Tuina in a Frozen Shoulder Rat Model: An Efficient and Reproducible Protocol
Summary
This study develops an efficient and reproducible Tuina protocol for treating frozen shoulder established in a rat model. This approach will help to study the Tuina therapy treatment method for frozen shoulders.
Abstract
Frozen shoulder (FS) is a common condition with no defined optimal therapy. Tuina therapy, a traditional Chinese medicine (TCM) technique used to treat FS patients in Chinese hospitals, has demonstrated excellent results, but its mechanisms are not fully understood. Building on a previous study, this work aimed to develop a Tuina protocol for an FS rat model. We randomly divided 20 SD rats into control (C; n = 5), FS model (M; n = 5), FS model Tuina treatment (MT; n = 5), and FS model oral treatment (MO; n = 5) groups. This study used the cast immobilization method to establish the FS rat model. The effect of Tuina and oral dexamethasone on the glenohumeral range of motion (ROM) was evaluated, and the histological findings were assessed. Our study showed that Tuina and oral dexamethasone were able to improve shoulder active ROM and preserve the structure of the capsule, with Tuina therapy proving to be more effective than oral dexamethasone. In conclusion, the Tuina protocol established in this study was highly effective for FS.
Introduction
Frozen shoulder (FS), also known as adhesive capsulitis of the shoulder, is a self-limiting disease characterized by shoulder pain and mobility deficits. It typically affects people between the ages of 30 and 70 years old, with a mean age of 50 years, and has a prevalence of around 5% in the Chinese population1. Females are reported to have a 1.6 times higher incidence of FS compared to males2. The prevalence of FS is higher in people with diabetes, glucose and lipid metabolism disorders or other related diseases, ranging between 10% and 36%2,3. Current clinical treatments for FS include physiotherapy, steroid medications, and surgical treatments4.
Tuina, a traditional Chinese medicine (TCM) therapy, has been shown to effectively relieve shoulder pain in FS patients, improving their quality of life5,6. However, the underlying mechanisms of this treatment are not well understood. Thus, using animal models to study the effects and mechanisms of Tuina in treating FS is crucial.
The rat shoulder joint has a complex structure similar to that of the human shoulder and is often used in mechanistic studies of FS7. The FS rat model is characterized by a decline in glenohumeral ROM and capsule fibrosis8. Furthermore, this model allows for the observation of the shoulder capsule and enables pathological research while repairing the injury9. Moreover, oral corticosteroids are often used as the control group in FS treatment research10. This study aims to develop a Tuina protocol for the FS rat model and demonstrates the feasibility of conducting animal experiments in Tuina research by comparing the efficacy of Tuina therapy and oral dexamethasone.
Protocol
This study was approved by the Ethics Committee of the Affiliated Hospital of Shandong University of Traditional Chinese Medicine (Number: AWE-2022-023).
1. Experimental animals
- Twenty male Sprague-Dawley (SD) rats (7 weeks old, 250-280 g) were housed in standard conditions (room temperature [RT] 20-24 °C, humidity 40%-60 %, and a 12 h/12 h light/dark cycle).
2. Grouping method
- Group the SD rats into control group (C), FS model control group (M), FS model Tuina treatment group (MT), and FS model oral treatment group (MO), each consisting of 5 rats. Keep 5 rats per cage (same group).
- After 7 days of acclimation, immobilize one shoulder of the rats in the M, MT, and MO groups using plaster cast immobilization for 3 weeks to mimic FS as described in the next section.
- Administer Tuina therapy to the rats in the MT group for 2 weeks, as described in section 4 (Figure 1).
- Calculate the required dosage of dexamethasone for each kilogram of rats (0.0675 mg/day) based on the adult dosage (0.75 mg/day) and the ratio of rat to human body surface area (0.018).
- Administer daily intragastric dexamethasone solution to rats in the MO group at 0.067 mg/ kg/day at 7:00 AM for 2 weeks.
NOTE: Use this grouping method to confirm the effect of the Tuina protocol in this study. Perform the grouping method according to experimental purposes in different studies.
3. Development of the FS model
- Anesthetize rats using tribromoethanol (250 mg/kg, by intraperitoneal injection)11.
NOTE: In accordance with the requirements of the institution's ethics committee, a stock solution composed of tribomoethanol (10 g) and tert-amyl alcohol (10 mL) was stored at 4 °C. Before use, it was diluted to 2% with distilled water. - Apply plaster-soaked bandages to the right shoulder and chest of the rats, keeping the right forelimb at 90° of internal rotation of the shoulder joint for 3 weeks (Figure 2)12.
NOTE: Monitor rats to ensure they can perform normal physiological activities such as walking, eating, and drinking. Refix the plaster bandage if the rats can not perform normal physiological activities. - Confirm the successful establishment of the FS model by observing the development of symptoms such as stiffness in the right shoulder joint, contraction of the right upper limb, muscular atrophy, and limping in rats13.
4. Tuina method
NOTE: Throughout the procedure, the investigator must wear personal protective equipment. Only a single professional Tuina doctor must perform all manipulations (Figure 3, Figure 4, and Figure 5).
- Train by the Intelligent-massage technique parameter determination system, which includes a mechanoreceptor and a computer (Figure 3A).
- Perform manipulations on the mechanoreceptor and force parameters in three directions displayed through software (Figure 3B).
- Use the thumb finger to perform the rotatory-kneading method in a rotatory movement at a strength of 0.5 kg and a frequency of 100-120 times/min (Figure 3C).
- Use the thumb fingertip to perform the point-pressing method at a strength of 0.5 kg (Figure 3D).
- Perform Tuina on the rats by maintaining the mechanical display mentioned in steps 4.1.2 and 4.1.3 for 1 min.
- Hold the rat until it calms down (~2 min). Then, perform the manipulation. Place the rat in the lateral recumbent position, but the position may change based on different manipulation methods.
- Use the right index finger and middle finger to clamp the right forelimb of the rat and flex and extend it several times to determine the positions of the rat's shoulder joint, elbow joint, and humerus.
- Knead the right shoulder, forelimb, and back of the rat by clockwise rotation with the thumb pulp at a strength of 0.5 kg and a frequency of 100-120 times/min for 3 min (Figure 4A-C).
- Manipulate the forelimb muscles in the lateral recumbent position.
- Manipulate the shoulder and back muscles in the prone position.
- Press acupoint LI15 (Jianyu), SI11 (Tianzong), HT01 (Jiquan,) and LI11 (Quchi) vertically with the thumb fingertip 30 times per acupoint at a strength of 0.5 kg (Figure 4D-G).
- Use the rat-acupoint atlas to define the location of each acupoint (Figure 5)14,15.
- Press LI15, located in the depression anterior-inferior to the acromial end, in the prone position.
- Press SI11, located in the depression to the infraspinatus fossa at the midpoint of the scapular spine, in the prone position.
- Press HT01, located at the axilla center, in the supine position.
- Press LI11 located in the depression medial to the extensor carpi radialis at the lateral end of the cubital crease in the lateral recumbent position.
- Hold the shoulder joint with the left thumb and middle finger, and stretch the forelimb in the adduction, abduction, anterior extension, and posterior extension positions for 10 s (Figure 4H-K).
NOTE: This stretching method must be carried out without resistance in rats. - Pause the Tuina procedure if the rat becomes agitated. Stroke the rat for 10 s to calm it down, and then proceed with the trial.
- Perform the procedure daily for 2 weeks.
5. Measurement of glenohumeral ROM
NOTE: It is important to complete the measurement process as quickly as possible to prevent degeneration of the joint capsule tissue.
- Remove the scapula and proximal two-thirds of the humerus en bloc after sacrificing the rat with an excessive dosage of tribromoethanol (3x initial dose, by intraperitoneal injection), exposing the lower edge of the scapula.
- Insert an injection needle (1.2 cm x 0.45 mm) along the humeral shaft into the humeral head.
- Insert two injection needles vertically into the upper and lower corners of the scapula on plastic foam wrapped with a sterile surgical sheet.
- Attach a thin thread to the injection needle on the humeral shaft and pull it at the other end with a 5 g force to make it parallel to the humeral shaft. Measure the angle between the lower edge of the scapula and the humeral shaft (Figure 6).
NOTE: To ensure reliable results, have a separate investigator conduct the measurements. - Report data as means ±standard deviation (SD) using a statistical analysis software application.
NOTE: SPSS software (SPSS, version 25.0) was used here. - Analyze the differences between groups using one-way analysis of variance (ANOVA).
- Obtain bar graphics using appropriate software.
NOTE: GraphPad Prism 8 was used here. - Assess capsule pathology using H&E and Masson staining after measurement.
6. Section preparation
- After evaluating glenohumeral ROM, fix whole samples in 4% PFA for 3 days, followed by decalcification in EDTA (pH 7.2) solution for an additional 2 months.
- After dehydration, slice embedded tissue blocks containing the samples into 5 µm slices16.
- Dry the slice at 65 °C for 60 min.
- Dewax the slice.
- Soak the slice in xylene I, xylene II, and xylene III for 7 min, followed by a descending ethanol series (anhydrous ethanol, 5 min; 95% ethanol, 2 min; 80% ethanol, 2 min, and 70% ethanol, 2 min), and finally in ultrapure water for 2 min.
7. H&E staining
- Stain the sections using hematoxylin for 5 min, rinse with 1% hydrochloric acid ethanol for 3 s, and wash with running water for 5 min.
- Stain the section with eosin for 3 min and wash with tap water.
- Soak the section in an ethanol series (95% ethanol I, 3 s; 95% ethanol II, 3 s; anhydrous ethanol I, 3 s, and anhydrous ethanol II, 1 min) and then immerse in a xylene series (xylene I, 1 min; xylene II, 1 min).
- Place a drop of neutral gum sealant on each sample. Seal each sample with a cover glass.
- Collect images using an inverted fluorescence microscope (Scale bar = 100 µm).
8. Masson staining
- Using an immunohistochemical pen, draw a circle around the sections and then incubate the sections in Bouin's solution for 2 h at 37 °C to mordant. Subsequently, wash the sections with water until the yellow color disappears.
- Treat the samples with lapis lazuli blue dye for 3 min and then wash them with distilled water.
- After staining the sections with hematoxylin (Mayer) for 2 min, treat the sections for 3 s in the acidic ethanol differentiation solution. Then, wash the sections in running water for 10 min.
- Stain the sections with ponceau magenta dye solution for 10 min and subsequently wash them with water.
- Immerse the sections in the phosphomolybdic acid solution for 10 min.
- Add aniline blue staining solution to the sections for 5 min, and then wash them with a weak acid working solution for 2 min.
- Dehydrate and make the sections transparent as described in step 7.3.
- Place a drop of neutral gum sealing agent on each section and cover it with a cover glass. Leave the sections in a fume hood to dry.
- Collect images as described in step 7.5.
Representative Results
The physical activity of rats was observed to evaluate the success or failure of the FS model. A previous study showed that cast immobilization significantly reduced the distance traveled and walking speed compared to normal rats17. Another research suggested that FS did not affect the distance traveled, and limping was the most common presenting symptom13. This study showed stiffness in the right shoulder joint, contraction of the right upper limb, muscular atrophy and limping in rats after modeling. These lesions in the MT and MO groups were resolved completely by 2 weeks of intervention. But there was no significant change in the M group.
The primary criterion for evaluating the effectiveness of Tuina in FS is the measurement of glenohumeral ROM18. We observed that the average values of glenohumeral ROM were 149.3° ± 5.9° in the C group, 111.1° ± 3.9° in the M group, 128.5° ± 2.8° in the MT group, and 119.56° ± 2.9° in the MO group. As depicted in Figure 7, the glenohumeral ROM of rats in the M group was significantly lower than that in the C group (P < 0.0001). Moreover, the ROM in the MT group and MO group was significantly higher than that in the M group (P < 0.05, P < 0.0001). However, the ROM in the MO group was significantly lower than that in the MT group (P < 0.0001). This finding suggests that Tuina can significantly improve shoulder joint function in FS rats.
Additionally, H&E staining and Masson staining can further demonstrate the effects of Tuina in preserving the structure and reducing fibrosis in capsule. To facilitate observation, the capsule of the glenohumeral joint was used for histological findings. The shoulder joint capsule comprises synovial and fibrous layers19. H&E staining revealed synoviocyte proliferation, flattened synovial folds, erythrocyte stasis, and vascular proliferation in the M group, which are typical features of FS (Figure 8A,B). These features diminished to some extent after Tuina and oral dexamethasone therapy (Figure 8C,D). Compared to the MT group, the MO group also showed much synovial cells. Masson staining showed the arrangement of fiber bundles in each group (yellow arrows). The capsule comprises a loose network of reticular fibers with fiber bundles arranged in a neat direction (Figure 8E). In the M group, the fiber bundles were arranged disorderly, indicating capsule fibrosis (Figure 8F). The capsules of rats in the MT group showed that the fiber bundles are neatly and clearly stratified but remain slightly disordered in the MO group (Figure 8G,H).
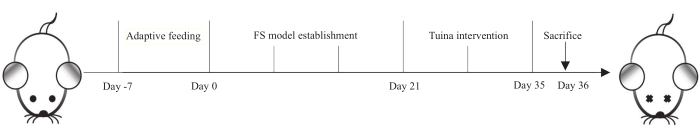
Figure 1: Protocol for establishing the FS model and Tuina intervention. The rats were on adaptive feeding for 7 days, FS model establishment for 21 days, and Tuina therapy was performed daily for 14 days. On day 36, all rats were sacrificed. Please click here to view a larger version of this figure.
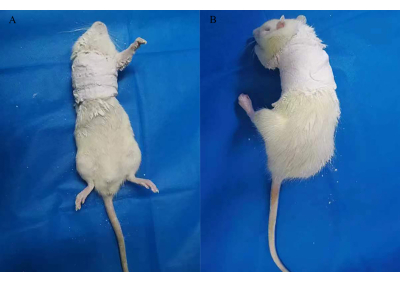
Figure 2: Cast immobilization for establishing a rat model of FS. Please click here to view a larger version of this figure.
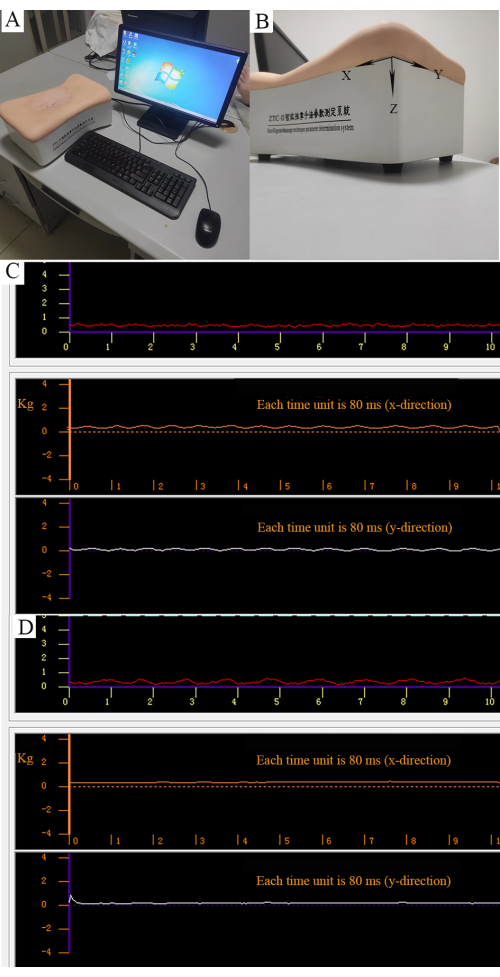
Figure 3: Quantitative control of manipulation. (A) Intelligent massage technique parameter determination system. (B) Three forces can be measured as parallel force along the X direction, longitudinal force along the Y direction, and vertical force along the Z direction. (C) Strength of the rotatory-kneading method. The red curve represents the stabilized vertical force (0.5 kg). The orange curve represents the regular parallel force. The white curve represents the regular longitudinal force. (D) The strength of the point-pressing method. The red curve represents the vertical force (0.5 kg). Orange and white curves represent nonparallel and longitudinal forces. Please click here to view a larger version of this figure.
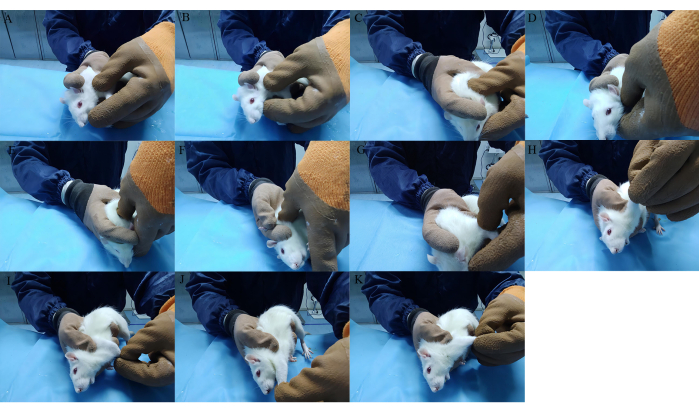
Figure 4: Manipulation used in Tuina therapy. (A-C) Knead muscles of the right shoulders, forelimbs, and back. (D-G) Point-press LI15, SI11, HT01, and LI11. (H-K) Stretch the forelimb in adduction, abduction, anterior extension, and posterior extension positions. Please click here to view a larger version of this figure.
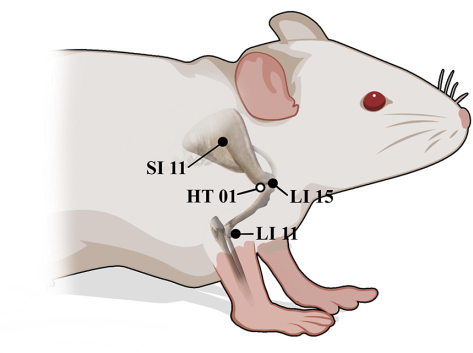
Figure 5: Anatomical positions of LI15, SI11, HT01, and LI11 in rats. ● Lateral surface, ○ Medial surface. Please click here to view a larger version of this figure.
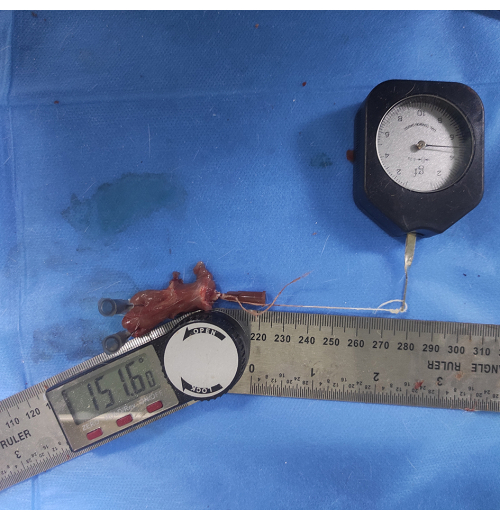
Figure 6: Measurement of glenohumeral ROM. A thin thread is attached to an injection needle inserted into the humeral shaft and pulled at the other end with a 5 g force to make it parallel to the humeral shaft. The angle between the lower edge of the scapula and the humeral shaft is measured as glenohumeral ROM. Please click here to view a larger version of this figure.
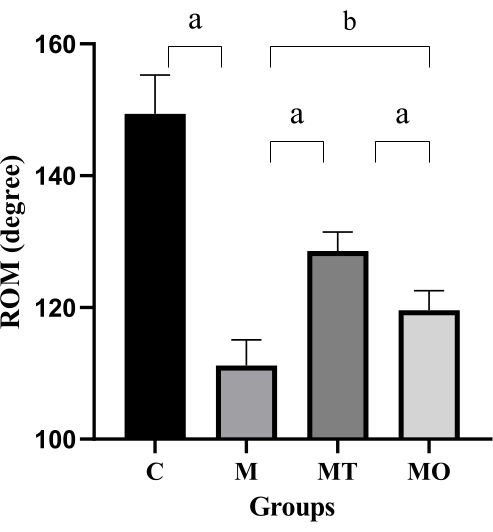
Figure 7: Glenohumeral ROM across three groups of rats. Values are means ± S.D., n = 5. Significant differences are indicated by one-way ANOVA (aP < 0.001 and bP < 0.0001). Please click here to view a larger version of this figure.
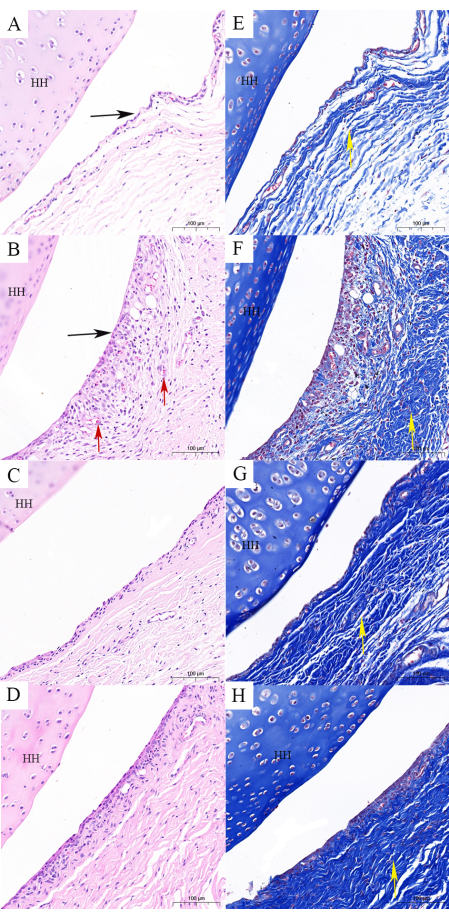
Figure 8: Histological findings of the shoulder capsule. (A,E) The control group contains a normal capsule structure (H&E and Masson staining). (B,F) The FS model group illustrates changes in the structure of the capsule as follows: flattened synovial folds, capsule fibrosis, and disturbed fiber bundles (H&E and Masson staining). (C,G). The FS model combined with Tuina group illustrates that the structure of the capsule is close to normal, and fibrosis is not obvious (H&E and Masson staining). (D,H) The FS model combined with oral dexamethasone shows that the structure of the capsule is close to normal, and fibrosis is obvious (H&E and Masson staining). Scale bar = 100 µm. HH: head of the humerus; black arrow: synovial folds; red arrow: erythrocyte stasis and vascular proliferation; yellow arrow: fiber bundles. Please click here to view a larger version of this figure.
Discussion
The first critical step is model selection. Due to the difficulty in implementing the primary FS model, cast immobilization and surgical internal fixation are often used to establish FS rat models9,12. The most severe restriction of shoulder mobility and fibrosis of the capsule were observed in the FS model established by cast immobilization for 3 weeks12,20. In this study, the success rates of the FS model were excellent, with 100% success.
The second critical step is the manipulations used in this protocol. Three manipulations (kneading, pressing, and stretching) were used in this study. The soft tissue kneading manipulation was applied to the shoulder, scapula and upper arm to relax the muscles. Pressing manipulation was performed by applying pressure to acupoints such as LI15, SI11, HT01, and LI11, which are most commonly used in clinical practice for FS5,21. LI15, SI11, and HT01 are located in positions around the shoulder capsule and can be effective in improving ROM and shoulder function22. LI11 is often used for upper extremity motor impairment and is located in the same meridian as LI15. This acupoint matching method helps improve the efficacy of LI1523. Following full relaxation, stretching techniques were used to restore functional activities.
The possible problem in this protocol is that rats exhibit intense resistance during Tuina, which may be caused by fear rather than exceeding the tolerance of the rats. At this point, the manipulations should be stopped until the rats calm down (stroking for 10 s calms down the rats). In addition, the extent of stretching should be adjusted according to the symptoms of the rats. Initially, the limitation of the shoulder joint was obvious, and the stretching amplitude was small. Along with the intervention, the shoulder joint function of the rats gradually recovered and the amplitude of the stretch progressively increased. The standard is that rats can accept the stretching method without resistance. Finally, rats have a certain degree of aggression, and Tuina requires prolonged contact with rats, so it is important to wear personal protective equipment.
The quantitative control of manipulation is the most difficult in Tuina experiments. While a massage manipulation simulator can be used to control the strength and frequency of a single manipulation, this method is limited when multiple manipulations and treatment sites are involved24,25. In clinical practice, Tuina is typically performed directly by practitioners, and in this study, it was difficult to intervene with medical equipment. To control the stimulation, the intelligent massage technique parameter determination system can be used to standardize the training of Tuina. After training, the investigator can apply the same force to each rat to an extent. The major limitation of this protocol is that manipulations cannot be completely controlled.
TCM Tuina therapy has a rich history of use across China, with various doctors in hospitals using different manipulation and treatment site combinations. Therefore, it is important to establish replicable and effective protocols for both animal experiments and clinical studies. In this study, the manipulations and acupoints used were based on a previous study by our team, combining our clinical experience with the characteristics of the FS animal model21. This study demonstrated the effectiveness of the developed Tuina protocol in improving shoulder joint function and reducing capsule fibrosis in FS rats. These findings provide a foundation for further investigations into the mechanisms underlying Tuina treatment. Moreover, the protocol can be useful for researchers interested in exploring the efficacy of alternative medical treatments for FS.
A previous study found that the mechanism of Tuina intervention on fibrosis may be related to the down-regulation of TGF-β and CTGF while regulating the balance of MMP-1/TIMP-1, thereby alleviating the production of extracellular matrix (ECM)26. The effect of Tuina on the fibrosis of the shoulder capsule may be achieved through the regulation of various mechanisms. However, further research is required to fully understand the mechanisms involved in this improvement.
Divulgaciones
The authors have nothing to disclose.
Acknowledgements
This work was supported by the 2020 Science and Technology Development Plan in Jinan City (Grant Number 202019059), the Traditional Chinese Medicine Science and Technology Project of Shandong Province (Grant Number 2021Q080), and the Qilu School of Traditional Chinese Medicine Inherit Project (Grant Number [2022]93).
Materials
| 4% paraformaldehyde | Solarbio | P1110 | |
| Embedding machine | Changzhou Paisijie Medical Equipment Co., Ltd | BM450A | |
| Ethylene Diamine Tetraacetic Acid (EDTA) | Solarbio | E1171 | |
| Hematoxylin eosin (HE) staining kit | Sparkjade | EE0012 | |
| Intelligent-massage technique parameter determination system | Shanghai Dukang Intrument Equipment Co. Ltd | ZTC- |
|
| Microtome | Leica | 531CM-Y43 | |
Modified Masson Trichrome Staining Solution |
Shanghai yuanye Bio-Technology Co., Ltd | R20381-8 | Bouin 50 mL; lapis lazuli blue dye 50 mL; Hematoxylin (Mayer) 50 mL; acidic ethanol differentiation solution 50 mL; ponceau magenta dye solution 50 mL; phosphomolybdic acid solution 50 mL; aniline blue staining solution 50 mL; weak acid 50 mL |
| Tribromoethanol | Macklin | T903147-5 |
Referencias
- Li, W., LU, N. Z., Xu, H. L., Wang, H. F., Huang, J. Case control study of risk factors for frozen shoulder in China. International Journal of Rheumatic Diseases. 18 (5), 508-513 (2015).
- Degreef, I., Steeno, P., De Smet, L. A survey of clinical manifestations and risk factors in women with Dupuytren’s disease. Acta Orthopaedica Belgica. 74 (4), 456-460 (2008).
- Tighe, C. B., Oakley, W. S. The prevalence of a diabetic condition and adhesive capsulitis of the shoulder. Southern Medical Journal. 101 (6), 591-595 (2008).
- Cho, C. H., Bae, K. C., Kim, D. H. Treatment strategy for frozen shoulder. Clinics in Orthopedic Surgery. 11 (3), 249-257 (2019).
- Liu, M., et al. Effects of massage and acupuncture on the range of motion and daily living ability of patients with frozen shoulder complicated with cervical spondylosis. American Journal of Translational Research. 13 (4), 2804-2812 (2021).
- Ai, J., Dong, Y. K., Tian, Q. D., Wang, C. L., Fang, M. Tuina for periarthritis of shoulder: A systematic review protocol. Medicina. 99 (11), e19332 (2020).
- Norlin, R., Hoe-Hansen, C., Oquist, G., Hildebrand, C. Shoulder region of the rat: anatomy and fiber composition of some suprascapular nerve branches. The Anatomical Record. 239 (3), 332-342 (1994).
- Okajima, S. M., et al. Rat model of adhesive capsulitis of the shoulder. Journal of Visualized Experiments: JoVE. (139), 58335 (2018).
- Zhao, H. K., et al. Tetrandrine inhibits the occurrence and development of frozen shoulder by inhibiting inflammation, angiogenesis, and fibrosis. Biomedicine & Pharmacotherapy. 140, 111700 (2021).
- nar, B. M., Battal, V. E., Bal, N., Güler, &. #. 2. 2. 0. ;. &. #. 2. 1. 4. ;., Beyaz, S. Comparison of efficacy of oral versus intra-articular corticosteroid application in the treatment of frozen shoulder: An experimental study in rats. Acta Orthopaedica et Traumatologica Turcica. 56 (1), 64-70 (2022).
- Dias, Q. M., Rossaneis, A. C., Fais, R. S., Prado, W. A. An improved experimental model for peripheral neuropathy in rats. Brazilian Journal of Medical and Biological Research. 46 (3), 253-256 (2013).
- Kim, D. H., et al. Characterization of a frozen shoulder model using immobilization in rats. Journal of Orthopaedic Surgery and Research. 11 (1), 160 (2016).
- Feusi, O., et al. Platelet-rich plasma as a potential prophylactic measure against frozen shoulder in an in vivo shoulder contracture model. Archives of Orthopaedic and Trauma Surgery. 142 (3), 363-372 (2022).
- Yin, C. S., et al. A proposed transpositional acupoint system in a mouse and rat model. Research in Veterinary Science. 84 (2), 159-165 (2008).
- Guo, X. R., et al. Study on the regulatory mechanism of electroacupuncture based on thyroid pathway for mammary gland hyperplasia rats. Zhongguo Zhen Jiu. 38 (8), 857-863 (2018).
- Feldman, A. T., Wolfe, D. Tissue processing and hematoxylin and eosin staining. Methods in Molecular Biology. 1180, 31-43 (2014).
- Taguchi, H., et al. A rat model of frozen shoulder demonstrating the effect of transcatheter arterial embolization on angiography, histopathology, and physical activity. Journal of Vascular and Interventional Radiology: JVIR. 32 (3), 376-383 (2021).
- Oki, S., et al. Generation and characterization of a novel shoulder contracture mouse model. Journal of Orthopaedic Research. 33 (11), 1732-1738 (2015).
- Kubo, H., et al. Histologic examination of the shoulder capsule shows new layer of elastic fibres between synovial and fibrous membrane. Journal of Orthopaedics. 22, 251-255 (2020).
- Cho, C. H., Lho, Y. M., Hwang, I., Kim, D. H. Role of matrix metalloproteinases 2 and 9 in the development of frozen shoulder: human data and experimental analysis in a rat contracture model. Journal of Shoulder and Elbow Surgery. 28 (7), 1265-1272 (2019).
- Wang, J. M., et al. Efficacy and safety of Tuina and intermediate frequency electrotherapy for frozen shoulder: MRI-based observation evidence. American Journal of Translation Research. 15 (3), 1766-1778 (2023).
- Ben-Arie, E., et al. The effectiveness of acupuncture in the treatment of frozen shoulder: A systematic review and meta-analysis. Evidence-Based Complementary and Alternative Medicine: eCAM. 2020, 9790470 (2020).
- Zou, F., et al. The impact of electroacupuncture at hegu, shousanli, and quchi based on the theory "Treating flaccid paralysis by Yangming alone" on stroke patients’ EEG: A pilot study. Evidence-Based Complementary and Alternative Medicine: eCAM. 2020, 8839491 (2020).
- Lv, T. T., et al. Using RNA-Seq to explore the repair mechanism of the three methods and three-acupoint technique on DRGs in sciatic nerve injured rats. Pain research & Management. 2020, 7531409 (2020).
- Niu, F., et al. Spinal tuina improves cognitive impairment in cerebral palsy rats through inhibiting pyroptosis induced by NLRP3 and Caspase-1. Evidence-Based Complementary and Alternative Medicine: eCAM. 2021, 1028909 (2021).
- Na, Z., et al. The combination of electroacupuncture and massage therapy alleviates myofibroblast transdifferentiation and extracellular matrix production in blunt trauma-induced skeletal muscle fibrosis. Evidence-Based Complementary and Alternative Medicine: eCAM. 2021, 5543468 (2021).

