suyeoka, G., Liu, X. A laser-induced mouse model of chronic ocular hypertension to characterize visual defects. Journal of Visualized Experiments: JoVE. 78 (78), (2013).
- Gao, J., et al. Differential effects of experimental glaucoma on intrinsically photosensitive retinal ganglion cells in mice. Journal of Comparative Neurology. 530 (9), 1494-1506 (2022).
- Thomson, B. R., et al. Angiopoietin-1 knockout mice as a genetic model of open-angle glaucoma. Translational Vision Science and Technology. 9 (4), (2020).
- Feng, L., et al. Sustained ocular hypertension induces dendritic degeneration of mouse retinal ganglion cells that depends on cell type and location. Investigative Ophthalmology and Visual Science. 54 (2), 1106-1117 (2013).
- Grannonico, M., et al. Longitudinal analysis of retinal ganglion cell damage at individual axon bundle level in mice using visible-light optical coherence tomography fibergraphy. Translational Vision Science and Technology. 12 (5), (2023).

