Harvest and Primary Culture of Leptomeningeal Lymphatic Endothelial Cells
Summary
Leptomeningeal lymphatic endothelial cells (LLECs), a recently identified intracranial cell type, have poorly understood functions. This study presents a reproducible protocol for harvesting LLECs from mice and establishing in vitro primary cultures. This protocol is designed to enable researchers to delve into the cellular functions and potential clinical implications of LLECs.
Abstract
Leptomeningeal lymphatic endothelial cells (LLECs) are a recently discovered intracranial cellular population with a unique distribution clearly distinct from peripheral lymphatic endothelial cells. Their cellular function and clinical implications remain largely unknown. Consequently, the availability of a supply of LLECs is essential for conducting functional research in vitro. However, there is currently no existing protocol for harvesting and culturing LLECs in vitro.
This study successfully harvested LLECs using a multi-step protocol, which included coating the flask with fibronectin, dissecting the leptomeninges with the assistance of a microscope, enzymatically digesting the leptomeninges to prepare a single-cell suspension, inducing the expansion of LLECs with vascular endothelial growth factor-C (VEGF-C), and selecting lymphatic vessel hyaluronic receptor-1 (LYVE-1) positive cells through magnetic-activated cell sorting (MACS). This process ultimately led to the establishment of a primary culture. The purity of the LLECs was confirmed through immunofluorescence staining and flow cytometric analysis, with a purity level exceeding 95%. This multi-step protocol has demonstrated reproducibility and feasibility, which will greatly facilitate the exploration of the cellular function and clinical implications of LLECs.
Introduction
The newly discovered leptomeningeal lymphatic endothelial cells (LLECs) form a meshwork of individual cells within the leptomeninges, exhibiting a distinct distribution pattern when compared to peripheral lymphatic endothelial cells1,2. The cellular functions and clinical implications associated with LLECs remain largely uncharted territory. In order to pave the way for functional research on LLECs, it is imperative to establish an in vitro model for their study. Therefore, this study has devised a comprehensive protocol for the isolation and primary culture of LLECs.
Mice are the preferred animal model due to their suitability for genetic manipulation in disease research. Previous studies have successfully isolated lymphatic endothelial cells from various mouse tissues, including lymph nodes3, mesenteric tissue4, dermal tissue5, collecting lymphatics6, and lung tissue7. These isolation procedures have primarily relied on techniques such as magnetic-activated cell sorting (MACS) and flow cytometry sorting8,9,10. Additionally, research efforts have led to the establishment of rat arachnoid cell lines and rat lymphatic capillary cell lines11,12. Despite the existence of explant culture techniques for leptomeninges13, there exists an urgent need for a standardized protocol for the isolation and culture of LLECs. Consequently, this study has successfully harvested and cultured LLECs by meticulously dissociating leptomeninges under the guidance of a microscope and promoting LLECs expansion through the use of vascular endothelial growth factor-C (VEGF-C). The distinctive marker for lymphatic endothelial cells is lymphatic vessel hyaluronic receptor-1 (LYVE-1)14. This multi-step protocol selectively isolates LYVE-1-positive LLECs using MACS and subsequently verifies their purity through flow cytometric analysis and immunofluorescent staining.
The primary steps of this multi-step protocol can be summarized as follows: flask coating, dissociation of leptomeninges, enzymatic digestion of leptomeninges, cell expansion, magnetic cell selection, and subsequent culture of LLECs. Finally, the purity of the isolated LLECs is confirmed through flow cytometric analysis and immunofluorescent staining. The overarching aim of this study is to present a reproducible, multi-step protocol for the isolation of LLECs from mouse leptomeninges and their subsequent in vitro culture. This protocol is poised to greatly facilitate investigations into the cellular functions and clinical implications of LLECs.
Protocol
This research received approval from the Animal Experiment Ethics Committee of Kunming Medical University (kmmu20220945). All experiments adhered to established animal care guidelines. Leptomeningeal lymphatic endothelial cells (LLECs) were harvested from male C57Bl/6J mice aged 6-8 weeks and weighing between 20-25 g. These mice were procured from Kunming Medical University in Kunming, China. The entire experimental procedure was conducted under strict sterile conditions. All the centrifugation steps are performed at room temperature unless otherwise specified.
1. Preparation of reagents and instruments
NOTE: All steps involving solutions must be conducted within a class II biohazard cabinet.
- Prepare the washing buffer by mixing phosphate-buffered saline (PBS) with calcium and magnesium, 10% fetal bovine serum (FBS), and 1% penicillin-streptomycin (P/S) (see Table of Materials). Keep this buffer cold at 4 °C. It is essential to prepare it fresh on the day of use and degas the buffer.
- Prepare the digestive enzymes by combining 10 mL of PBS (without calcium and magnesium) with 2 mL of 0.25% trypsin, 1 mL of 20 mg/mL papain, and 200 µL of 1 mg/mL collagenase I (see Table of Materials).
NOTE: Ensure the use of aliquots of appropriate volumes to prevent repeated freeze-thaw cycles. Store these aliquots at -20 °C. - Prepare the culture medium by utilizing the commercially available endothelial cell growth medium kit, which includes VEGF-C (100 ng/mL) and 1% P/S (see Table of Materials).
- Prepare the stopping buffer by supplementing Dulbecco's modified Eagle's medium (DMEM) with 10% FBS to stop the digestion process.
- Prepare sterile surgical instruments: these instruments should include scissors, serrated-tip tweezers, and fine-point tweezers.
2. Flask coating
- Coat the T25 flask with a 100 µg/mL fibronectin solution (see Table of Materials) and incubate overnight at 37 °C. Prior to use, remove the coating solution using a pipette.
- Wash the flask three times with PBS, subsequently aspirate the PBS, and allow the T25 flask to air dry.
3. Leptomeninges dissociation
NOTE: Always use pre-cooled buffers and solutions at 4 °C.
- Anesthetize the mice with excess inhalation of 4% isoflurane and then sacrifice them by swift decapitation using scissors that have been previously cleaned with 70% ethanol.
- Carefully incise the skin along the midline, starting from the opening at the back of the skull and extending toward the frontal area.
- Delicately remove the skull, lifting it gently with the scissors to avoid damaging the leptomeninges, ensuring the entire brain, including the leptomeninges, is obtained.
- Submerge the whole brain in a washing buffer and gently flush it to remove surface blood.
- Transfer the brain into a sterile Petri dish without chopping, and extract the leptomeninges from the brain's surface using fine-point tweezers under a microscope.
- Cut the leptomeninges tissue into fragments using sterile micro-scissors.
4. Leptomeninges enzymatic digestion
- Add 10 mL of the enzyme mix (step 1.2) to the fragments and incubate at 37 °C for 15 min. Gently agitate to ensure the fragments detach from the bottom of the tube. Afterward, add 10 mL of stopping buffer (step 1.4) to halt the digestion.
- Centrifuge at 300 x g for 5 min at 4 °C and carefully remove the supernatant using a pipette.
- Add 10 mL of cold PBS, and pass the mixture through a 70 µm strainer into a sterile 50 mL tube to filter out any clumps.
5. Cell expansion
- Plate 1 x 105 cells per cm2 into a fibronectin-coated T25 flask (step 2) with 5 mL of culture medium.
- Incubate the cells at 37 °C with 5% CO2 for 24 h. Afterward, remove the medium to eliminate non-attached cells.
- Sustain the culture by replacing 50% of the medium every two days. Repeat this process two or three times.
6. Magnetic cell selection
NOTE: Work swiftly, maintain cell coldness, and utilize pre-cooled solutions to prevent non-specific cell labeling.
- Preparation of instruments and reagents: attach a magnetic separator to a magnetic separator stand (see Table of Materials). Connect a selection column to the magnetic separator, and position a 70 µm cell strainer atop the selection column. Place a 50 mL tube beneath the selection column to collect the flow.
- Enzymatic digestion: once the cells reach 80% confluence, aspirate the medium and rinse the cells with PBS. Add 0.25% trypsin to detach adherent cells and incubate at 37 °C for 5 min. Stop the digestion by adding the stopping buffer. Centrifuge the cells at 300 x g for 5 min at room temperature and remove the supernatant.
- Antibody incubation: resuspend 1 x 107 total cells in 100 µL of PBS and add 10 µL of LYVE-1 antibody (see Table of Materials). Mix thoroughly and incubate for 30 min in the dark at 4 °C.
- Spin the cells at 300 x g for 5 min and discard the supernatant. Rinse the cells by introducing 1 mL of PBS and centrifuging at 300 x g for 5 min. Completely remove the supernatant.
- Microbeads labeling: resuspend the cells in 100 µL of PBS and add 20 µL of Microbeads (see Table of Materials). Mix well and incubate for 30 min in the dark at 4 °C.
- Spin the cells at 300 x g for 5 min and decant the supernatant. Subsequently, cleanse the cells with 1 mL of PBS through centrifugation at 300 x g for 5 min. Finally, completely remove the supernatant.
- Magnetic negative exclusion: resuspend the cells in 4 mL of PBS. Pass the cells through a 70 µm cell strainer to eliminate clumps. Prepare the selection column (see Table of Materials) by rinsing it with 3 mL of PBS, then apply the cell suspension into the selection column.
- Perform washing steps with 3 mL of PBS and collect LYVE-1-negative cells into a 50 mL tube positioned below the selection column to allow them to pass through.
- Magnetic positive selection: pipette 6 mL of PBS into the selection column. Instantly flush out the magnetically labeled cells by firmly pushing the plunger into the selection column to obtain LYVE-1-positive LLECs.
NOTE: Always wait until the selection column reservoir is empty before proceeding to the next step.
7. LLECs culture
- Centrifuge the LYVE-1-positive LLECs at 300 x g for 5 min, and then carefully remove the supernatant.
- Plate 1 x 105 cells per cm2 into a fibronectin-coated T25 flask with 5 mL of culture medium.
- Maintain the culture by replacing 50% of the medium every other day. When the cells reach 80% confluence, detach the cells with 0.25% trypsin and perform cell passage. Utilize the cells for subsequent experiments after 2-3 passages.
8. Flow cytometric analysis
NOTE: The Flow cytometric analysis was conducted following the previously described procedure15.
- Add 0.25% trypsin to detach adherent cells and incubate at 37 °C for 5 min. Then, add the stopping buffer to halt the digestion.
- Centrifuge the cells at 300 x g for 5 min and completely aspirate the supernatant. Resuspend 1 x 106 cells in 100 µL of PBS.
- Add 1 µL of LYVE-1 antibody. Mix thoroughly and incubate for 10 min in the dark at room temperature.
- Resuspend the cells in an appropriate amount of PBS buffer for analysis using flow cytometry and FlowJo software (see Table of Materials).
9. Immunofluorescent staining
NOTE: The immunofluorescent staining was conducted following the procedure described previously16.
- Wash the cells with PBS three times. Fix the cells with 4% paraformaldehyde (PFA) for 10 min at room temperature. Discard the PFA and wash the cells with PBS three times.
- Apply the block buffer for 1 h at room temperature. Remove the block buffer from the coverslips.
- Add the LYVE-1 antibody in the staining buffer to the cells and incubate in the dark. Wash the cells three times with PBS.
- Add an appropriate secondary antibody (see Table of Materials) in the staining buffer to the cells and incubate in the dark. Wash the cells three times with PBS.
- Counterstain with 4',6-diamidino-2-phenylindole (DAPI). The cells are now ready for immunofluorescence microscopy.
Representative Results
This study presents a reproducible, multi-step protocol for harvesting lymphatic endothelial cells (LLECs) from mice and subsequently establishing their primary culture in vitro. The key steps involve flask preparation and fibronectin coating, dissociation of leptomeninges, obtaining a single-cell suspension through enzymatic digestion, and inducing LLECs expansion with VEGF-C. LYVE-1-positive LLECs are then selectively isolated using magnetic-activated cell sorting (MACS). Finally, immunofluorescence staining and flow cytometric analysis are performed to assess LLECs purity, with MTT assay results demonstrating robust LLECs growth rates (Supplementary Figure 1). The primary steps of this multi-procedural protocol are illustrated in the flowchart, with the entire process taking approximately 2-3 weeks (Figure 1).
To harvest LLECs, it is essential to dissociate leptomeninges and promote LLECs expansion while preserving cell viability through efficient surgical techniques and maintaining a cold environment (Figure 2A-E). After obtaining the whole brain along with the leptomeninges under sterile conditions, a gentle flush washing step is performed to remove blood-red cells (Figure 2F). Next, leptomeninges are carefully extracted from the brain's surface under a microscope (Figure 2G). These leptomeninges primarily consist of collagen fibers, so they are fragmented to expedite enzymatic digestion (Figure 2H). Subsequently, enzymatic digestion of these fragments is carried out, and any remaining clumps are filtered through a 70 µm strainer to eliminate larger clusters of collagen fibers (Figure 2I). Finally, cells are plated in a fibronectin-coated T25 flask, and VEGF-C is added to induce expansion (Figure 2J). After 24 h, the culture medium is removed to eliminate non-attached cells. These steps facilitate the harvesting of cells from leptomeninges and promote expansion, which is critical for obtaining sufficient LYVE-1-positive LLECs later in the process.
While VEGF-C induces LLEC expansion, heterogeneous cellular populations can still grow together. To isolate pure LYVE-1-positive LLECs, MACS is employed as LYVE-1 is a recognized marker for lymphatic endothelial cells. The purity of LLECs is assessed through flow cytometry, with results indicating that the percentage of LYVE-1-positive cells in passage 2 is not significantly different from passage 3 after MACS, demonstrating a purity greater than 95% (Figure 3A). To further validate the specificity of LYVE-1-positive LLECs, three additional lymphatic endothelial cell markers-podoplanin (PDPN), vascular endothelial growth factor receptor-3 (VEGFR-3), and Prospero-related homeobox1 (PROX1) were used for identification17,18,19. Immunofluorescence staining confirmed that LYVE-1 co-stained with these three markers (Figure 3B). Moreover, the identity of LLECs was established in brain slices under physiological conditions, showing co-staining of LYVE-1 with PROX1, VEGFR-3, and PDPN (Supplementary Figure 2A). LYVE-1-positive cells did not express F4/80 and Platelet-Derived Growth Factor beta (PDGFR-β), effectively distinguishing LLECs from macrophages and fibroblasts (Supplementary Figure 2B). In summary, this protocol allows the harvesting of highly pure LLECs capable of in vitro culture.
Leptomeningeal cells before MACS exhibit heterogeneity, differing from the colonies of lymphatic endothelial cells. Their morphology ranges from single round spheres to fused fiber shapes (Figure 4A-D). However, post-MACS, LLECs exhibit typical spindle and cobblestone-shaped endothelial-like features (Figure 4E-H). Based on these results, LLECs are effectively harvested through this multi-procedural protocol and maintained in a healthy growth state.
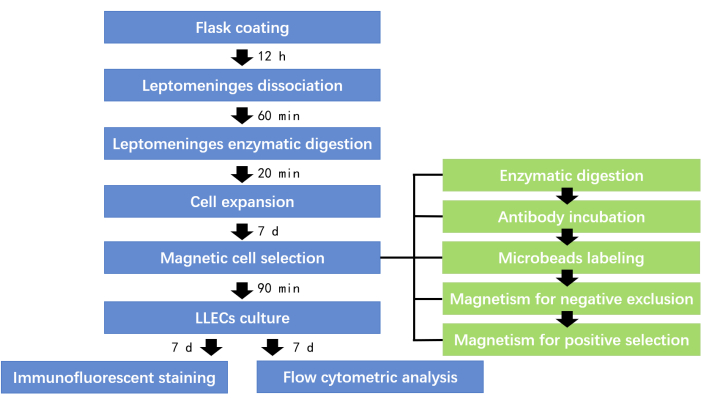
Figure 1: Schematic representation of the multi-procedural protocol. This flowchart illustrates the multi-procedural protocol for the harvesting and culture of LLECs. Please click here to view a larger version of this figure.
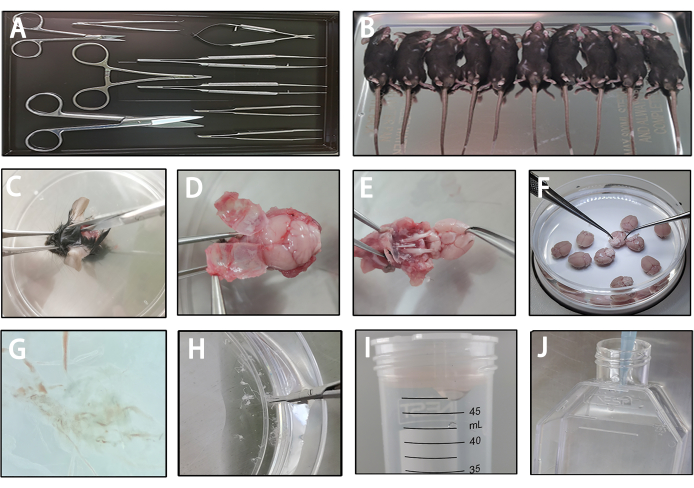
Figure 2: Harvesting of leptomeninges cells. (A) Preparation of sterile surgical instruments. (B) Mice were anesthetized via inhalation of 4% isoflurane and subsequently cleaned with 70% ethanol after euthanization. (C) Decapitation of the mice and careful midline incision of the skin, starting from the back of the skull towards the frontal area. (D) Delicate removal of the skull to preserve the integrity of the leptomeninges. (E) Retrieval of the entire brain containing the leptomeninges. (F) Washing of the whole brain in a buffer solution with gentle flushing to remove surface blood. (G) Dissection of leptomeninges from the brain's surface using fine-point tweezers. (H) Cutting of leptomeninges into fragments with sterile micro-scissors, followed by the addition of 10 mL of enzyme mix and incubation at 37 °C for 15 min. (I) Resuspension of the pellet in 10 mL of PBS and filtration of clumps through a 70 µm strainer. (J) Plating of cells into a coated T25 flask. Please click here to view a larger version of this figure.
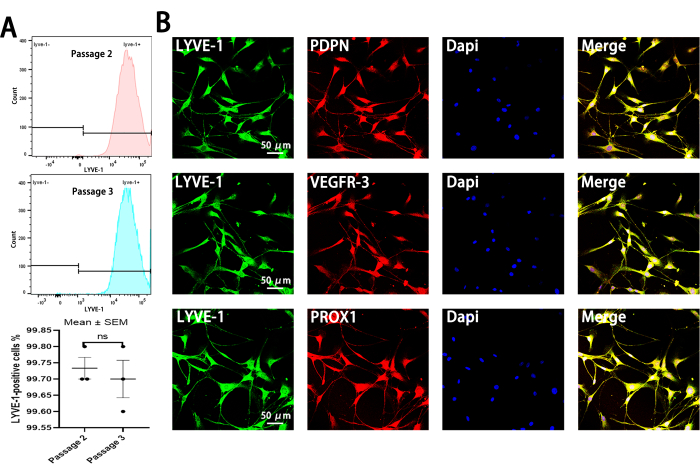
Figure 3: Assessment of LLEC purity after MACS. (A) Representative histograms from flow cytometric analysis demonstrating the expression of LYVE-1-positive cells in passages 2 and 3 following MACS. The percentage of LYVE-1-positive cells exceeds 95% after MACS. Data are representative of three independent experiments (Mean ± SEM). Error bars indicate SEM. (B) Representative immunofluorescence image showing co-staining of LYVE-1 with PDPN, VEGFR-3, and PROX1. Scale bars = 50 µm. Please click here to view a larger version of this figure.
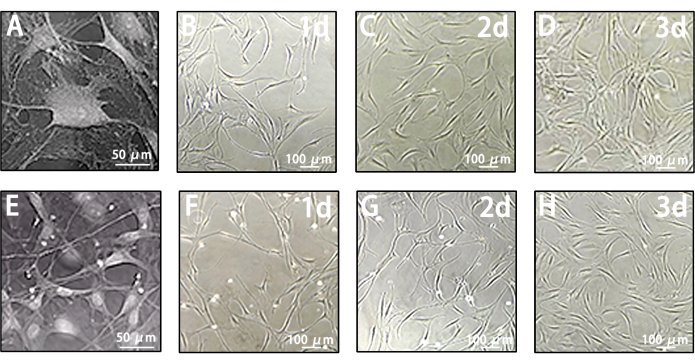
Figure 4: Growth characteristics of leptomeningeal cells and LLECs. (A) Morphology of leptomeningeal cells captured in representative images. Scale bar = 50 µm. (B–D) Representative images depicting the morphology of leptomeningeal cells on day 1, day 2, and day 3 prior to MACS. Scale bars = 100 µm. (E) Morphology of LLECs captured in representative images. Scale bar = 50 µm. (F–H) Representative images illustrating the morphology of LLECs on day 1, day 2, and day 3 following MACS. Scale bars = 100 µm. Please click here to view a larger version of this figure.
Supplementary Figure 1: Growth rate of LLECs and SVEC4-10. MTT assay results show a significant decrease in the growth rate of LLECs in passage 1 compared to the SVEC4-10 group. There were no significant differences between the passage 2 and passage 3 LLECs groups compared to the SVEC4-10 group. Data represent results from six independent experiments (Mean ± SEM). Error bars indicate SEM. Please click here to download this File.
Supplementary Figure 2: Characterization and discrimination of LLECs in brain slices. (A) Representative immunofluorescence image illustrating co-staining of LYVE-1 with PROX1, VEGFR-3, and PDPN in brain slices under physiological conditions. Scale bars = 2 µm. (B) Representative immunofluorescence image demonstrating that LYVE-1 does not co-stain with F4/80 and PDGFR-β in brain slices under physiological conditions. Scale bars = 20 µm. Please click here to download this File.
Supplementary Figure 3: Growth of LLECs under VEGF-C stimulation. (A) Representative images of LLECs under 1 ng/mL VEGF-C stimulation. (B) Representative images of LLECs under 100 ng/mL VEGF-C stimulation. (C) Representative images of LLECs under 500 ng/mL VEGF-C stimulation. Scale bars = 50 µm. Please click here to download this File.
Supplementary Figure 4: Expression of CD31, PDPN, and VEGFR-3 after harvesting. (A) Representative histograms from flow cytometric analysis show that CD31-positive cells are less than 5%. (B) Representative histograms from flow cytometric analysis indicate that PDPN-positive cells exceed 95%. (C) Representative histograms from flow cytometric analysis demonstrate that VEGFR-3-positive cells exceed 95%. Please click here to download this File.
Supplementary Figure 5: Morphology of LLECs and distinction from CD31. (A) Representative images of LLECs morphology revealed by hematoxylin-eosin staining. Scale bar = 20 µm. (B) Representative immunofluorescence images illustrate that LYVE-1 and PDPN do not co-stain with CD31. Scale bars = 50 µm. Please click here to download this File.
Supplementary Figure 6: Proliferation rate of LLECs. Representative results from the CCK-8 assay show a significant decrease in the proliferation rate of LLECs in passage 1 and passage 6 compared to passages 2 and 5, respectively. Data represent results from six independent experiments (Mean ± SEM). Error bars indicate SEM. Please click here to download this File.
Discussion
The existing protocol for harvesting and culturing LLECs in vitro has not been previously reported. This study introduces a reproducible, multi-procedural protocol for harvesting and culturing LLECs from mouse leptomeninges.
While this multi-procedural protocol is reproducible, there are several key considerations. For example, fibronectin-coated T25 flasks promote the adhesion of LLECs and function by eliminating non-adherent cells, thereby ensuring a more homogenous cellular population. Additionally, the time duration and temperature during surgical procedures for dissociating leptomeninges are critical factors affecting cell viability. Therefore, it is crucial to maintain a time duration within 1 h and keep the buffer sufficiently cold. Another critical point pertains to the presence of red blood cells in the leptomeninges, from which the release of hemoglobin may harm LLECs. To mitigate this, it is essential to gently flush and wash to remove red blood cells, preventing their cytotoxic effects. Finally, LLECs require stimulation with VEGF-C20, particularly at a concentration of 100 ng/mL. Under this stimulation, LLECs exhibit characteristic endothelial-like morphology rather than forming tubes (Supplementary Figure 3A-C). Consequently, this study employed a culture medium containing 100 ng/mL VEGF-C to induce expansion and maintain homogeneity in cellular populations.
The multi-procedural protocol described herein comes with troubleshooting steps and some limitations. Firstly, this protocol takes 2-3 weeks to complete, making it impractical for frequent repetition. Secondly, surgical and enzymatic procedures may result in the presence of dead cells that are not eliminated before plating, potentially affecting cell culture. This possibility will be explored in future protocol implementations. Thirdly, while the purity of harvested LLECs (VEGFR-3 and PDPN-positive cells) exceeds 95%, some heterogeneous cell populations persist, including CD31-positive cells, which constitute less than 5% (Supplementary Figure 4A-C). This is a common challenge in the culture of primary cells. High-resolution images from hematoxylin-eosin staining demonstrate that LLECs display characteristic endothelial-like shapes but do not express CD31 (Supplementary Figure 5A,B). Lastly, CCK-8 assay results indicate a significant decrease in the proliferation rate in LLECs at passage 1 and 6 compared to passage 2 and 5, respectively (Supplementary Figure 6). Passage 1 may lead to LLECs damage due to antibody treatment and the physical stress during sorting, while passage 6 shows reduced proliferation as the number of passages increases. Therefore, subsequent cells after passage 6 are unsuitable for relevant experiments. Given the distribution of fibroblasts in the meninges21, approximately 5% of contaminated cells may contain fibroblasts. To address this, the addition of geneticin and immunomagnetic cell sorting can be used to remove contaminated fibroblasts22,23. Monitoring the contamination rate of fibroblasts in each passage of LLECs will help eliminate the impact of fibroblasts on LLECs' growth24. Considering the heterogeneity between mice and humans, the harvesting and culturing of primary human LLECs should be pursued for potential clinical applications in future research.
In summary, there is currently no established protocol for harvesting and culturing LLECs in vitro. This study introduces a reproducible, multi-procedural protocol for harvesting LLECs from mice leptomeninges and subsequently establishing their primary culture in vitro. This work will facilitate further exploration of cellular functions and potential clinical applications for LLECs.
Divulgaciones
The authors have nothing to disclose.
Acknowledgements
The study was supported by grants from the National Natural Science Foundation of China (81960226, 81760223), the Natural Science Foundation of Yunnan Province (202001AS070045, 202301AY070001-011), and the Scientific Research Foundation of Yunnan Province Department of Education (2023Y0784).
Materials
| Block buffer | Beyotime | P0102 | Store aliquots at –4 °C |
| Collagenase I | Solarbio | C8140 | Store aliquots at –20 °C |
| DAPI | Beyotime | P0131 | Store aliquots at –20 °C |
| DMEM | Solarbio | 11995 | Store aliquots at –4 °C |
| D-PBS | Solarbio | D1041 | Store aliquots at –4 °C |
| EGM-2 MV Bullet Kit | Lonza | C-3202 | Store aliquots at –4 °C |
| FBS | Solarbio | S9010 | Store aliquots at –20 °C |
| Fibronectin | Solarbio | F8180 | Store aliquots at –20 °C |
| FlowJo Software | BD Biosciences | V10.8.1 | |
| LYVE-1 antibody | eBioscience | 12-0443-82 | Store aliquots at –4 °C |
| Magnetic separator | Miltenyi | 130-042-302 | Sterile before use |
| Magnetic separator stand | Miltenyi | 130-042-303 | Sterile before use |
| Microbeads | Miltenyi | 130-048-801 | Store aliquots at –4 °C |
| P/S | Solarbio | P1400 | Store aliquots at –20 °C |
| Papain | Solarbio | G8430-25g | Store aliquots at –20 °C |
| PBS | Solarbio | D1040 | Store aliquots at –4 °C |
| PDPN antibody | Santa | sc-53533 | Store aliquots at –4 °C |
| PFA | Solarbio | P1110 | Store aliquots at –4 °C |
| PROX1 antibody | Santa | sc-81983 | Store aliquots at –4 °C |
| Selection column | Miltenyi | 130-042-401 | Sterile before use |
| Trypsin | Gibco | 25200072 | Store aliquots at –20 °C |
| VEGF-C | Abcam | ab51947 | Store aliquots at –20 °C |
| VEGFR-3 antibody | Santa | sc-514825 | Store aliquots at –4 °C |
Referencias
- Shibata-Germanos, S., et al. Structural and functional conservation of non-lumenized lymphatic endothelial cells in the mammalian leptomeninges. Acta Neuropathologica. 139 (2), 383-401 (2020).
- Suárez, I., Schulte-Merker, S. Cells with many talents: lymphatic endothelial cells in the brain meninges. Cells. 10 (4), 799 (2021).
- Jordan-Williams, K. L., Ruddell, A. Culturing purifies murine lymph node lymphatic endothelium. Lymphatic Research and Biology. 12 (3), 144-149 (2014).
- Jones, B. E., Yong, L. C. Culture and characterization of bovine mesenteric lymphatic endothelium. In vitro Cellular & Developmental Biology. 23 (10), 698-706 (1987).
- Podgrabinska, S., et al. Molecular characterization of lymphatic endothelial cells. Proceedings of the National Academy of Sciences of the United States of America. 99 (25), 16069-16074 (2002).
- Jablon, K. L., et al. Isolation and short-term culturing of primary lymphatic endothelial cells from collecting lymphatics: A techniques study. Microcirculation. 30 (2-3), e12778 (2023).
- Lapinski, P. E., King, P. D. Isolation and culture of mouse lymphatic endothelial cells from lung tissue. Methods in Molecular Biology. 2319, 69-75 (2021).
- Lokmic, Z. Isolation, Identification, and culture of human lymphatic endothelial cells. Methods in Molecular Biology. 1430, 77-90 (2016).
- Thiele, W., Rothley, M., Schmaus, A., Plaumann, D., Sleeman, J. Flow cytometry-based isolation of dermal lymphatic endothelial cells from newborn rats. Lymphology. 47 (4), 177-186 (2014).
- Lokmic, Z., et al. Isolation of human lymphatic endothelial cells by multi-parameter fluorescence-activated cell sorting. Journal of Visualized Experiments. 99, e52691 (2015).
- Janson, C., Romanova, L., Hansen, E., Hubel, A., Lam, C. Immortalization and functional characterization of rat arachnoid cell lines. Neurociencias. 177, 23-34 (2011).
- Romanova, L. G., Hansen, E. A., Lam, C. H. Generation and preliminary characterization of immortalized cell line derived from rat lymphatic capillaries. Microcirculation. 21 (6), 551-561 (2014).
- Park, T. I., et al. Routine culture and study of adult human brain cells from neurosurgical specimens. Nature Protocols. 17 (2), 190-221 (2022).
- Okuda, K. S., et al. lyve1 expression reveals novel lymphatic vessels and new mechanisms for lymphatic vessel development in zebrafish. Development. 139 (13), 2381-2391 (2012).
- Bokobza, C., et al. Magnetic Isolation of microglial cells from neonate mouse for primary cell cultures. Journal of Visualized Experiments. 185, e62964 (2022).
- Wang, J. M., Chen, A. F., Zhang, K. Isolation and primary culture of mouse aortic endothelial cells. Journal of Visualized Experiments. 118, e52965 (2016).
- Breiteneder-Geleff, S., et al. Angiosarcomas express mixed endothelial phenotypes of blood and lymphatic capillaries: podoplanin as a specific marker for lymphatic endothelium. The American Journal of Pathology. 154 (2), 385-394 (1999).
- Petrova, T. V., Koh, G. Y. Organ-specific lymphatic vasculature: From development to pathophysiology. The Journal of Experimental Medicine. 215 (1), 35-49 (2018).
- Wilting, J., et al. The transcription factor Prox1 is a marker for lymphatic endothelial cells in normal and diseased human tissues. The FASEB Journal. 16 (10), 1271-1273 (2002).
- Yin, X., et al. Lymphatic endothelial heparan sulfate deficiency results in altered growth responses to vascular endothelial growth factor-C (VEGF-C). The Journal of Biological Chemistry. 286 (17), 14952-14962 (2011).
- DeSisto, J., et al. Single-cell transcriptomic analyses of the developing meninges reveal meningeal fibroblast diversity and function. Developmental Cell. 54 (1), 43-59 (2020).
- Chen, Z., et al. A method for eliminating fibroblast contamination in mouse and human primary corneal epithelial cell cultures. Current Eye Research. , 1-11 (2023).
- Starzonek, C., et al. Enrichment of human dermal stem cells from primary cell cultures through the elimination of fibroblasts. Cells. 12 (6), 949 (2023).
- Lam, C. H., Romanova, L., Hubel, A., Janson, C., Hansen, E. A. The influence of fibroblast on the arachnoid leptomeningeal cells in vitro. Brain Research. 1657, 109-119 (2017).

