The Micronucleus Assay on Cryopreserved Whole Blood
Summary
Here we present an optimized protocol for the cytokinesis-block micronucleus assay on cryopreserved whole blood samples. This optimized method of cryopreservation of whole blood for micronucleus analysis is a reliable technique for large-scale sampling and multi-center studies and can be used for other blood-related assays as well.
Abstract
The in vitro cytokinesis-block micronucleus (CBMN) assay is a widely used technique in radiobiology research, biological dosimetry, genotoxicity studies, and in vitro radiosensitivity testing. This cytogenetic method is based on the detection of micronuclei in binucleated cells resulting from chromosomal fragments lagging during cell division. Fresh whole blood samples are the most preferred sample type for the CBMN assay. However, the disadvantages of working with fresh blood samples include immediate processing after blood collection and the limited number of repeated analyses that can be performed without extra blood sampling.
As the need for fresh blood samples can be logistically challenging, CBMN assay on cryopreserved whole blood samples would be of great advantage, especially in large-scale patient studies. This paper describes a protocol to freeze whole blood samples and to perform the CBMN assay on these frozen blood samples. Blood samples from healthy volunteers have been frozen and thawed at different time points and then, subjected to a modified micronucleus assay protocol. The results demonstrate that this optimized procedure allows the performance of the CBMN assay on frozen blood samples. The described cryopreservation protocol may also be very useful for other cytogenetic assays and a variety of functional assays requiring proliferating lymphocytes.
Introduction
Ever since its discovery, the use of ionizing radiation (IR) has been a matter of debate among researchers because of its adverse effects on living beings. The detrimental effect is usually manifested by DNA damage such as double-stranded breaks (DSBs), and the failure to repair these DSBs leads to chromosomal aberrations and mutations, which are important hallmarks of cancer1,2. Such chromosomal aberrations can be examined by cytogenic assays such as the cytokinesis-block micronucleus (CBMN) assay. Micronuclei are lagging chromosomal fragments that cannot be incorporated into daughter nuclei and hence, are left behind during mitosis.
CBMN is a commonly used, reliable cytogenetic technique to assess chromosomal damage in individuals exposed to in vivo or in vitro ionizing radiation. Either fresh whole blood or isolated peripheral blood mononuclear cells (PBMCs) can be used in the CBMN assay. Fresh whole blood is mostly the biological material of choice since the isolation and processing of PBMCs can be time-consuming and is accompanied by a loss of serum plasma that acts as the supporting medium for cell survival and growth. To achieve a good binucleated cell yield, fresh whole blood should be processed immediately after collection. However, the need for immediate processing can be logistically challenging during time constraints. Moreover, when many samples are supposed to be acquired over an extended period or collected at points away from the processing centers, storage of fresh blood samples can be a limiting factor3,4.
Further, to allow repeated MN analysis in the same individual/patient, freezing of blood samples would be beneficial. One way to store lymphocytes for later application of the CBMN assay is by freezing isolated PBMCs5,6. This technique, however, requires several processing steps before the PBMCs can be frozen. Therefore, cryopreservation of whole blood would represent a simple and time-efficient alternative to the cryopreservation of isolated PBMCs. Little information is available concerning the use of frozen whole blood for cytogenetic assays or assays that require the proliferation of lymphocytes. Only one paper reports the use of cryopreserved whole blood for metaphase analysis7.
As cryopreservation of whole blood would offer many advantages in the field of biomonitoring, biodosimetry, and radiosensitivity assessment, our group optimized a cryopreservation protocol for whole blood that allows the application of the CBMN assay8. We demonstrated that lymphocytes present in cryopreserved whole blood cultures retain their genomic integrity and proliferation capacity for at least 1 year. In this methods paper, we describe in detail the cryopreservation procedure and CBMN assay protocol, which was optimized by Beyls et al.8, and report findings obtained for frozen blood samples of 30 healthy individuals. For the CBMN assay, the blood cultures were irradiated in vitro with doses of 0.5, 1, and 2 Gy to assess the MN response in lymphocytes of cryopreserved whole blood samples.
Protocol
For this study, blood samples were collected by venipuncture from 30 healthy donors aged between 17 and 65 years. MN data from 20 donors were reused from the paper of Beyls et al.8 Collection of the blood samples is in accordance with the guidelines of the Ethics Committee of Ghent University Hospital (registration number:2019/1565), Belgium. Written informed consent was obtained from all the participants. See the Table of Materials for details related to all materials and reagents used in this protocol.
1. Collection of blood samples
- Before starting, ensure that written informed consent of the participants is available and ethical guidelines are followed properly .
- Collect blood into Li-Heparin tubes and store at room temperature until ready for processing for cryopreservation.
2. Cryopreservation of whole blood
NOTE: All the steps until cell harvesting are performed aseptically in sterile laminar airflow.
- To cryopreserve 1 mL of blood, make 1 mL of freezing medium by mixing 800 µL of Fetal Calf Serum (FCS) and 200 µL of dimethyl sulfoxide (DMSO).
NOTE: The freezing medium is prepared in a volume equal to the blood samples that need to be frozen. - For cryopreservation of whole blood, transfer the blood sample from the heparinized tube to 15 or 50 mL centrifuge tubes
NOTE: The capacity of the tube depends on the volume of the blood. - Vortex the blood at low speed with continuous but dropwise addition of an equal volume of freezing medium.
- Transfer 2 mL of blood-freeze mix into cryovials (2 mL) where each aliquot has 1 mL of blood sample mixed with 1 mL of freezing mixture.
- Stepwise gradual freezing
- Transfer cryovials to a cryobox containing isopropanol and put in a freezer (-80 °C) for overnight.
- Transfer the cryovials to liquid nitrogen for long-term use.
3. Thawing of cryopreserved blood
NOTE: To limit the total time of the thawing process, a maximum of 8 cryovials (2 mL each) should be handled at a time.
- Preparation
- Prewarm 200 mL of sterile Phosphate-buffered Saline (PBS) to 37 °C before thawing the blood.
- Prepare a working solution of the required culture medium under sterile conditions. Ensure that medium per culture includes 1.6 mL of complete medium: Rosewell Park Memorial Institute (RPMI) media supplemented with penicillin/streptomycin (0.5%), 0.2 mL of FCS, 20 µL of sodium pyruvate, and 2 µL of β-mercaptoethanol.
NOTE: Since a significant number of blood cells can be lost while thawing, the thawed blood is cultured in small wells like 24-well plates with a total volume of 2 mL. One multi-well plate per dose is used. - For each culture well, indicate the donor code and A/B on the lid along with the date and dose, as suggested below (Figure 1).
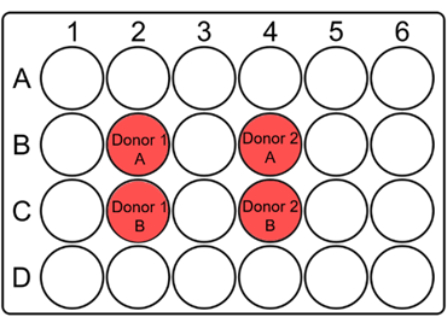
Figure 1: Suggested layout of a 24-well plate to culture whole blood for the micronucleus assay. Mark the wells and indicate the donor codes at appropriate places on the lid. For each patient sample, duplicate wells should be adjacent to each other. Note that it is a suggestive layout for a 10 x 10 collimator and can be changed according to the size of the collimator or samples. Please click here to view a larger version of this figure.
- Steps for thawing and culturing whole blood
- Warm the water bath to 37 °C.
- Take out the required cryovials from the liquid nitrogen (the number depends on the requirements such as the number of doses).
NOTE: One cryovial contains 1 mL of blood and 1 mL of freezing medium. Therefore, two cultures can be set up with blood from one cryovial. - Partially thaw the cryovials in the warm water bath (until small ice crystals are still seen).
- Take out the vials from the water bath and clean the outside of the vials with sterile tissue paper.
- In a sterile laminar airflow hood , open the vials very slowly to prevent air bubbles formed inside the tubes from spilling out.
- Resuspend the contents of each vial and individually transfer them to a 15 mL conical centrifuge tube (1 tube per vial).
- To the respective tubes, add dropwise 1 mL of warm (37 °C) PBS while gently swirling the tube. Resuspend with a P1000 pipette.
- For dilution and uniform washing, add dropwise 7 mL of prewarmed PBS (37 °C) and resuspend with a P1000 pipette.
- Centrifuge the blood for 8 min at 180 × g (with setting at acceleration 1, brake 1) at room temperature.
- During this waiting step, fill the empty wells (other than the one where cells will be cultured) with 500 µL of PBS.
- Place the well plates back in the incubator to prewarm.
- Once the centrifugation is completed, carefully remove the supernatant, leaving behind approximately 1 mL to avoid disturbing the cell pellet.
- Resuspend the pellet in the remaining supernatant with a P1000 pipette.
- Add dropwise 8 mL of warm PBS (37 °C) into the respective tubes while gently swirling the tubes. Afterwards, resuspend with a P1000 pipette.
- Centrifuge the resuspended blood for 8 min at 180 × g (with setting at acceleration 9, brake 9).
- During this waiting step, transfer 200 µL of culture medium to each of the marked wells.
- Keep the plates back in the incubator to remain at 37 °C.
- After centrifugation, remove the supernatant in a continuous motion (to avoid disturbing the loose cell pellet), but leave behind approximately 80 µL of the supernatant.
- To the pellet, add 280 µL of FCS (room temperature) and resuspend (to a total volume of 360 µL).
- Transfer 180 µL of the cell suspension to each well and resuspend (= 0.5 mL of "blood"/culture). The total volume in each well is now 380 µL, resulting in a 2 mm medium layer.
- Incubate the plates in a CO2 incubator at 37 °C for at least 10 min before irradiation.
4. G0 MN assay
- Take out the plates from the incubator and irradiate the cell culture with required doses such as 0.5, 1, and 2 Gy X-rays (220 kV, 13 mA, 0.15 mm Cu) at room temperature. Use sham irradiated samples as controls to identify spontaneous MN yields.
- Immediately after irradiation, add 1.62 mL of culture medium (37 °C) to each well.
- To stimulate cell division in T-lymphocytes, add 40 µL of Phytohemagglutinin (PHA) to each well and resuspend thoroughly.
- Place these plates back in the incubator (5% CO2, 37 °C).
NOTE: This will be the starting time of the assay. - Block the cytokinesis 23 h post stimulation by adding 8 µL of cytochalasin B (6 µg/mL) per well and resuspend properly.
- Harvest the cell cultures 70 h post stimulation/culture time. Resuspend and transfer the cells to a 15 mL tube. Rinse each well with 2 mL of PBS and add this PBS to the respective 15 mL tube.
- Centrifuge the tubes for 8 min at 180 × g, at room temperature.
- Discard the supernatant but leave approximately 500 µL over the pellet.
- Vortex the pellet (at full speed) and while vortexing, slowly add dropwise 2 mL of cold potassium chloride (KCl, 4 °C).
- Immediately centrifuge the cells at 180 × g for 8 min.
- Discard the supernatant, leaving approximately 500 µL of the supernatant.
- Vortex the pellet (at full speed) and while vortexing, slowly add dropwise 2 mL of cold fixative 1 (Methanol/Acetic acid/Ringer solution in a ratio of 4:1:5).
- Leave the sample tubes overnight at 4 °C (minimum 12 h and maximum 96 h).
- Centrifuge for 8 min at 180 × g.
- Discard the supernatant.
- Vortex the pellet (at full speed) and while vortexing, slowly add dropwise 2 mL of cold fixative 2 (methanol/acetic acid in a ratio of 4:1).
- Centrifuge for 8 min at 180 × g.
- Discard the supernatant.
- Vortex the pellet (at full speed) and then add slowly (dropwise), while vortexing, 2 mL of cold fixative 2 to the pellet.
- Leave the sample tubes at 4 °C (minimum 12 h and maximum 96 h).
- Clean the slides with isopropanol and label them properly.
NOTE: Use printed stickers to label as marker ink is easily wiped off while processing. - Centrifuge the sample tubes for 8 min at 180 × g.
- Transfer the supernatant to another tube to concentrate the cells according to the size of the pellet.
- Vortex the pellet.
- Drop 40 µL of the fixed cells on a dry and clean slide.
- Let the slides dry at room temperature. Store the slides in a box or stain immediately for observation.
5. Acridine orange (AO) staining
- Immerse the slides in AO stain for 1 min, followed by a quick wash in the distilled water, and then, place the slides in phosphate buffer (pH 6.8) for 1 min.
- Take out the slides from the buffer solution and with clean tissue paper, dry the back of the slides, and put the slides on a clean tissue paper.
- Drop 20 µL of the phosphate buffer on top of the slide and gently cover it with a clean coverslip, avoiding air bubbles.
- Seal these slides with silicone cement.
NOTE: Since AO is light-sensitive and fades over time, for a nice contrast and fluorescence, scoring of the slides is recommended within 5 days of staining. Always store slides in a cold room (4 °C), when not examined.
6. Scoring stained slides
- Place your slide under a fluorescence microscope and examine 1,000 binucleated cells (BNs). Manually count MNs, which are one of the six biomarkers of toxicity. To do so, have two independent scorers examine 500 BN cells/slide under the same magnification.
NOTE: Following are some highlights of the scoring criteria, as recommended by Fenech to score the stained slides9- Select a BN cell by looking for nicely rounded cells with intact cytoplasm, with two well-distinguished nuclei of approximately the same size, regular shape, and staining pattern. Ensure that the cytoplasm of the BN cell is distinguishable from the cytoplasm of the adjacent cell.
- Score a MN, noting that it is morphologically identical to but smaller than the main nuclei; even the largest MN cannot be more than 1/3 of the diameter of the main nuclei. Score a MN only if it does not touch the main nuclei or at least the micronuclear boundary is distinguishable from the boundary of the main nuclei.
Representative Results
To validate the reproducibility of the protocol, we performed the MN assay on cryopreserved blood samples of 30 healthy volunteers aged between 17 and 65 years. The mean age of the group is 35 years. The cryopreservation time ranged from 1 week to 154 weeks. After exposing the whole blood cell culture to different radiation doses (0.5, 1, and 2 Gy), the micronuclei yield in 1,000 BN cells was examined under a microscope. Nicely rounded binucleated cells (as can be seen in Figure 2) indicate a successful retrieval of healthy viable cells from cryopreserved whole blood samples. Of note, the radiation response of lymphocytes remained stable after long-term storage at ultra-low temperatures (liquid nitrogen). This observation is in line with the response expected from fresh blood samples.
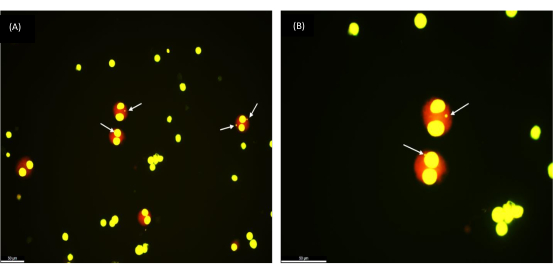
Figure 2: Micronuclei as observed in binucleated cells retrieved from a frozen whole blood sample that was cryopreserved 1 year ago. (A) Magnification 200x, (B) Magnification 400x; Arrows pointing to the MNs. Scale bar = 50 µm. Please click here to view a larger version of this figure.
With increased doses of radiation (0.5, 1, and 2 Gy), a linear-quadratic increase in micronucleus yield was observed (Table 1 and Figure 3). The MN yields in sham irradiated control samples (0 Gy) represent the background MN yields that are mainly the result of lagging chromosomes.
| Dose (Gy) | 0 | 0.5 | 1 | 2 |
| Average | 18 | 107 | 247 | 601 |
| SD | 10.8 | 23.9 | 53.3 | 101.1 |
| CV (%) | 59.9 | 22.3 | 21.6 | 16.8 |
| Range | 5-41 | 62-140 | 139-324 | 395-755 |
Table 1: Range and coefficient of variation along with average MN yield, as observed in cryopreserved whole blood samples of 30 healthy donors, indicative of inter-individual variability. Abbreviations: CV = coefficient of variation; SD = standard deviation.
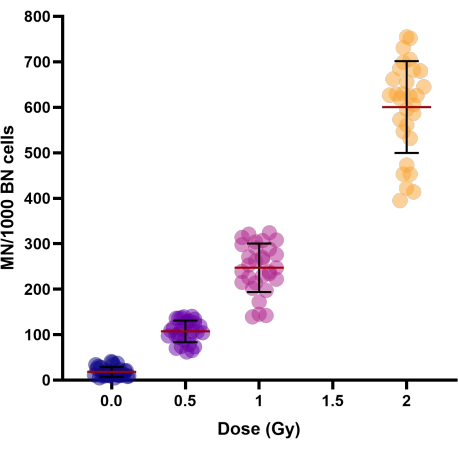
Figure 3: Micronuclei yields as observed in control and irradiated cryopreserved whole blood samples of 30 donors. Cryopreservation periods ranged from 1 week to 154 weeks. Scatter plot graph shows individual values. Lines in clusters represent the Mean ± SD of the group. Abbreviations: MN = micronuclei; BN = binucleated. Please click here to view a larger version of this figure.
To investigate the interindividual variation in the MN yield of persons, the coefficient of variation (CV) was calculated (see Table 1). For radiation-induced MN, a CV < 25% was obtained for all doses (0.5, 1, and 2 Gy), which indicates good reproducibility of the modified protocol.
Discussion
The modified protocol for the application of the CBMN assay is a relatively easy and convenient way to store blood samples in bulk. The procedure outlines all the minute but important details that need to be taken care of during cryopreservation and the CBMN assay. Other lab protocols normally use 10% DMSO in the freezing mixture, while our freezing mixture contains 20% DMSO along with 80% FCS7. As this freezing mixture is added in equal volumes to the whole blood sample, the final concentration is also 10% DMSO. To improve the cell survival rate and stimulate cell division, we add 1% sodium pyruvate and 0.1% beta-mercaptoethanol to the complete culture medium (cRPMI). This is in accordance with the cell-culture protocol established by our research group6,8.
Although the problem of cell clumping while thawing could not be resolved completely in this protocol, cell segregation was better than the other conventional protocols. Instead of consistent, abrupt addition of culture media to recover cells post thawing, we obtained better results when prewarmed PBS (37 °C) was added dropwise during the washing steps. This improvement helps in reducing cellular stress and minimizes clumping with a proven high recovery of cells. Furthermore, no clear difference in cell viability could be observed when PBS was used over RPMI. It has been validated by the group that the length of the cryopreservation period (up to 1 year) will not affect MN yields, both in irradiated and non-irradiated samples6,8. Studies suggest that good cell viability and proliferation of PBMCs can be achieved with gradual freezing at low temperatures (liquid nitrogen), followed by gradual addition of prewarmed medium while thawing10,11. Researchers have shown that the sub-populations of T-lymphocytes in thawed whole blood are comparable to those observed in thawed PBMCs12. Further, it is proven that cell subtypes recovered from cryopreserved whole blood samples are similar to those observed in fresh whole blood samples13,14.
If we examine the indicative results obtained in the report, we see that the linear quadratic increase with dose (Figure 3) is in agreement with other literature reports on Linear energy transfer (LET)-induced micronuclei15,16. The variability in MN yields observed in the cryopreserved whole blood samples (Table 1) are in range with the one reported for fresh whole blood cultures8,17,18. The protocol described in detail here was validated on fresh and cryopreserved whole blood of 20 healthy volunteers8. The nuclear division index (NDI), which is an important parameter of cell proliferation observed in that report8 was in agreement with the one suggested for fresh whole blood19,20. Taken together, this optimized protocol of cryopreservation of whole blood and modified micronucleus assay provides a better cell yield and hence, adaptation of this protocol is recommended for radiosensitivity assessments. Since the reproducibility of the protocol has already been validated8, it is suggested to have applicability in large-scale and multi-center studies.
Divulgaciones
The authors have nothing to disclose.
Acknowledgements
The authors would like to thank L. Pieters, T. Thiron, and G. De Smet for their technical support. We are thankful to all the volunteers who donated blood for the study. The work was financially supported by the Research Foundation- Flanders (FWO) under Grant (T000118N).
Materials
| 15 mL centrifuge tubes | Greiner | 188271 | |
| 24-well cell suspension plate | VWR | 734-2779 | |
| 96% alcohol | ChemLab | CL00.1807.2500 | |
| Acetic acid | Merck life science | 8,18,75,52,500 | |
| Acridine orange | Merck life science | 235474-5g | |
| CaCl2 | Merck life science | C5670-100g | |
| cover slips | VWR | 631-1365 | 22 x 50 |
| Cryobox (Mr.Frosty) | Nalgene, Sigma Aldrich | ||
| Cryovials 2ml | Novolab | A04573 | |
| Cytochalsin B | Merck life science | C6762-10 | 10 mg |
| Dimethyl sulphoxide (DMSO) | Merck life science | D4540-500ml | |
| Fetal calf serum (FCS) | Thermo Fischer scientific | 10270-106 | |
| Fixative 1 | Methanol/acetic acid/ringer in a ratio of 4:1:5 | ||
| Fixative 2 | Methanol/acetic acid in a ratio of 4:1 | ||
| GURR buffer | Thermo Fischer scientific | 10582013 | phosphate buffer (pH 6.8) |
| KCl | Merck life science | 1,04,93,60,250 | 75 mM |
| KH2PO4 | Merck life science | 1,04,87,30,250 | |
| Li-heparin tubes | BD Life sciences | 367526-LH170 I.U. | BD Vacutainer |
| Methanol | fisher scinetific | M/4000/17 | |
| Na2HPO2 | Merck life science | 10,65,80,500 | |
| NaCl | Merck life science | S7653-1kg | |
| Object slides | VWR | MENZAA00000112E04 | |
| Penicillin/Streptomycin | Thermo Fischer scientific | 15140-122 | 10,000 U/mL + 10,000 µg/mL |
| Phytohemagglutinin (PHA-M) | Thermo Fischer scientific | 10576-015 | |
| Ringer solution | contains NaCl, KCl, CaCl2 dissolved in distilled water | ||
| RPMI-1640 | Thermo Fischer scientific | 52400041 | |
| Silicon rubber adhesive sealent | collall | CF-100 | |
| Sodium pyruvate | Thermo Fischer scientific | 11360039 | |
| Sterile warm PBS (37 °C) | contains NaCl, Na2HPO2, KH2PO4 dissolved in distilled water | ||
| β-mercaptoethanol | Thermo Fischer scientific | 31350-010 |
Referencias
- Grade, M., Difilippantonio, M. J., Camps, J. Patterns of chromosomal aberrations in solid tumors. Recent Results in Cancer Research. 200, 115-142 (2015).
- Jiao, Y., Cao, F., Liu, H. Radiation-induced cell death and its mechanisms. Health Physics. 123 (5), 376-386 (2022).
- Roederer, M., et al. The genetic architecture of the human immune system: a bioresource for autoimmunity and disease pathogenesis. Cell. 161 (2), 387-403 (2015).
- Bankoglu, E. E., et al. Effect of cryopreservation on DNA damage and DNA repair activity in human blood samples in the comet assay. Archives of Toxicology. 95, 1831-1841 (2021).
- Zijno, A., Saini, F., Crebelli, R. Suitability of cryopreserved isolated lymphocytes for the analysis of micronuclei with the cytokinesis-block method. Mutagenesis. 22, 311-315 (2007).
- Sioen, S., Cloet, K., Vral, A., Baeyens, A. The cytokinesis-block micronucleus assay on human isolated fresh and cryopreserved peripheral blood mononuclear cells. Journal of Personalized Medicine. 10 (3), 125 (2020).
- Cheng, L., Wang, L. E., Spitz, M. R., Wei, Q. Cryopreserving whole blood for functional assays using viable lymphocytes in molecular epidemiology studies. Cancer Letters. 166 (2), 155-163 (2001).
- Beyls, E., Baeyens, A., Vral, A. The cytokinesis-block micronucleus assay for cryopreserved whole blood. International Journal of Radiation Biology. 97 (9), 1252-1260 (2021).
- Fenech, M. Cytokinesis-block micronucleus cytome assay. Nature Protocols. 2 (5), 1084-1104 (2007).
- Ramachandran, H., et al. Optimal thawing of cryopreserved peripheral blood mononuclear cells for use in high-throughput human immune monitoring studies. Cells. 1 (3), 313-324 (2012).
- Hønge, B. L., Petersen, M. S., Olesen, R., Møller, B. K., Erikstrup, C. Optimizing recovery of frozen human peripheral blood mononuclear cells for flow cytometry. PLoS One. 12 (11), e0187440-e0187517 (2017).
- Alam, I., Goldeck, D., Larbi, A., Pawelec, G. Flow cytometric lymphocyte subset analysis using material from frozen whole blood. Journal of Immunoassay and Immunochemistry. 33 (2), 128-139 (2012).
- Langenskiöld, C., Mellgren, K., Abrahamsson, J., Bemark, M. Determination of blood cell subtype concentrations from frozen whole blood samples using TruCount beads. Cytometry Part B: Clinical Cytometry. 94B, 660-666 (2018).
- Braudeau, C., et al. An easy and reliable whole blood freezing method for flow cytometry immuno-phenotyping and functional analyses. Cytometry Part B: Clinical Cytometry. 100 (6), 652-665 (2021).
- Thierens, H., Vral, A., De Ridder, L. Biological dosimetry using the micronucleus assay for lymphocytes: interindividual differences in dose response. Health Physics. 61 (5), 623-630 (1991).
- Vral, A., Fenech, M., Thierens, H. The micronucleus assay as a biological dosimeter of in vivo ionising radiation exposure. Mutagenesis. 26 (1), 11-17 (2011).
- Vral, A., Thierens, H., Baeyens, A., De Ridder, L. The micronucleus and G2-phase assays for human blood lymphocytes as biomarkers of individual sensitivity to ionizing radiation: limitations imposed by intraindividual variability. Radiation Research. 157 (4), 472-477 (2002).
- Pajic, J., et al. Inter-individual variability in the response of human peripheral blood lymphocytes to ionizing radiation: comparison of the dicentric and micronucleus assays. Radiation and Environmental Biophysics. 54, 317-325 (2015).
- Fenech, M., et al. Intra- and inter-laboratory variation in the scoring of micronuclei and nucleoplasmic bridges in binucleated human lymphocytes: results of an international slide-scoring exercise by the HUMN project. Mutation Research. 534 (1-2), 45-64 (2003).
- Miszczyk, J., Rawojć, K. Effects of culturing technique on human peripheral blood lymphocytes response to proton and X-ray radiation. International Journal of Radiation Biology. 96 (4), 424-433 (2020).

