Ex Vivo Calcium Imaging for Drosophila Model of Epilepsy
Summary
Here, we present a protocol for ex vivo calcium imaging in GCaMP6-expressing adult Drosophila to monitor epileptiform activities. The protocol provides a valuable tool for investigating ictal events in adult Drosophila through ex vivo calcium imaging, allowing for exploration of the potential mechanisms of epilepsy at the cellular levels.
Abstract
Epilepsy is a neurological disorder characterized by recurrent seizures, partially correlated with genetic origin, affecting over 70 million individuals worldwide. Despite the clinical importance of epilepsy, the functional analysis of neural activity in the central nervous system is still to be developed. Recent advancements in imaging technology, in combination with stable expression of genetically encoded calcium indicators, such as GCaMP6, have revolutionized the study of epilepsy at both brain-wide and single-cell resolution levels. Drosophila melanogaster has emerged as a tool for investigating the molecular and cellular mechanisms underlying epilepsy due to its sophisticated molecular genetics and behavioral assays. In this study, we present a novel and efficient protocol for ex vivo calcium imaging in GCaMP6-expressing adult Drosophila to monitor epileptiform activities. The whole brain is prepared from cac, a well-known epilepsy gene, knockdown flies for calcium imaging with a confocal microscope to identify the neural activity as a follow-up to the bang-sensitive seizure-like behavior assay. The cac knockdown flies showed a higher rate of seizure-like behavior and abnormal calcium activities, including more large spikes and fewer small spikes than wild-type flies. The calcium activities were correlated to seizure-like behavior. This methodology serves as an efficient methodology in screening the pathogenic genes for epilepsy and exploring the potential mechanism of epilepsy at the cellular level.
Introduction
Epilepsy, a complex chronic neurological disorder characterized by the recurrence of spontaneous and unprovoked seizures and aberrant neuronal network activity, has affected over 70 million individuals worldwide, making it one of the most common neurological diseases1 and leading to the heavy burdens of families and society. In consideration of the impact of epilepsy, many studies have been conducted to identify the etiology of seizures, of which genetics has been approved as a primary cause of many types of epilepsies or epileptic syndromes2. For the past decades, advances in genomic technologies have led to a rapid increase in the discovery of novel epilepsy-associated genes, which play a crucial role in seizure occurrence, including ion channels and non-ion channel genes3,4. However, the underlying mechanisms and functional analysis between the genes and epileptic phenotypes are incompletely understood. Identifying epilepsy-associated genes and mechanisms offers the possibility to the management of patients efficiently5,6.
Cytosolic calcium signals are pivotal elements in neuronal activity and synaptic transmission. Calcium imaging, including brain slices7, in vivo8,9, and ex vivo10, has been utilized to monitor neuronal activity11 as a marker for neuronal excitability since the 1970s12,13. Recent advancements in imaging technology, in combination with the genetically encoded calcium indicators (GECIs), such as GCaMP6, have revolutionized the study of epilepsy at both brain-wide and single-cell resolution levels14,15,16, which has a high level of spatiotemporal precision. Changes in calcium concentration and transients were observed in action potentials and synaptic transmission, respectively14, indicating the alteration of intracellular calcium levels exhibits a strict correlation with the electrical excitability of neurons17,18. Calcium imaging has also been applied as a developmental seizure model9 and performed in Drosophila for screening anticonvulsive compounds19.
Drosophila melanogaster has been emerging as a powerful model organism in scientific research, such as epilepsy, for its sophisticated molecular genetics and behavioral assays20,21,22. Moreover, the advanced genetic tools in Drosophila have contributed to the expression of genetically encoded calcium indicator GCaMP6. For instance, the Gal4 and UAS-based binary transcriptional systems enable specific expression of the GCaMP6 in a spatially and temporally controlled manner. Since Drosophila is a tiny organism, in vivo calcium imaging requires proficient operation skills to perform a surgical intervention, in which only a small part of the dorsal of the brain was exposed through a small window14,23. At the same time, ex vivo calcium imaging in the intact brain of Drosophila can be used to monitor the regions of interest (ROIs) of the whole brain.
In this study, we present ex vivo calcium imaging in GCaMP6-expressing adult Drosophila to monitor epileptiform activities. CACNA1A is a well-known epilepsy gene, cac belongs to Cav2 channel, which is a homolog to CACNA1A. We began by dissecting the brains of cac knockdown flies tub-Gal4>GCaMP6m/cac-RNAi and imaging them using a confocal microscope with xyt scanning mode. We then analyzed the changes in calcium signals of ROIs by calculating indicators that quantify spontaneous seizure-like events, such as %ΔF/F value and calcium events of GCaMP6 fluorescence. Additionally, we performed mechanical stimulus by vortex machine to induce seizure behavior tests on cac-knockdown flies as well to validate the results of calcium imaging. Overall, this protocol provides a valuable tool for investigating ictal events in adult Drosophila through ex vivo calcium imaging, allowing for exploration of the potential mechanisms of epilepsy at the cellular levels.
Protocol
1. Protocol for bang-sensitive assay
- Establish the experimental flies by crossing the tub-Gal4 driver line with the UAS-cac-RNAi line via the Gal4/UAS system21. Collect the virgin flies of the tub-Gal4 line and the male flies of the UAS-cac-RNAi line. Then, transfer the virgin and male flies into the same vial to harvest the offspring.
NOTE: The tub-Gal4 driver line will allow achieving global knockdown of the cac gene. Use the UAS-cac-RNAi line as the control group. - After 3-5 days of eclosion, gently anesthetize the flies using the 100% CO2 (CO2 anesthesia equipment) and collect both the tub-Gal4>UAS-cac-RNAi flies and the UAS-cac-RNAi flies with a brush. Transfer these flies to new, clean food vials 1 day before testing to ensure they are well-prepared.
- Gently anesthetize the flies using the CO2. Once anesthetized, carefully place 4-6 flies into individual fresh vials. Allow the flies to recover from anesthesia for about 1 h before proceeding with the testing.
NOTE: For each genotype, a minimum of five trials were tested, and each trial consisted of six vials of flies. - Set up the recording equipment. Position a camera (720-1080P, 30-60 FPS at least, AF locked) on a tripod in front of a whiteboard. Adjust the camera's focus manually using an empty vial to ensure clear and accurate footage.
- Use a vortex mixer to perform the mechanically induced seizure-like behavior. Place each vial containing 4-6 flies onto the vortex mixer and run it at the highest setting (2800 rpm) for exactly 10 s. After the vortexing, promptly place the vial on the whiteboard.
- Observe the flies and record any seizure-like behavior they exhibit.
NOTE: Seizure-like behavior observed in flies consists of three stages: seizure (manifesting as vibrating wings), paralysis, and recovery24. The ratio of seizure flies to tested flies was defined as seizure rate.- Measure the recovery time as the time required after vortexing until flies regain the ability to stand upright25.
2. Protocol for calcium imaging of ex vivo brain
- Establish the tub-Gal4>UAS-GCaMP6m and tub-Gal4>UAS-GCaMP6m/cac-RNAi line flies, respectively. Generate the flies using the Gal4/UAS binary expression system21 to allow monitoring of calcium activity in the ex vivo brain.
- Prepare the dissection solution as described in the provided recipe (Table 1)26.
- Prepare the external solution as described in Table 1. Adjust to pH 7.2 with NaOH and adjust osmolarity to 250-255 mOsm/L.
NOTE: The external solution can be stored at 4 °C for 1 week. - For preparing the dissection solution, add 9 units of papain suspension and L-lysine (2 mg/mL) to 600 µL of the external solution. Vortex the solution and wait 15 min until the solution is clear.
NOTE: It is recommended to use the dissection solution within 4 h.
- Prepare the external solution as described in Table 1. Adjust to pH 7.2 with NaOH and adjust osmolarity to 250-255 mOsm/L.
- Perfuse the external solution with oxygenated saline (95% oxygen and 5% carbon dioxide) for 5 min before use. Use the pipette to transfer about 10 µL of the dissection solution to a Petri dish. This solution will provide the necessary conditions for brain dissection and imaging.
- Carefully dissect the brains of both the tub-Gal4>UAS-GCaMP6m and tub-Gal4>UAS-GCaMP6m/cac-RNAi lines using the syringe needles and microscope. Follow the established protocol for brain dissection27.
- Use a pipette to transfer the prepared brain into a recording dish containing 5 mL of fresh external solution and immobilize the brain using a C-sharp holder in the recording dish for 3-5 min for recovery.
NOTE: The C-sharp holder was made of a metal semicircle with 7 mm diameter crossed by parallel fibers 0.5 mm apart. - Confocal imaging setup and acquisition
- For confocal imaging, capture each brain with a 20x objective and xyt Scanning Mode. Identify the mushroom body neurons with an additional 4.5x digital amplification based on 20x optical amplification.
NOTE: Each ex vivo brain can be imaged for more than 1 h. - Acquire the whole brain-GCaMP6m emission at 488 nm laser excitation with 16 µw laser power. Set the scanning parameters to a speed of 400, with a pixel size of 256 pixels x 256 pixels. Use a 5.3 Hz acquisition rate and record for a duration of 3 min.
- For confocal imaging, capture each brain with a 20x objective and xyt Scanning Mode. Identify the mushroom body neurons with an additional 4.5x digital amplification based on 20x optical amplification.
- Identify 5-8 ROIs in every brain and analyze the fluorescence. Manually determine the cell body of mushroom body neurons as the region of interest28.
- Label the identified neurons and measure their fluorescence intensities by ImageJ. Analyze the Fluorescence data of GCaMP6m via %ΔF/F = (F1-F0)/(F0-B). Define the intracellular fluorescence, increasing between 2 and 2.5 standard deviations above the baseline, as small spikes, and intracellular fluorescence, increasing greater than 2.5 standard deviations above the baseline, as large spikes. Analyze the frequency of calcium spikes by Student's t-test.
NOTE: F0 was defined as the baseline by the mean fluorescence intensity of the initial 5 frames in ROIs, F1 as the fluorescence intensity of the given time point, and B as the background without fluorescence. Temporal physiological activities of neurons are also helpful in distinguishing them from noise signs.
- Label the identified neurons and measure their fluorescence intensities by ImageJ. Analyze the Fluorescence data of GCaMP6m via %ΔF/F = (F1-F0)/(F0-B). Define the intracellular fluorescence, increasing between 2 and 2.5 standard deviations above the baseline, as small spikes, and intracellular fluorescence, increasing greater than 2.5 standard deviations above the baseline, as large spikes. Analyze the frequency of calcium spikes by Student's t-test.
Representative Results
Using this protocol, we found that cac knockdown flies showed significantly higher rates of seizure-like behavior than the WT flies (17.00 ± 2.99 [n = 6] vs 4.50 ± 2.03 [n = 6]; P = 0.0061; Student's t-test, Figure 1A). Most tub-Gal4>UAS-cac-RNAi flies recovered within 1-5 s, while UAS-cac-RNAi flies recovered within 2 s. The recovery percentage of cac knockdown flies within 1 s was significantly lower than the WT flies (88.08 ± 1.89 [n = 6] vs 96.50 ± 1.82 [n = 6]; P = 0.0093; Student's t-test, Figure 1B). As shown in Figure 2, calcium signals were observed in the mushroom body of flies. Small spikes occurred less in cac knockdown flies (tub-Gal4>UAS-GCaMP6m/cac-RNAi, 2.97 ± 0.96 [n = 13] vs. tub-Gal4>UAS-GCaMP6m, 5.33 ± 1.31 [n = 13]; P < 0.0001; Student's t-test), whereas large spikes occurred more in cac knockdown flies (tub-Gal4>UAS-GCaMP6m/cac-RNAi, 2.87 ± 0.63 [n = 13] vs. tub-Gal4>UAS-GCaMP6m, 1.72 ± 0.62 [n = 13]; P < 0.0001). These results indicate that the calcium signals observed in flies correlate highly with neuronal activity and showed consistency with the seizure-like behavior in cac knockdown flies.
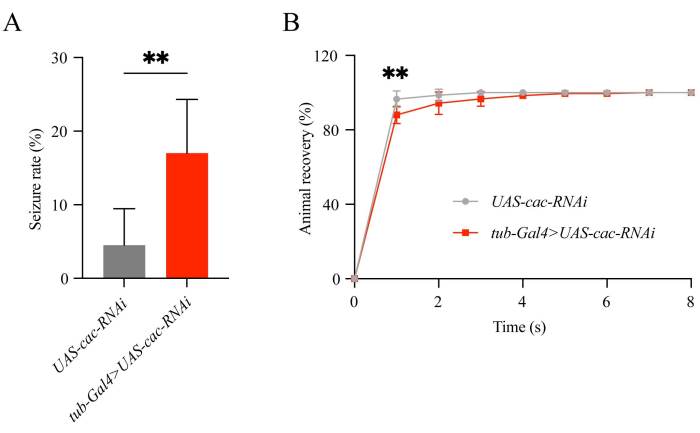
Figure 1: Classical seizure rates and recovery time in flies. (A) Seizures occurred at a higher rate in cac knockdown flies. (B) The recovery time from seizure. Please click here to view a larger version of this figure.
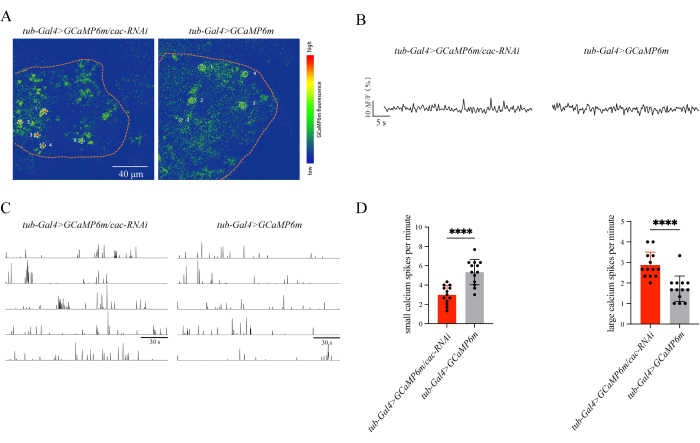
Figure 2: Representative calcium signals in the mushroom body of flies. (A) Calcium signals in selected 5 cells in the mushroom body of flies. (B) Typical trace: Change in fluorescence (ΔF/F) over time from an individual cell of the mushroom body in knockdown and wild-type files. (C) Global or neuronal calcium events occurring in the mushroom body of flies distinguished into small and large spikes. (D) Calcium spikes in the whole brain of knockdown flies and controls. This figure has been modified with permission from Liu et al.29. Please click here to view a larger version of this figure.
| External solution for recording and making dissection solution. | |
| Sodium chloride | 101 mM |
| Potassium chloride | 3 mM |
| Calcium chloride | 1 mM |
| Magnesium chloride | 4 mM |
| Glucose | 5 mM |
| Sodium phosphate monobasic | 1.25 mM |
| Sodium bicarbonate | 20.7 mM |
| Dissection solution | |
| External solution | 600 μL |
| Papain suspension | 9 U |
| L-lysine | 2 mg/mL |
Table 1: Composition of external and dissection solutions.
Discussion
The calcium ion serves as a crucial second messenger, playing a pivotal role in a range of physiological and pathophysiological responses to both chemical and electrical perturbations. Furthermore, the topological element of the presynaptic P/Q channels, encoded by the human CACNA1A gene, has been identified as responsible for mediating the discharge of various neurotransmitters, including glutamate30,31,32, and is closely linked to epilepsy33,34. Previously, the behavior of Cac knockdown flies did not exhibit the severity reported in some mutants and even displayed suppressive effects in certain double mutant flies35 . The findings of this study reveal that cac knockdown flies are more prone to seizures than their wild-type counterparts. This observation corresponds with the fact that patients and other animal models carrying CACNA1A loss-of-function variants are afflicted with epilepsy28,36. Globally, the knockdown of cac could directly disrupt neuromuscular transmission in motor neurons. Although locomotion in adults and larvae was found to be non-predictive of seizure susceptibility, the contribution of motor neurons' cac in seizure behavior needs to be further investigated37. Consistently, in ex vivo calcium imaging, we noticed a greater proportion of calcium events in the mushroom body of cac knockdown flies than that of the wild-type (WT) flies, which indicated a higher frequency of neuronal firing9. The mushroom body of flies is considered loosely analogous to the hippocampus and cortex in the mammalian brain. Since seizures usually occur in the hippocampus and cortex of humans, the mushroom body is used for studying seizure-related neural calcium activity in this study. In conclusion, this study established the consistency of the bang-sensitive seizure-like assay and calcium imaging in Drosophila.
In the field of calcium imaging, utilization of the calcium indicator GCaMP6 in combination with confocal microscopy enables the monitoring of alterations in calcium concentration within various contexts such as in vivo, in cell cultures, in brain slices, and in ex vivo intact brain tissue of the Drosophila model. However, it is commonly understood that the in vivo imaging of calcium fluxes within the central nervous system of Drosophila necessitates a refined surgical intervention to achieve optical access to the brain; this hindrance may inhibit the practicality and progress of this technique in individual laboratories8,16. Zebrafish is also an important animal model to study epilepsy. The in vivo calcium imaging method was also established previously36. However, only zebrafish larvae can be used for in vivo imaging recording. On the other hand, although cell culture and brain slice models can provide higher-resolution images, the integrity of the brain and its neural networks cannot be fully replicated in cell culture or brain slices due to structural disruption. The current study addresses these issues by implementing isolated intact Drosophila brain tissues for calcium imaging, thus avoiding the need for complex surgical techniques and preserving the integrity of the brain and neural networks without disruption. The ex vivo approach also yields a superior signal-to-noise ratio when compared to in vivo imaging techniques.
The present study elucidates various pivotal procedures in the protocol concerning the generation of the tub-Gal4>UAS-GCaMP6m/cac-RNAi line flies. Initially, the double balancer played a crucial role in obtaining the desirable flies. Marked balancers allow researchers to follow alleles and chromosomes through complex multi-generational crosses38. Subsequently, for the efficient separation of the intact brain tissue and improved microscopic visualization, it was imperative to use Papain suspension to soften the surface fiber of the brain. Furthermore, to stabilize the brain and prevent neuronal damage during experimentation, the use of a C-sharp holder with nylon crosshairs was deemed necessary27.
In conclusion, calcium imaging applied here alongside the bang-sensitive seizure-like behavior assay in Drosophila is a powerful system for screening the epilepsy-associated genes and exploring the potential mechanism of epilepsy at the cellular level. Certainly, this technique cannot study the real-time calcium signals correlated to animal behavior like other in vivo methods.
Divulgaciones
The authors have nothing to disclose.
Acknowledgements
This work was supported by the Guangdong Basic and Applied Basic Research Foundation (grant no. 2022A1515111123 to Jing-Da Qiao) and plan to enhance scientific research in GMU (Jing-Da Qiao). This work was also supported by the Guangzhou Medical University Student Innovation Ability Enihancement Plan (Funding No. 02-408-2304-02038XM).
Materials
| Brushes | Panera | AAhc022-2 | for handling flies |
| Calcium chloride (CaCl2) | Sigma-Aldrich | C4901 | |
| Confocal microscope | SP8; Zeiss, Jena, Germany. | N/A | for calcium imaging |
| CO2 anesthesia machine | N/A | N/A | for Anesthetizing the flies. |
| C-sharp holder | N/A | N/A | handmade, for mounting the brain |
| Culture vials | Biologix | 51-0500 | 2.5 cm diameter, 9.5 cm height |
| Fiji software | National Institutes of Health, Bethesda, MD, USA | version: 2.14.0 | for analysis |
| Fly morgue | N/A | N/A | handmade, for handling flies |
| Fly stocks | cac-RNAi | 27244 | from Bloomington Drosophila Stock Center |
| Fly stocks | GCaMP6m | 42750 | from Bloomington Drosophila Stock Center |
| Fly stocks | tub-Gal4 | N/A | from the Sion-Frech Hoffmann Institute, Guangzhou Medical University |
| Glucose | Sigma-Aldrich | G8270 | |
| High-resolution camera | N/A | N/A | for recording the seizure-like behavior assay |
| L-lysine | Sigma-Aldrich | L5626 | |
| Magnesium chloride solution (MgCl2) | Sigma-Aldrich | M1028 | |
| Papain suspension | Worthington Biochemical | LS003126 | |
| Petri dishes | Sigma-Aldrich | SLW1480/02D | for dissection |
| Pipette | Thermo Scientific | 4640010, 4640030, 4640050, 4640060 | for transporting a measured volume of liquid and diseccected brain |
| Potassium chloride (KCl) | Sigma-Aldrich | P4504 | |
| Recording dish | Thermo Scientific | 150682- Glass Based Dish | for holding the brain and calcium imaging |
| Sodium bicarbonate (NaHCO3) | Sigma-Aldrich | S5761 | |
| Sodium chloride (NaCl) | Sigma-Aldrich | S5886 | |
| Sodium hydroxide (NaOH) | Fisher Scientific | S25550 | |
| Sodium phosphate monobasic (NaH2PO4) | Sigma-Aldrich | S8282 | |
| Stereo-binocular microscope | SHANG GUANG | XTZ-D | for handling flies and dissection |
| Syringe needles | pythonbio | HCL0693 | for dissection |
| Tripod | WEIFENG | 45634732523 | for recording the seizure-like behavior assay |
| Vortex mixer | Lab dancer, IKA, Germany/Sigma-Aldrich | Z653438 | for performing the seizure-like behavior assay |
| Whiteboard | N/A | N/A | handmade, foam pad or paper for background |
Referencias
- Thijs, R. D., Surges, R., O’brien, T. J., Sander, J. W. Epilepsy in adults. Lancet. 393 (10172), 689-701 (2019).
- Ellis, C. A., Petrovski, S., Berkovic, S. F. Epilepsy genetics: Clinical impacts and biological insights. Lancet Neurol. 19 (1), 93-100 (2020).
- Wang, J., et al. Epilepsy-associated genes. Seizure. 44, 11-20 (2017).
- Oliver, K. L., et al. Genes4epilepsy: An epilepsy gene resource. Epilepsia. 64 (5), 1368-1375 (2023).
- Rogawski, M. A., Loscher, W., Rho, J. M. Mechanisms of action of antiseizure drugs and the ketogenic diet. Cold Spring Harb Perspect Med. 6 (5), 022780 (2016).
- Ademuwagun, I. A., Rotimi, S. O., Syrbe, S., Ajamma, Y. U., Adebiyi, E. Voltage gated sodium channel genes in epilepsy: Mutations, functional studies, and treatment dimensions. Front Neurol. 12, 600050 (2021).
- Leweke, F. M., Louvel, J., Rausche, G., Heinemann, U. Effects of pentetrazol on neuronal activity and on extracellular calcium concentration in rat hippocampal slices. Epilepsy Res. 6 (3), 187-198 (1990).
- Yang, W., Yuste, R. In vivo imaging of neural activity. Nat Methods. 14 (4), 349-359 (2017).
- Hewapathirane, D. S., Dunfield, D., Yen, W., Chen, S., Haas, K. In vivo imaging of seizure activity in a novel developmental seizure model. Exp Neurol. 211 (2), 480-488 (2008).
- Ishimoto, H., Sano, H. Ex vivo calcium imaging for visualizing brain responses to endocrine signaling in drosophila. J Vis Exp. 136, 57701 (2018).
- Chen, T. W., et al. Ultrasensitive fluorescent proteins for imaging neuronal activity. Nature. 499 (7458), 295-300 (2013).
- Moisescu, D. G., Ashley, C. C., Campbell, A. K. Comparative aspects of the calcium-sensitive photoproteins aequorin and obelin. Biochim Biophys Acta. 396 (1), 133-140 (1975).
- Blinks, J. R., Prendergast, F. G., Allen, D. G. Photoproteins as biological calcium indicators. Pharmacol Rev. 28 (1), 1-93 (1976).
- Tian, L., et al. Imaging neural activity in worms, flies and mice with improved gcamp calcium indicators. Nat Methods. 6 (12), 875-881 (2009).
- Svoboda, K., Helmchen, F., Denk, W., Tank, D. W. Spread of dendritic excitation in layer 2/3 pyramidal neurons in rat barrel cortex in vivo. Nat Neurosci. 2 (1), 65-73 (1999).
- Rochefort, N. L., Jia, H., Konnerth, A. Calcium imaging in the living brain: Prospects for molecular medicine. Trends Mol Med. 14 (9), 389-399 (2008).
- Russell, J. T. Imaging calcium signals in vivo: A powerful tool in physiology and pharmacology. Br J Pharmacol. 163 (8), 1605-1625 (2011).
- Neher, E., Sakaba, T. Multiple roles of calcium ions in the regulation of neurotransmitter release. Neuron. 59 (6), 861-872 (2008).
- Streit, A. K., Fan, Y. N., Masullo, L., Baines, R. A. Calcium imaging of neuronal activity in drosophila can identify anticonvulsive compounds. PLoS One. 11 (2), 0148461 (2016).
- Parker, L., Howlett, I. C., Rusan, Z. M., Tanouye, M. A. Seizure and epilepsy: Studies of seizure disorders in drosophila. Int Rev Neurobiol. 99, 1-21 (2011).
- Del Valle Rodriguez, A., Didiano, D., Desplan, C. Power tools for gene expression and clonal analysis in drosophila. Nat Methods. 9 (1), 47-55 (2011).
- Liu, C. Q., et al. Efficient strategies based on behavioral and electrophysiological methods for epilepsy-related gene screening in the drosophila model. Front Mol Neurosci. 16, 1121877 (2023).
- Wang, Y., et al. Genetic manipulation of the odor-evoked distributed neural activity in the drosophila mushroom body. Neuron. 29 (1), 267-276 (2001).
- Wang, J., et al. Unc13b variants associated with partial epilepsy with favourable outcome. Brain. 144 (10), 3050-3060 (2021).
- Ganetzky, B., Wu, C. F. Indirect suppression involving behavioral mutants with altered nerve excitability in drosophila melanogaster. Genética. 100 (4), 597-614 (1982).
- Roemmich, A. J., Schutte, S. S., O’dowd, D. K. Ex vivo whole-cell recordings in adult drosophila brain. Bio Protoc. 8 (14), 2467 (2018).
- Gu, H., O’dowd, D. K. Whole cell recordings from brain of adult drosophila. J Vis Exp. (6), 248 (2007).
- Qiao, J., Yang, S., Geng, H., Yung, W. H., Ke, Y. Input-timing-dependent plasticity at incoming synapses of the mushroom body facilitates olfactory learning in drosophila. Curr Biol. 32 (22), 4869-4880 (2022).
- Liu, C. -. Q., Lin, Y. -. M., Zhang, X. -. X., Peng, R. -. C., Qiao, J. -. D. Protective effect of CACNA1A deficiency against seizure in the CACNA1A-CELSR2 digenic knockdown flies. Research Square. , (2023).
- Uchitel, O. D., Inchauspe, C. G., Urbano, F. J. D. i., Guilmi, M. N. Cav2.1 voltage activated calcium channels and synaptic transmission in familial hemiplegic migraine pathogenesis. J Physiol Paris. 106 (1-2), 12-22 (2012).
- Le Roux, M., et al. Cacna1a-associated epilepsy: Electroclinical findings and treatment response on seizures in 18 patients. Eur J Paediatr Neurol. 33, 75-85 (2021).
- Alehabib, E., et al. Clinical and molecular spectrum of p/q type calcium channel cav2.1 in epileptic patients. Orphanet J Rare Dis. 16 (1), 461 (2021).
- Li, X. L., et al. Cacna1a mutations associated with epilepsies and their molecular sub-regional implications. Front Mol Neurosci. 15, 860662 (2022).
- Indelicato, E., Boesch, S. From genotype to phenotype: Expanding the clinical spectrum of cacna1a variants in the era of next generation sequencing. Front Neurol. 12, 639994 (2021).
- Saras, A., Tanouye, M. A. Mutations of the calcium channel gene cacophony suppress seizures in drosophila. Plos Genetics. 12 (1), e1005784 (2016).
- Cozzolino, O., et al. Evolution of epileptiform activity in zebrafish by statistical-based integration of electrophysiology and 2-photon ca2+ imaging. Cells. 9 (3), 769 (2020).
- Mituzaite, J., Petersen, R., Claridge-Chang, A., Baines, R. A. Characterization of seizure induction methods in drosophila. eNeuro. 8 (4), (2021).
- Miller, D. E., Cook, K. R., Hawley, R. S. The joy of balancers. Plos Genetics. 15 (11), e1008421 (2019).

