Functional Assessment of Antibody-Secreting B Cells Using ELISpot Assay
Abstract
Source: Tzeng, S. J. The Isolation, Differentiation, and Quantification of Human Antibody-secreting B Cells from Blood: ELISpot as a Functional Readout of Humoral Immunity. J. Vis. Exp. (2016)
This video demonstrates the use of the ELISpot assay to assess the function of antibody-secreting B cells, ASCs. The antibodies secreted by the ASCs interact with the membrane-bound polyclonal antibody fragments, which are detected by the enzyme-conjugated IgG or IgM antibodies. The colored spot produced in each well indicates the presence of the specific antibody's isotype, which correlates with the B cells' secretory activity.
Protocol
All procedures involving human participants have been performed in compliance with the institutional, national, and international guidelines for human welfare and have been reviewed by the local institutional review board.
Human peripheral blood must be obtained from healthy donors under informed consent, and the use of blood samples must conform to the approved guidelines established by review boards. In this study, the protocol to use human blood in a demonstration of the results of flow cytometry (Figure 1) and ELISpot assays (Figure 2).
1. Isolation and Purification of Human Peripheral Blood B Cells
- Draw ~ 10 mL of blood from the median cubital vein (in the cubital fossa anterior to the elbow) into a 15-mL tube containing K2EDTA (1.5 to 2.0 mg/mL blood) and immediately invert the tube several times to prevent clot formation.
- Add 35 mL of autoclaved (121 °C, 15 min) red blood cell (RBC) lysis buffer (150 mM NH4Cl, 10 mM KHCO3, and 1 mM EDTA; pH 7.4) to the tube containing the fresh blood sample (≥ 3:1 vol/vol) and incubate at room temperature (RT) for no longer than 5 min.
NOTE: The appearance of light transmission through the tube indicates the completion of RBC lysis. - Centrifuge at 600 x g at RT for 5 min. Ensure that the pellet is white in color.
- Resuspend the pellet with 10 mL of autoclaved phosphate-buffered saline (PBS, 137 mM NaCl, 2.7 mM KCl, 4.3 mM Na2HPO4, and 1.47 mM KH2PO4; pH 7.4) and centrifuge as in step 1.3.
- Discard the supernatant, resuspend the pellet with 10 mL of RPMI 1640 medium (supplements: 10% fetal calf serum, 100 U/mL penicillin/streptomycin, 0.25 µg/mL amphotericin B, and 2 mM L-glutamine), and then plate the cells into a 10-cm culture dish (10 mL of blood per dish). Place the dish in a 37 °C incubator with 5% CO2 for 30 min.
- Gently swirl the culture dish a few times and place the culture medium (suspension cells) into 15-mL plastic conical tubes. Discard the adherent cells (mostly macrophages) on the culture dishes.
- Centrifuge at 600 x g at RT for 5 min. Discard the supernatant.
- Resuspend the pellet with 1 mL of RPMI 1640 medium. Count the cell number with a hemocytometer or an automated cell counter.
NOTE: The viability of isolated leukocytes is normally greater than 90% by trypan blue exclusion. - Centrifuge as in step 1.7 and resuspend the cells in ~ 200 µL of cold PBS buffer (0.5% bovine serum albumin (BSA) and 2 mM EDTA) at the concentration of 5 – 10 x 106 cells/mL.
- Add 5 µL of biotinylated anti-human Ab cocktail specific to blood cells (for the negative selection of B cells) per 106 cells and incubate on ice for 30 min.
NOTE: The anti-human Ab cocktail should at least include Abs specific to CD2 (or CD3), CD14, and CD16. - Add a 10-fold excess volume of sterile PBS to the cells, centrifuge at 600 x g for 5 min, and discard the supernatant.
- Add equal amounts of streptavidin-conjugated microbeads (5 µL per 106 cells) to the pellet and mix thoroughly.
- Incubate on ice for 30 min in a 15-mL plastic conical tube.
- Add 2 mL of PBS buffer into the tube.
- Place the tube into a magnetic stand and incubate at RT for 8 min. The brown microbeads will gradually attach to the tube wall next to the magnet.
- With the tube remaining in the magnetic stand, carefully transfer the supernatant into a new sterile 15-mL tube.
- Repeat steps 1.14 to 1.16 and combine the two supernatants that contain the untouched B cells. Discard the microbeads.
- Centrifuge at 600 x g at RT for 5 min. Discard the supernatant.
- Resuspend the pellet in RPMI 1640 medium for downstream experiments.
NOTE: Typically, 1 – 5 x 105 B cells with a purity greater than 95% from 10 mL of peripheral blood can be isolated.
2. Purification and Separation of Memory and Naïve B Cells from Isolated B Cells
- Use cells purified from Step 1 and determine the cell number using a hemocytometer or an automatic cell counter.
- Resuspend the cells in 100 µL of cold PBS buffer after centrifugation at 600 x g and RT for 5 min.
- Add 1 – 2 µg of biotinylated CD27 mAb per 106 cells and incubate on ice for 30 min.
- Add 10 mL of PBS buffer to the tube and centrifuge at 600 x g for 5 min.
- Discard the supernatant and resuspend the cells in 50 µL of PBS buffer.
- Add equal amounts of streptavidin magnetic microbeads (1 – 2 µg/106 cells) to cells in a 15-mL plastic conical tube.
- Gently mix well and incubate on ice for 30 min.
- Add 2 – 3 mL of PBS buffer to the tube.
- Place the tube into a magnetic stand and incubate at RT for 8 min to allow the brown microbeads to attach to the side closest to the magnet.
- With the tube in the magnetic stand, carefully transfer the supernatant fraction into a new sterile tube. This fraction contains the enriched CD27– naïve B cells.
- Add 5 mL to the tube containing the microbeads and gently resuspend the microbeads (i.e., the enriched fraction of CD27+ memory B cells).
- Centrifuge at 600 x g at RT for 5 min. Resuspend the pellets with RPMI 1640 medium for the downstream experiments.
NOTE: Typically, ~ 30 – 60% of B cells can be purified as CD27+ memory cells from the PBMCs of a healthy donor.
3. Cell Sorting for the Collection of Naïve B Cells, Memory B Cells, and PBs/PCs
- Using the cells purified from Step 1, determine the cell number using a hemocytometer or an automatic cell counter.
- Resuspend the cells in cold PBS buffer at the concentration of 107 per mL in a 5-mL polystyrene tube.
- Add 1 – 2 µg of human IgG per 106 cells and incubate on ice for 10 min for the Fc block.
- Add 1 µg each of anti-CD19-APC (clone: HIB19), anti-CD27-eFluor450 (clone: O323), and anti-CD38-PE (clone: HIT2) per 106 cells; mix well and incubate on ice for 30 min.
- In the last 5 min in step 3.4, add 5 µL of the commercial 7-amino actinomycin D (7-AAD).
- Add 2 mL of PBS to the tube, vortex, and centrifuge at 600 x g for 5 min.
- Resuspend the cells in sorting buffer (sterile PBS with 2% BSA and 2 mM EDTA) at a concentration of 1 – 5 x 107 cells per mL in a 15-mL tube.
- Filter the cells through a nylon mesh cell strainer (40 µm pore size) to eliminate cell clumps.
- Separate the cells with a flow cytometric sorter equipped with three lasers: violet (405 nm), blue (488 nm), and red (640 nm).
NOTE: The blue laser alone is sufficient for 3-color flow cytometry. - Sort the cells into three 15-mL tubes (containing 5 mL of RPMI medium) for the simultaneous collection of naïve B cells (CD19+CD27–), memory B cells (CD19+CD27+), and PBs/PCs (CD19+CD27+/hiCD38+).
NOTE: Sorted naïve and memory B cells can be cultured as described in step 4.
4. In Vitro Differentiation of Isolated Human CD19+ B Cells, CD19+CD27+ Memory B Cells, and CD19+CD27- Naïve B Cells
- Using the cells purified in steps 1.19, 2.10, 2.11, and 3.10, determine the cell number with a hemocytometer or an automatic cell counter.
- Resuspend the cells with RPMI 1640 medium at a concentration of 1 – 10 x 105 per mL and aliquot them into the wells of a 12-well plate.
- Add CpG (ODN 2006) at 5 µg/106 cells/mL.
- Culture the cells in a 37 °C incubator with 5% CO2 for 5 days.
- Harvest the cells from each well, place them separately into 15-mL tubes, add 5 mL of PBS to each tube, and centrifuge them at 600 x g and RT for 5 min.
- Count the cells using a hemocytometer or an automatic cell counter. Resuspend the cells at a concentration of 1 – 10 x 105 per mL with RPMI 1640 medium.
5. ELISpot Assay
- Add 30 µL of 35% ethanol in distilled water to each well of the ELISpot plates for 30 s.
NOTE: When pipetting, avoid touching the membrane in the wells at all times. - Invert the ELISpot plates to remove the ethanol.
- Put 150 µL of autoclaved ddH2O into each well and incubate them at RT for 5 min to flush off the residual ethanol; follow with a wash of sterile PBS at RT for 3 min.
NOTE: Steps 5.1 to 5.3 may be optional, depending on the manufacture of the plates. - Put 50 µL of 5 µg/mL polyclonal F(ab')2 fragment of anti-human Ig (IgG + IgM + IgA) (in PBS) into each well of the ELISpot plates and incubate them at 4 °C overnight (preferred) or 37 °C for 2 h.
NOTE: Seal the edges of plates with Parafilm until use. - Invert the plates to remove unbound Abs, add 200 µL of PBS to each well, and incubate them twice at RT for 3 min each time.
- Add 200 µL of PBS with 5% BSA (or RPMI 1640 medium) to each well for blocking, and incubate them at RT for 2 h.
- Invert the plates to remove the blocking buffer. Wash each well twice with 200 µL of PBS, as in step 5.5.
- Add 100 µL of RPMI 1640 medium to each well and incubate them at 37 °C.
- When ready to seed the cells, invert the plates to remove the RPMI 1640 medium.
- Seed 100 µL (5 x 104), 50 µL (2.5 x 104), and 25 µL (1.25 x 104) of the cells (from steps 1.19, 2.10, 2.11, 3.10, and 4.6) into the wells of an ELISpot plate. With RPMI 1640 medium, bring the volume to 150 µL/well.
NOTE: A minimum of one or two 2-fold serial dilutions for plating the cells is recommended. - Incubate the plates in a 37 °C incubator with 5% CO2 for 8 – 14 h. Avoid moving the plates during incubation.
- Invert the plates to remove the cells and RPMI 1640 medium.
- Add 200 µL/well of PBS-T (PBS with 0.05% Tween 20) and incubate them 5 times at RT, each time for 3 min. Invert the plate to remove the wash buffer between each of the 5 washes.
- Add either goat anti-human IgG-alkaline phosphatase (AP), Fcγ-specific Abs (for IgG detection, 1:5,000 in PBS-T) or goat anti-human IgM-AP, Fcµ fragment-specific Abs (for IgM detection, 1:5,000 in PBS-T) into the designated wells and incubate at RT for 2 h in the dark.
- Wash each well twice with 200 µL of PBS, as in step 5.5.
- Add 50 µL/well of bromochloroindolyl phosphate-nitro blue tetrazolium (BCIP/NBT) substrate solution. Purple-colored spots normally appear in 5 – 15 min.
- Add 100 µL/well of ddH2O to prevent the over-development of spots.
- Rinse the plates with running tap water after the complete development of all spots.
- Remove the underdrain of the plates and allow them to air dry in the dark.
- Count the spots using an automated plate reader with an image acquisition/analysis unit (e.g., an automatic scanner or manually via a dissecting microscope).
NOTE: The plates can be stored at RT in the dark and analyzed later.
Representative Results
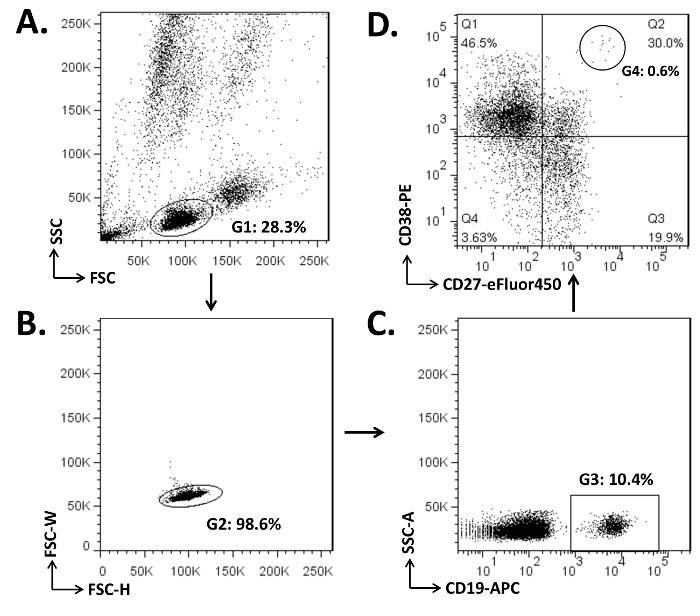
Figure 1. Illustration of the Gating Strategy to Separate the Naïve B cells, Memory B cells, and PBs in the PBMCs. (A) Cells (2 x 106) were stained with 2 µg each of CD19-APC, CD27-eFluor450, and CD38-PE mAbs (step 3.4). The lymphocyte compartment was gated (G1) on the dot plot for forward scattering (FSC) versus side scattering (SSC). (B) Cell doublets, which are formed by two cells stuck together, were excluded (those outside G2) on the plot for FSC-W (width) versus FSC-H (height). (C) CD19+ B cells were gated as G3 on the plot for SSC-A (area) and CD19-APC. (D) Of the CD19+ cells, the dot plot of CD27 and CD38 parameters showed naïve B cells (CD19+CD27–; Q1 and Q4), memory B cells (CD19+CD27+; Q2 and Q3), and PBs (CD19+CD27+/hiCD38+; G4, 0.6% of Q2).
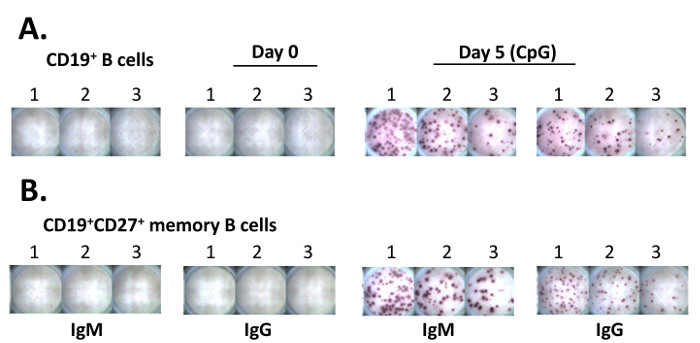
Figure 2. ELISpot Results from a Representative Plate. (A) Purified CD19+ B cells were placed into three adjacent wells (50,000 cells in well # 1, 25,000 cells in well # 2, and 12,500 cells in well # 3, respectively) of the ELISpot plate. Cells were then cultured in RPMI 1640 medium overnight (~14 h). The ELISpot assay was performed after completing the culture (steps 5.12 to 5.18). To analyze the results, the ELISpot plate was scanned to acquire images via an automatic analyzer equipped with the scanner and software. (B) Purified CD19+CD27+ memory cells (106/mL) were cultured in the presence of 5 µg/mL of CpG for 5 days. The cells were treated as in (A) for the ELISpot assay.
Divulgations
The authors have nothing to disclose.
Materials
| BD Vacutainer K2E | BD Biosciences | 367525 | 10 ml tube |
| Ficoll-Paque Plus | GE Healthcare | 17-1440-02 | Endotoxin-free |
| Trypan blue 0.5% solution | Biological Industries | 03-102-1B | |
| IMag Human B lymphocyte enrichment set | BD Biosciences | 558007 | |
| Biotinylated CD27 mAb | Biolegend | 302804 | Clone O323 |
| Streptavidin magnetic microbeads | BD Biosciences | 9000810 | |
| 15 ml Falcon tubes | BD Falcon | 352196 | |
| Blue nylon mesh cell strainer, 40 μm | BD Falcon | 352340 | |
| Anti-human CD19-APC | Biolegend | 302212 | Clone HIB19 |
| Anti-human CD27-eFluor 450 | eBioscience | 48-0279-42 | Clone O323 |
| Anti-human CD38-PE-Cy7 | Biolegend | 303516 | Clone HIT2 |
| Anti-human CD38-PE-Cy7 | BD Biosciences | 560677 | Clone HIT2 |
| Anti-human CD45-FITC | Biolegend | 304006 | Clone HI30 |
| Anti-human CD45-FITC | BD Biosciences | 555482 | Clone HI30 |
| Anti-mouse/rat/human CD27-PerCP Cy5.5 | Biolegend | 124213 | Clone LG.3A10 |
| Anti-human CD27-PerCP Cy5.5 | BD Biosciences | 65429 | Clone L128 |
| Anti-human CD19-FITC | Miltenyi Biotec | 130-098-064 | Clone LT19 |
| Anti-human CD19-FITC | GeneTex | GTX75599 | Clone LT19 |
| Anti-human CD20-FITC | BD Biosciences | 555622 | Clone 2H7 |
| Biotinylated anti-human CD27 | Biolegend | 302804 | Clone O323 |
| Biotinylated anti-human CD27 | eBioscience | 13-0279-80 | Clone O323 |
| 7-aminoactinomycin D (7-AAD) | BD Biosciences | 559925 | |
| CpG (ODN 2006) | InvivoGen | tlrl-2006 | Type B CpG |
| Recombinant human IL-2 | PeproTech | 200-02 | |
| Recombinant human IL-10 | PeproTech | 200-10 | |
| Recombinant human IL-21 | PeproTech | 200-21 | |
| Recombinant human sCD40L | PeproTech | 310-02 | |
| Protein A of S. aureus Cowan (SAC) | Sigma-Aldrich | 82526 | |
| Pokeweed mitogen (PWM) | Sigma-Aldrich | L9379 | |
| MultiScreen filter plates, 0.45 µm pore size | Merck Millipore | MSIPS4510 | Sterile, clear 96-well filter plate with hydrophobic PVDF membrane |
| BCIP/NBT solution | Sigma-Aldrich | B6404 | |
| BCIP/NBT single reagent, alkaline phosphatase substrate | Merck Millipore | ES006 | |
| Human IgG | Jackson ImmunoResearch | 009-000-003 | |
| Human IgG, Fc fragment | Jackson ImmunoResearch | 009-000-008 | |
| F(ab')2 fragment of goat anti-human Ig (IgG+IgM+IgA) | Jackson ImmunoResearch | 109-006-127 | |
| Goat anti-human IgG-alkaline phosphatase, Fcγ fragment specific | Jackson ImmunoResearch | 109-055-008 | |
| Goat anti-human IgM-alkaline phosphatase, Fcµ fragment specific | Jackson ImmunoResearch | 109-055-095 | |
| Goat anti-human IgG-peroxidase, Fcγ fragment specific | Jackson ImmunoResearch | 109-035-008 | |
| Goat anti-human IgM-peroxidase, Fcµ fragment specific | Jackson ImmunoResearch | 109-035-095 | |
| BD ELISPOT AEC substrate kit | BD Biosciences | 551951 | |
| C.T.L. ImmunoSpot analyzer | C.T.L. |

