Langendorff Retrograde Heart Perfusion: An Ex Vivo Technique to Perfuse Isolated Mouse Heart
Abstract
Source: Wu, K. et al. Modifications of the Langendorff Method for Simultaneous Isolation of Atrial and Ventricular Myocytes from Adult Mice. J. Vis. Exp. (2021)
In this video, we perform Langendorff retrograde perfusion of an isolated mouse heart. During the procedure, the cardiac activity of the heart is maintained by perfusing it retrogradely with a perfusion solution via the coronary arteries using a cannula inserted into the ascending aorta. The model is used to study the cardiac pathophysiology of the heart.
Protocol
All procedures involving animal models have been reviewed by the local institutional animal care committee and the JoVE veterinary review board.
1. Solution and Perfusion Apparatus Preparation
- Assemble a constant flow Langendorff apparatus (commercially available or self-made). Figure 1 provides a simple schematic.
- Prepare the solutions according to the reagents given in Table 1 and Table 2.
- Turn on the circulating water bath and adjust a suitable input temperature (42.7 °C in our laboratory) to ensure that the perfusate circulation system's outflow from the cannula reaches 37 °C.
- Clean the perfusion system by circulating deionized water. If a sterile condition is required, run 75% ethanol through the perfusion system for 15 min, followed by deionized water (at least 10 cycles to avoid alcohol toxicity effects on the heart).
- Measure the time and volume for one cycle of the perfusate circulation system to determine how many milliliters of oxygenated Tyrode's solution to be loaded in the following perfusion step.
- Sterilize all the surgical tools in the desired method.
- Set the flow rate of the perfusate perfusion system at 4 mL/min.
- Prepare two clean 35 mm Petri dishes—one containing Tyrode's solution (Petri dish 1), the other containing solution 1 (Petri dish 2).
- Prepare a 1 mL syringe filled with solution 1 and a 20 G blunt steel cannula needle with a notch from where the distance is 1 mm to the tip. Prepare a loose knot with 3-0 suture to the cannula shaft. Regulate the viewing field of the stereomicroscope and ensure that the cannula tip is below the liquid surface of Petri dish 2.
2. Animal Preparation
- Administer heparin (concentration, 1,000 IU/mL; 0.2 mL/mouse) intraperitoneally to the mice to avoid blood clots. After 10 min, anesthetize the mice with sodium pentobarbital (50 mg/kg, I.P. injection) and ensure that the mice stop responding to tail/toe pinches. Perform cervical dislocation 20 min after heparin administration.
- Transfer the mice onto the surgical platform, fix the mice in a supine position, sterilize the chest with 75% ethanol, and dry it with gauze or napkin.
3. Heart Excision and Aorta Cannulation
- Lift the skin of the xiphoid with tissue forceps and make a minor lateral incision through the skin with tissue scissors. Perform a blunt dissection between the skin and fascia and extend the incision of the skin in a V-shape toward the axillae on both sides.
- Continue the same incision trace through the ribcage, and then, deflect the rib cage upward by clamping the sternum with tissue forceps to fully expose the heart and lungs.
- Peel off the pericardium using a curved forceps. Tear the thymus gland toward both sides by two curved forceps if it covers the great vessels. Gently pull the base of the heart toward the tail with curved forceps until a "Y"-shaped blood vessel (the aorta and its branch arteries) can be seen.
- To reserve different lengths of the aorta for cannulation and comparison, transect the aorta at the left common carotid artery (green line in Figure 2) and cut the brachiocephalic artery at the same time in some mice. Transect at the ascending aorta in other mice (black line in Figure 2).
NOTE: For those who are new to this procedure, this step can also be done under a stereoscopic microscope. - Excise the heart, and immediately immerse in Petri dish 1 to wash and pump out the residual blood.
- Transfer the heart to Petri dish 2 and trim any surplus tissue using fine iris scissors if necessary. For those who are new to this procedure, do this step under the stereomicroscope to avoid erroneously cutting the aorta or other heart structures.
- Push the syringe to expel air bubbles before aorta cannulation. Perform retrograde aorta cannulation with the assistance of two straight tying forceps (smooth jaws) under the stereomicroscope. Ensure that the whole cannulation process is under the liquid surface.
- Avoid penetrating the aortic valves and adjust the depths to the ascending aorta (the mouse that the aorta was transected at the left common carotid artery) and the aortic root (the mouse that the aorta was transected at the ascending aorta), respectively.
NOTE: The depth of aorta cannulation (referred to as simply as depth) is defined here as the position where the cannula tip is in the aorta or where the aorta is ligated. - Ligate the aorta with the pre-knot 3-0 suture to the cannula notch. Gently press the syringe content to rinse the residual blood.
NOTE: The blood will leave the coronary arteries and effuse from the dorsal veins If the depth is properly positioned. The heart and the atrial appendages will expand and become pale. - Remove and connect the cannula to the Langendorff apparatus. Once again, take care to avoid any air bubbles entering the heart.
NOTE: Be aware that the time from thoracotomy to initial perfusion should not exceed 5 min. - For heart perfusion, first, prime and perfuse oxygenated Tyrode's solution for approximately 10 s (the volume of liquid running 10 s in the system used in this protocol is approximately 0.7 mL) if there is residual blood in the atria, and then switch to solution 1. However, just perfuse with solution 1 if there is no residual blood in the atria. Perfuse the heart for approximately 2 min.
Table 1. Solutions for adult mouse CM isolation.
| Solution | Contents (final concentration in mmol/L, if not specified differently) | Noted |
| Perfusion solution (solution 1) | 113 NaCl, 4.7 KCl, 0.6 KH2PO4, 0.6 Na2HPO4, 1.2 MgSO4, 12 NaHCO3, 10 KHCO3, 10 HEPES, 15 Taurine, 5 Glucose, 10 2,3-butanedione monoxime(BDM) | Which can be stored for 3 days at 4 °C. Glucose, Taurine and BDM are added on the day of experiment. Prepare 200 mL of perfusion solution for each heart. |
| Tyrode’s solution (solution 2) | 140 NaCl, 4 KCl, 1 MgCl2, 10 HEPES, 1 CaCl2, 5 Glucose | Which can be stored for 3 days at 4 °C. CaCl2 and Glucose are added on the day of experiment, adjust pH to 7.3-7.4 with saturated NaOH at room temperature (28-30°C) with a PH meter. |
Table 2. Solutions for adult mouse CM isolation and storage.
| Solution | Contents | Noted |
| Digestion solution(solution 3) | 25 mL solution 1, 6μL 100 mM/L CaCl2, 25mg collagenase II, 50μL (2.5 % 10×) trypsin | For each heart |
| Stop solution 1(solution 4) | 9 mL solution 1, 1 mL FBS (Fetal Bovine Serum), 4μL 100 mM/L CaCl2 | For each heart |
| Stop solution 2(solution 5) | 9.5 mL solution 1, 0.5 mL FBS (Fetal Bovine Serum), 4μL 100 mM/L CaCl2 | For each heart |
| Cell resuspension solution(solution 6) | 13 mL solution 1, 7μL 1M/L CaCl2, BSA (Bull Serum Albumin) at a dose that can form a thin layer covering the surface of the liquid | For each heart |
Representative Results
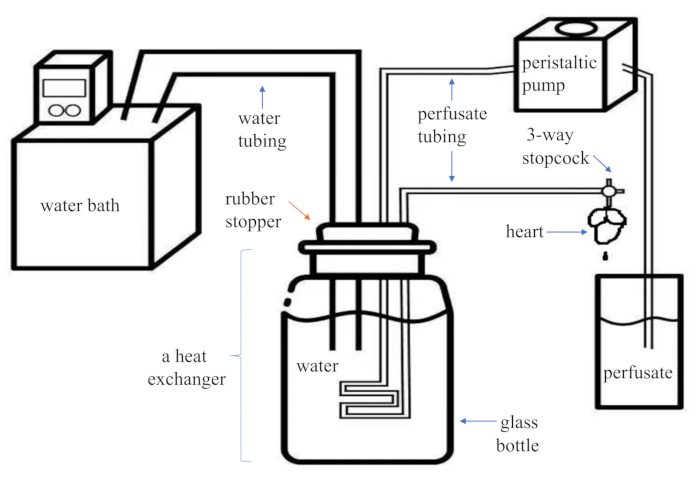
Figure 1. Schematic diagram of an assembled modified Langendorff system. This system is economical and portable for the users. It has two parts of plastic tubing-one for the water (inner diameter, 8 mm) and one for the perfusate (inner diameter, 1.5 mm)-of which the distal end is connected to a PE medical three-way stopcock with a Luer lock. A glass bottle containing water and with a rubber stopper form as a heat exchanger. The perfusate is pumped into the plastic tubing in the heat exchanger by the peristaltic pump and comes out from the three-way stopcock connected to the cannula.
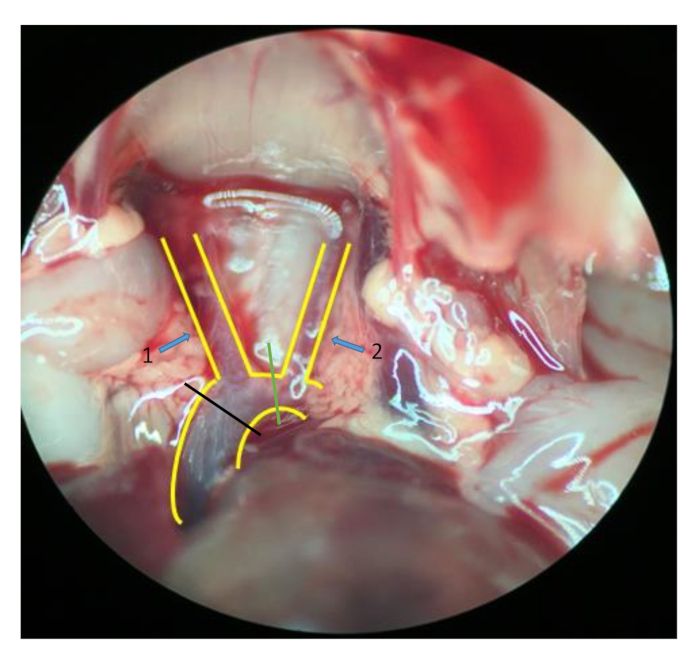
Figure 2: Aorta and tissues around. A "Y"-shaped blood vessel structure is the aorta and its branches: the brachiocephalic artery (arrow 1) and the left common carotid artery (arrow 2). Transect the aorta between the left common carotid artery (green line) and simultaneously cut the brachiocephalic artery in some mice; transect at the ascending aorta (black line) in other mice (stereomicroscope 10 × 2 magnification).
Divulgations
The authors have nothing to disclose.
Materials
| 2,3-butanedione monoxime (BDM) | Sigma-Aldrich | 31550 | |
| CaCl2 | Sigma-Aldrich | C4901 | |
| Glucose | Sigma-Aldrich | G7528 | |
| HEPES | Sigma-Aldrich | H3375 | |
| KCl | Sigma-Aldrich | P9333 | |
| KH2PO4 | Sigma-Aldrich | P5655 | |
| KHCO3 | Sigma-Aldrich | 237205 | |
| MgCl2 | Sigma-Aldrich | M8266 | |
| MgSO4 | Sigma-Aldrich | M7506 | |
| Na2HPO4 | Sigma-Aldrich | S7907 | |
| NaCl | Sigma-Aldrich | S7653 | |
| NaHCO3 | Sigma-Aldrich | S5761 | |
| Peristaltic pump | Longerpump | BT100-2J | |
| Taurine | Sigma-Aldrich | T0625 | |
| Water bath | JULABO | ED (v.2) |

