Automated System for Single Molecule Fluorescence Measurements of Surface-immobilized Biomolecules
Summary
In this article we describe how we obtain FRET traces from individual DNA molecules immobilized to a surface using an automated scanning confocal microscope.
Abstract
Protocol
1 – Assembling the microfluidic cell
The microfluidic cell is made by fusing a patterned, 2 mm thick, polydimethylsiloxane (PDMS) disk to a freshly cleaned quartz cover slip. The PDMS disk contains four 30 μl rectangular chambers that are accessed by inlet and outlet flexible capillaries connected to a buffer reservoir and a syringe pump, respectively, for the controlled flow of buffer. The flow cell is supported on its back side by a glass window and placed on a custom-made cell holder containing a water cooled thermoelectric element to regulate temperature.
- To make the patterned PDMS disk, mix 10 parts silicone elastomer base with 1 part curing agent, mix well and leave degassing for 1 hour in vacuum.
- Gently pour the elastomer mix onto the microfluidic cell mold. In our case, this is a 1″ silicon wafer with four strips (1×104 x 1×103 x 300 μm) made from negative photoresist. Leave this to cure on a hot plate at 70° C for two hours.
- Carefully peel the cured PDMS disk from the mold using a razor blade. Fuse the disk onto a 1” glass window (containing 8 x 2 mm-diameter pre-drilled holes at both ends of the channels) by plasma oxidizing both surfaces for 30 seconds. Fuse the glass windows onto the flat side of the PDMS disk (the side not containing the channels).
- Insert a 2-inch long flexible fused silica capillary tubing through each glass window’s hole until it reaches the channel. Inserting a needle first and then following with the capillary may facilitate this process. Seal the glass window holes using a fast curing silicone casting compound such as Kwik-Cast.
- Rinse the channels with ethanol and water, and plasma-activate the cell together with a freshly cleaned, 1-inch circular quartz cover slip for 30 seconds. We suggest cleaning this cover slip using piranha*. Fuse the cover slip to the side of the cell containing the channels, sealing the chambers.
- Leave the assembled cell to cure overnight at room temperature.
* Piranha is a solution of H2O2 and H2SO4 in 1:2.5 proportions. To clean the cover slips, immerse them in piranha at 90° C for 20 min.
2 – Activating the surface for molecule immobilization
For nucleic acid studies, the simplest surface immobilization scheme consists of first coating a glass or quartz cover slip with biotinylated bovine serum albumin (BSA) and then with streptavidin. This allows the biotinylated sample to be immobilized with high specificity for days at room temperature.
- Connect flexible tubing to the capillaries for easy buffer injection using a syringe and needle.
- Inject a solution of 0.1 mg/ml of biotinylated BSA in buffer A (10 mM TRIS-HCl, pH 8.0, 50 mM NaCl, filtered using 0.02 μm filters) into the channel and incubate for at least 30 min.
- Flow 300-500 μl of buffer A to remove any free biotinylated BSA from the channel, being careful not to trap any air bubbles inside the chamber.
- Inject a solution of 0.1 mg/ml of streptavidin in buffer A and incubate for 15 min. Then repeat Step 3.
- Flow through a 3-5 pM solution of the biotinylated sample. Perform a surface scan to check the coverage of fluorescent molecules. If necessary, increase the sample concentration to obtain better coverage. Incubate for 10 min and repeat Step 3.
The microfluidic cell is mounted on a motorized x-y stage allowing coarse (up to 25 mm) motion using optically encoded DC motors, and fine (1 nm) motion using two closed-loop piezo actuators (Physik Instrumente model M-014 or similar). This can be done either before or after activating the surface for sample immobilization.
3 – Preparing the Imaging Buffer (IB)
The IB consists of an enzymatic oxygen scavenging system and a triplet quencher, such as Trolox. Since the IB is flushed through constantly overnight, at least 10 ml should be prepared in advance.
- Prepare 10 ml of 0.8% w/v D-glucose, at least 35 mM TRIS-HCl pH 8.0 and 50 mM NaCl in deionized water. The higher concentration of buffer is important for two reasons: dissolving Trolox will acidify the solution, and the enzymatic reaction will also lower the pH of the solution over time. Since this solution has a long shelf life it can be stored as a stock solution.
- Dissolve 5 mg of Trolox in the buffer prepared in Step 1 by vortexing for a couple of minutes and then shaking for >10 min. Trolox is not very soluble in water at pH > 7, so do not rush this step. Filter the solution using a 0.2 μm filter and leave degassing in vacuum for 10 minutes.
- Prepare the “gloxy” enzymatic solution by mixing 60 μl water, 20 μl of 5X buffer A, 20 μl of catalase, 1 mg glucose oxidase and 100 μl of 10 mg/ml BSA. Filter the solution using a 0.22 μm centrifuge filter.
- Gently mix the buffer from Step 2 with the “gloxy” solution from Step 3 avoiding the formation of air bubbles.
- Flow the IB through the channel at a constant rate of 5 μl/min using a mechanical syringe pump to control the flow rate. Bubble argon gas into the IB reservoir to prevent atmospheric oxygen from re-dissolving into the buffer.
The surface scanning, molecule localization and acquisition of single molecule intensity traces is controlled by a LabView program that permits automated data collection1. The program scans 20×20 μm2 areas at 0.2 μm resolution, with a rate of 0.1 μm/ms. Data is immediately processed to yield intensity-weighted locations of all pixels above the background counts. Once the immobilized molecules are found, they are moved one by one into the confocal volume and the intensities of the donor and acceptor fluorophore are recorded as a function of time until both fluorophores photobleach. The stage is then programmed to move to a new origin 100 μm away, and the scanning process is repeated.
4 – Representative Results
Below are some images showing the assembled microfluidic cell before surface activation and after being mounted on the microscope ready for molecule immobilization. If the channel is activated successfully, surface scans should be similar to the scans depicted below showing emission of the donor dye (green) and acceptor (red) after direct donor excitation. These molecules are moved one by one into the probing volume and the fluorescence is recorded over time until both dyes photobleach, as shown in the single molecule traces. From traces like these one can obtain the FRET efficiency which informs on the instantaneous donor-acceptor separation, which in turn is used to explore the structure and dynamics of molecules of biological interest.
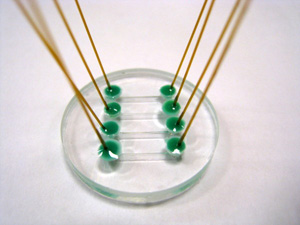
Figure 1: Microfluidic cell with four channels, each with inlet/outlet capillaries. Please click here to see a larger version of figure 1.
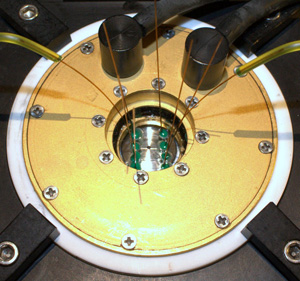
Figure 2: Cell mounted on the microscope ready for measurements. Please click here to see a larger version of figure 2.
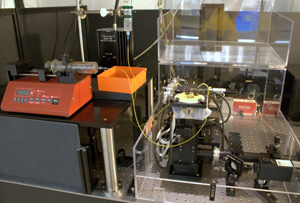
Figure 3: Setup for sm-FRET measurements. Please click here to see a larger version of figure 3.
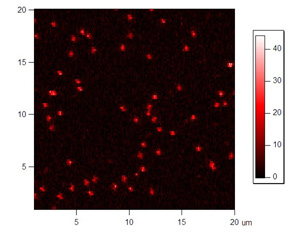
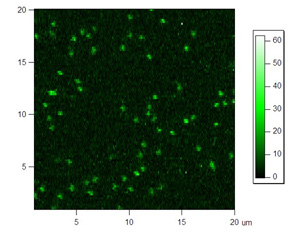
Figures 4 and 5: Red and green channel of a surface scan of immobilized FRET molecules excited with green laser. Please click to see a larger version of figure 4, or figure 5.
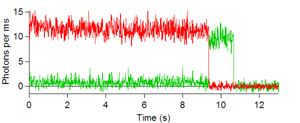
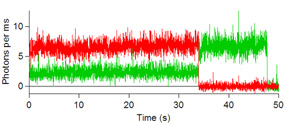
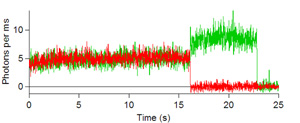
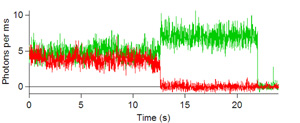
Figures 6, 7, 8, and 9: Intensity trajectories of donor (green) and acceptor (red) fluorophores labeled to a DNA molecule 8, 10, 16 and 18 basepairs apart. Please click to see a larger version of
figure 6, figure 7,figure 8, or figure 9.
Discussion
Although the above protocol has been optimized for our particular system and for a specific choice of dyes (TMR and ATTO647N), it is by no means the only proved method. Recently, an alternative oxygen scavenging system consisting of protocatechuic acid (PCA)/protocatechuate-3,4-dioxygenase (PCD) was shown to increase the photostability of certain dyes4. Likewise, other triplet-state quenchers such as β-mercaptoethanol (BME) can be used instead of Trolox, although these might not suppress long-lasting blinking of some dyes, in particular Cy55.
Although immobilization via biotin-streptavidin-biotinylated bovine serum albumin (BSA) is the preferred choice for nucleic acids studies, a polyethylene glycol (PEG)-coated surface is better suited for studies of proteins since it prevents non-specific adhesion1. Additionally, sub-diffraction-limited size vesicles can be used to encapsulate the biomolecule of interest in cases where it can be affected by surface interactions6.
In this article we have used a confocal setup equipped with silicone avalanche photodiodes to acquire the fluorescence intensity traces. This protocol works equally well with Total Internal Reflection Fluorescence microscopes (TIRF) equipped with CCD cameras. Regardless of the microscopy used, sm-FRET can inform on spatial resolutions approaching the angstrom when the donor and acceptor signal are properly corrected7.
Acknowledgements
We thank Chandran Sabanayagam for his help in developing the system, Joshua Edel for advice on designing the microfluidic device and the staff at the Rowland Institute at Harvard University for machining parts of the setup. We acknowledge useful discussions with members of Meller’s group at the Rowland Institute and at Boston University, and members of T. Ha’s group at UIUC for helpful information. Finally, we appreciate the financial support from the National Science Foundation (PHY-0701207) and the National Institute of Health (GM075893).
Materials
| Material Name | Type | Company | Catalogue Number | Comment |
|---|---|---|---|---|
| Fused silica capillary tubing | Polymicro Technologies | TSP150375 | ||
| Fused silica cover slip | Esco Products | R425000 | ||
| Tubing | Diba Industries | |||
| Biotinylated BSA | Sigma | A8549 | ||
| BSA | Sigma | A9085 | ||
| Streptavidin | Thermo Scientific | 0021122 | ||
| Sylgard 184 Elastomer Kit | DowCorning | 3097366-1004 | Silicone Elastomer Kit | |
| Trolox | Aldrich | 238813 | ||
| Catalase | Sigma | C3155 | ||
| Glucose Oxidase | Sigma | G2133 | ||
| D-Glucose | Sigma | G7528 | ||
| Kwik-Cast Sealant | World Precision Instruments | Kwik-Cast | Silicone Casting Compound |
References
- Selvin, P. R., Ha, T. Single-Molecule Techniques, a Laboratory Manual. CSHL Press. , (2008).
- Sabanayagam, C. R., Eid, J. S., Meller, A. High-throughput scanning confocal microscope for single molecule analysis. Applied Pys. Letters. , 84-87 (2004).
- Sabanayagam, C. R., Eid, J. S., Meller, A. Using Fluorescence resonance energy transfer to measure distances along individual DNA molecules: Corrections due to nonideal transfer. J. Chem. Phys. 122, 61103-61105 (2004).
- Echeverra, A., Aitken, C., Marshall, R. A., Puglisi, J. D. An Oxygen Scavenging System for Improvement of Dye Stability in Single-Molecule Fluorescence Experiments. Biophysical Journal. 94, 1826-1835 (2008).
- Rasnik, I., McKinney, S. A., Ha, T. Nonblinking and long-lasting single molecule fluorescence imaging. Nature Methods. 3 (11), 891-893 (2006).
- Boukobza, E., Sonnenfeld, A., Haran, G., Puglisi, J. D. Immobilization in Surface-Tethered Lipid Vesicles as a New Tool for Single Biomolecule Spectroscopy. J. Phys. Chem. 105, 12165-12170 (2001).
- Di Fiori, N., Meller, A. . Article in preparation. , .

